Do Oral Antiseptics Affect the Force Degradation of Elastomeric Chains?
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Study Protocol
2.3. Sample Size Calculation
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pithon, M.M.; Rodrigues, A.C.; Sousa, E.L.; Santos, L.P.; Soares Ndos, S. Do mouthwashes with and without bleaching agents degrade the force of elastomeric chains? Angle Orthod. 2013, 83, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.; Lobner, D. In vitro neuronal cytotoxicity of latex and nonlatex orthodontic elastics. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Pithon, M.M.; Macena, J.A.; Prado, M.C.; Pinto, H.B. Effects of Active Substances in Dentifrices on Force Degradation of Elastomeric Chain Elastics. J. Indian Orthod. Soc. 2014, 48, 27–32. [Google Scholar] [CrossRef]
- Freeman, D.H.; Johnston, W.M.; Brantley, M.A.; Firestone, A.R. Idealized force decay of orthodontic elastomeric chains follows Nutting Equation. Med. Devices Sens. 2021, 4, e10145. [Google Scholar] [CrossRef]
- Halimi, A.; Benyahia, H.; Doukkali, A.; Azeroual, M.F.; Zaoui, F. A systematic review of force decay in orthodontic elastomeric power chains. Int. Orthod. 2012, 10, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Andhare, P.; Datana, S.; Agarwal, S.S.; Chopra, S.S. Comparison of in vivo and in vitro force decay of elastomeric chains/modules: A systematic review and meta analysis. J. World Fed. Orthod. 2021, 10, 155–162. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, A.; Bruno, G.; Preo, G.; Gracco, A. Application of Nanotechnology in Orthodontic Materials: A State-of-the-Art Review. Dent. J. 2020, 8, 126. [Google Scholar] [CrossRef]
- Panagiotou, A.; Rossouw, P.E.; Michelogiannakis, D.; Javed, F. Role of Essential Oil-Based Mouthwashes in Controlling Gingivitis in Patients Undergoing Fixed Orthodontic Treatment. A Review of Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 10825. [Google Scholar] [CrossRef]
- Morrier, J.J. Leucomes et traitement orthodontique. Prévention, traitement [White spot lesions and orthodontic treatment. Prevention and treatment]. L’Orthodontie Fr. 2014, 85, 235–244. [Google Scholar]
- Derks, A.; Katsaros, C.; Frencken, J.E.; van’t Hof, M.A.; Kuijpers-Jagtman, A.M. Caries-inhibiting effect of preventive measures during orthodontic treatment with fixed appliances. A systematic review. Caries Res. 2004, 38, 413–420. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Tadakamadla, S.K.; Connelly, S.T.; Sforza, C.; Martín, C. Adverse events associated with home use of mouthrinses: A systematic review. Ther. Adv. Drug Saf. 2019, 10, 2042098619854881. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar]
- D’Amico, F.; Moro, M.; Saracino, M.; Marmiere, M.; Cilona, M.B.; Lloyd-Jones, G.; Zangrillo, A. Efficacy of Cetylpyridinium Chloride mouthwash against SARS-CoV-2: A systematic review of randomized controlled trials. Mol. Oral Microbiol. 2023, 38, 171–180. [Google Scholar] [CrossRef]
- Herrera, D.; Escudero, N.; Pérez, L.; Otheo, M.; Cañete-Sánchez, E.; Pérez, T.; Alonso, B.; Serrano, J.; Palma, J.C.; Sanz, M.; et al. Clinical and microbiological effects of the use of a cetylpyridinium chloride dentifrice and mouth rinse in orthodontic patients: A 3-month randomized clinical trial. Eur. J. Orthod. 2018, 40, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, F.A.; Van der Sluijs, E.; Ciancio, S.G. Can chemical mouthwash agents achieve plaque/gingivitis control? Dent. Clin. N. Am. 2015, 59, 799–829. [Google Scholar] [CrossRef] [PubMed]
- Behnaz, M.; Namvar, F.; Sohrabi, S.; Parishanian, M. Effect of Bleaching Mouthwash on Force Decay of Orthodontic Elastomeric Chains. J. Contemp. Dent. Pract. 2018, 19, 221–225. [Google Scholar] [CrossRef]
- Csekő, K.; Maróti, P.; Helyes, Z.; Told, R.; Riegler, F.; Szalma, J.; Gurdán, Z. The Effect of Extrinsic Factors on the Mechanical Behavior and Structure of Elastic Dental Ligatures and Chains. Polymers 2021, 14, 38. [Google Scholar] [CrossRef]
- Takeda, R.; Sawa, H.; Sasaki, M.; Orba, Y.; Maishi, N.; Tsumita, T.; Ushijima, N.; Hida, Y.; Sano, H.; Kitagawa, Y.; et al. Antiviral Effect of Cetylpyridinium Chloride in Mouthwash on SARS-CoV-2. Sci. Rep. 2022, 12, 14050. [Google Scholar] [CrossRef] [PubMed]
- Castelló, C.A.; Zamora-Martínez, N.; Paredes-Gallardo, V.; Tarazona-Álvarez, B. Effect of Mouthwashes on the Force Decay of Orthodontic Elastomeric Chains: A Systematic Review and Meta-Analysis; University of Valencia: Valencia, Spain, 2017; Volume 5, pp. 115–122. [Google Scholar]
- Javidi, P.; Bashardoust, N.; Shekarbaghani, A. Evaluation of force decay rate in orthodontic elastomeric chains in the environment of various mouthwashes: A systematic review. Dent. Res. J. 2023, 20, 39. [Google Scholar]
- ISO/TS 11405:2015; Dentistry—Testing of adhesion to tooth structure. ISO International Organization for Standardization: Geneva, Switzerland, 2015.
- Issa, A.R.; Kadhum, A.S.; Mohammed, S.A. The Effects of Zinc-Containing Mouthwashes on the Force Degradation of Orthodontic Elastomeric Chains: An In Vitro Study. Int. J. Dent. 2022, 2022, 3557317. [Google Scholar] [CrossRef]
- Eliades, T.; Eliades, G.; Watts, D.C. Structural conformation of in vitro and in vivo aged orthodontic elastomeric modules. Eur. J. Orthod. 1999, 21, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T.; Eliades, G.; Brantley, W.A.; Watts, D.C. Elastomeric ligatures and chains. In Orthodontic Materials: Scientific and Clinical Aspects, 1st ed.; Brantley, W.A., Eliades, T., Eds.; Thieme: Stuttgart, Germany, 2001; pp. 173–189. [Google Scholar]
- Eliades, T.; Eliades, G.; Silikas, N.; Watts, D.C. Tensile properties of orthodontic elastomeric chains. Eur. J. Orthod. 2004, 26, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, M.; Rashed, R.; Khodarahmi, N. Evaluation of the effects of three different mouthwashes on the force decay of orthodontic chains. Dent. Res. J. 2015, 12, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.V.; Madhavan, S.; Chacko, T.; Gopalakrishnan, S.; Jacob, J.; Parayancode, A. Comparative Assessment of Force Decay of the Elastomeric Chain With the Use of Various Mouth Rinses in Simulated Oral Environment: An In Vitro Study. J. Pharm. Bioallied Sci. 2019, 11, S269–S273. [Google Scholar] [CrossRef] [PubMed]
- Larrabee, T.M.; Liu, S.S.; Torres-Gorena, A.; Soto-Rojas, A.; Eckert, G.J.; Stewart, K.T. The effects of varying alcohol concentrations commonly found in mouth rinses on the force decay of elastomeric chain. Angle Orthod. 2012, 82, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, R.A.; Taha, S.S.; Saleem, A.I.; Al-Attar, A.M. The effect of mouth wash containing chlorhexidine on force degradation of colored elastomeric chains. Int. J. Sci. Res. 2015, 6, 892–898. [Google Scholar]
- Al–Ani, R.A. The effect of mouth wash containing alcohol on force degradation of colored elastomeric chains. Indian J. Public Health Res. Dev. 2019, 10, 3212–3218. [Google Scholar] [CrossRef]
- Baty, D.L.; Storie, D.J.; von Fraunhofer, J.A. Synthetic elastomeric chains: A literature review. Am. J. Orthod. Dentofac. Orthop. 1994, 105, 536–542. [Google Scholar] [CrossRef]
- Proffit, W.R.; Fields, H.W.; Larson, B.E.; Sarver, D.M. The Biologic Basis of Orthodontic Therapy. In Contemporary Orthodontics, 6th ed.; Proffit, W.A., Fields, H.W., Larson, B.E., Sarver, D.M., Eds.; Elsevier: St. Louis, MO, USA, 2018; pp. 248–275. [Google Scholar]
- Evans, K.S.; Wood, C.M.; Moffitt, A.H.; Colgan, J.A.; Holman, J.K.; Marshall, S.D.; Pope, D.S.; Sample, L.B.; Sherman, S.L.; Sinclair, P.M.; et al. Sixteen-week analysis of unaltered elastomeric chain relating in vitro force degradation with in vivo extraction space tooth movement. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 727–734. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Mahboobi, S.; Rakhshan, V. Effects of different stretching extents, morphologies, and brands on initial force and force decay of orthodontic elastomeric chains: An in vitro study. Dent. Res. J. 2020, 17, 326–337. [Google Scholar]
- Dadgar, S.; Sobouti, F.; Armin, M.; Ebrahiminasab, P.; Moosazadeh, M.; Rakhshan, V. Effects of 6 different chemical treatments on force kinetics of memory elastic chains versus conventional chains: An in vitro study. Int. Orthod. 2020, 18, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.; Singla, A.; Negi, A.; Jaj, H.S.; Bhandari, V. Dynamic force delivery and damping behavior of different brands of elastomeric chains using dynamic mechanical analysis. J. Indian Orthod. Soc. 2015, 49, 71. [Google Scholar] [CrossRef]
- Santos, R.L.; Pithon, M.M.; Romanos, M.T. The effect of different pH levels on conventional vs. super force chain elastics. Mater. Res. 2013, 16, 246–251. [Google Scholar] [CrossRef]
- Khaleghi, A.; Ahmadvand, A.; Sadeghian, S. Effect of citric acid on force decay of orthodontic elastomeric chains. Dent. Res. J. 2021, 18, 31. [Google Scholar]
- Ramachandraiah, S.; Sridharan, K.; Nishad, A.; Manjusha, K.K.; Abraham, E.A.; Ramees, M.M. Force Decay Characteristics of commonly used Elastomeric Chains on Exposure to various Mouth Rinses with different Alcohol Concentration: An in vitro Study. J. Contemp. Dent. Pract. 2017, 18, 813–820. [Google Scholar]
- Mirhashemi, A.; Farahmand, N.; Saffar Shahroudi, A.; Ahmad Akhoundi, M.S. Effect of four different mouth-washes on force-degradation pattern of orthodontic elastomeric chains. Orthod. Waves. 2017, 76, 67–72. [Google Scholar] [CrossRef]
- Sufarnap, E.; Harahap, K.I.; Terry, T. Effect of sodium fluoride in chlorhexidine mouthwashes on force decay and permanent deformation of orthodontic elastomeric chain. Padjadjaran J. Dent. 2021, 33, 74–80. [Google Scholar] [CrossRef]
- Teixeira, L.; Pereira, B.d.R.; Bortoly, T.G.; Brancher, J.A.; Tanaka, O.M.; Guariza-Filho, O. The environmental influence of Light Coke, phosphoric acid, and citric acid on elastomeric chains. J. Contemp. Dent. Pract. 2008, 9, 17–24. [Google Scholar] [CrossRef]
- Clemitson, I.R. Castable Polyurethane Elastomers, 2nd ed.; CRS Press: Hoboken, NJ, USA, 2015. [Google Scholar]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
- Nattrass, C.; Ireland, A.J.; Sherriff, M. The effect of environmental factors on elastomeric chain and nickel titanium coil springs. Eur. J. Orthod. 1998, 20, 169–176. [Google Scholar] [CrossRef] [PubMed]
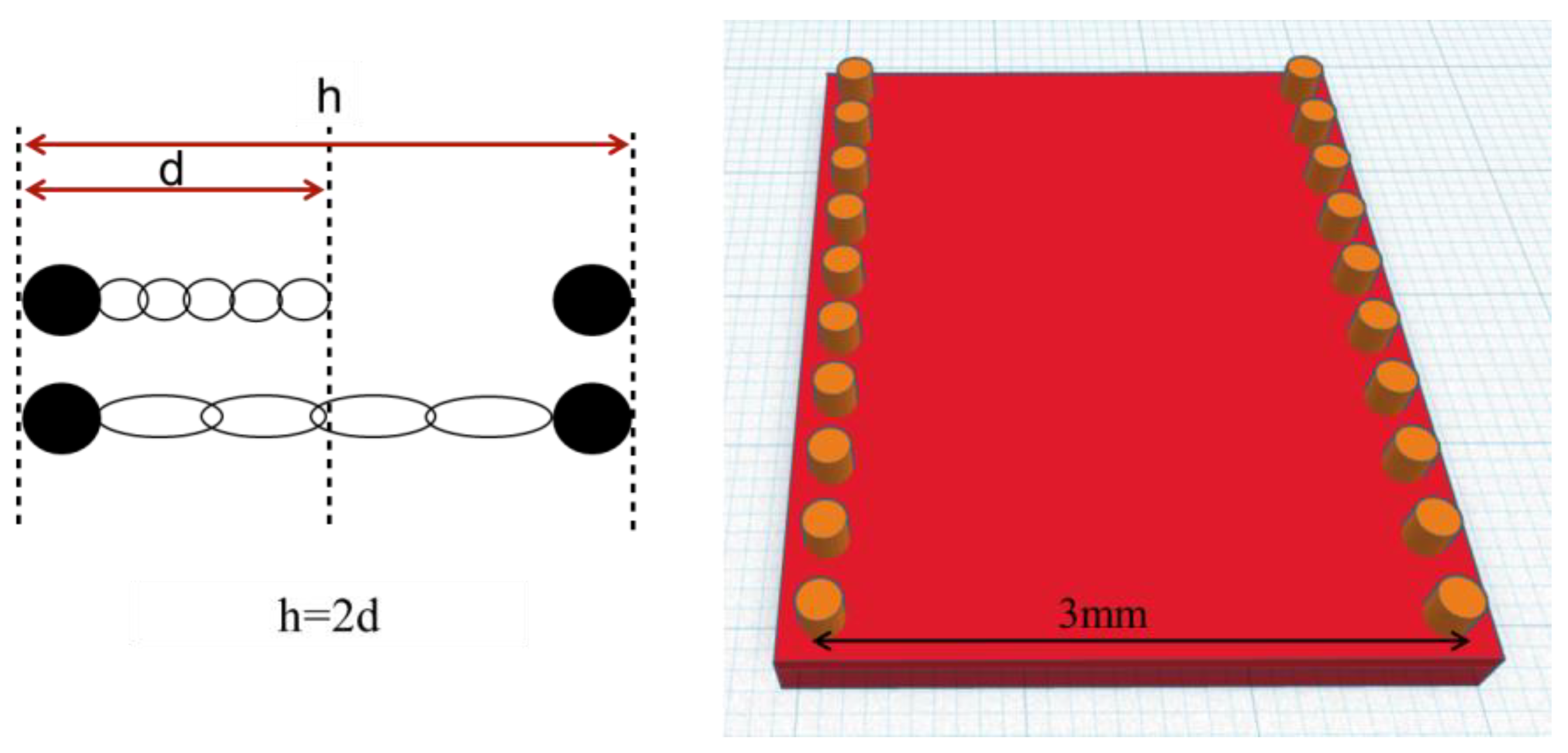

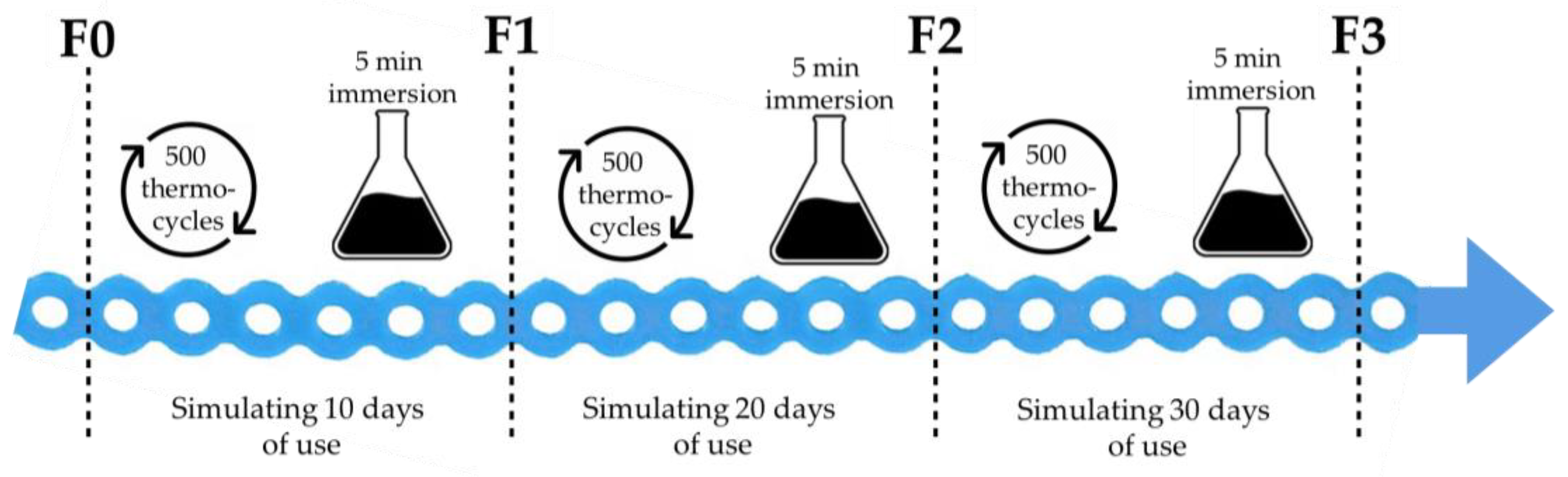
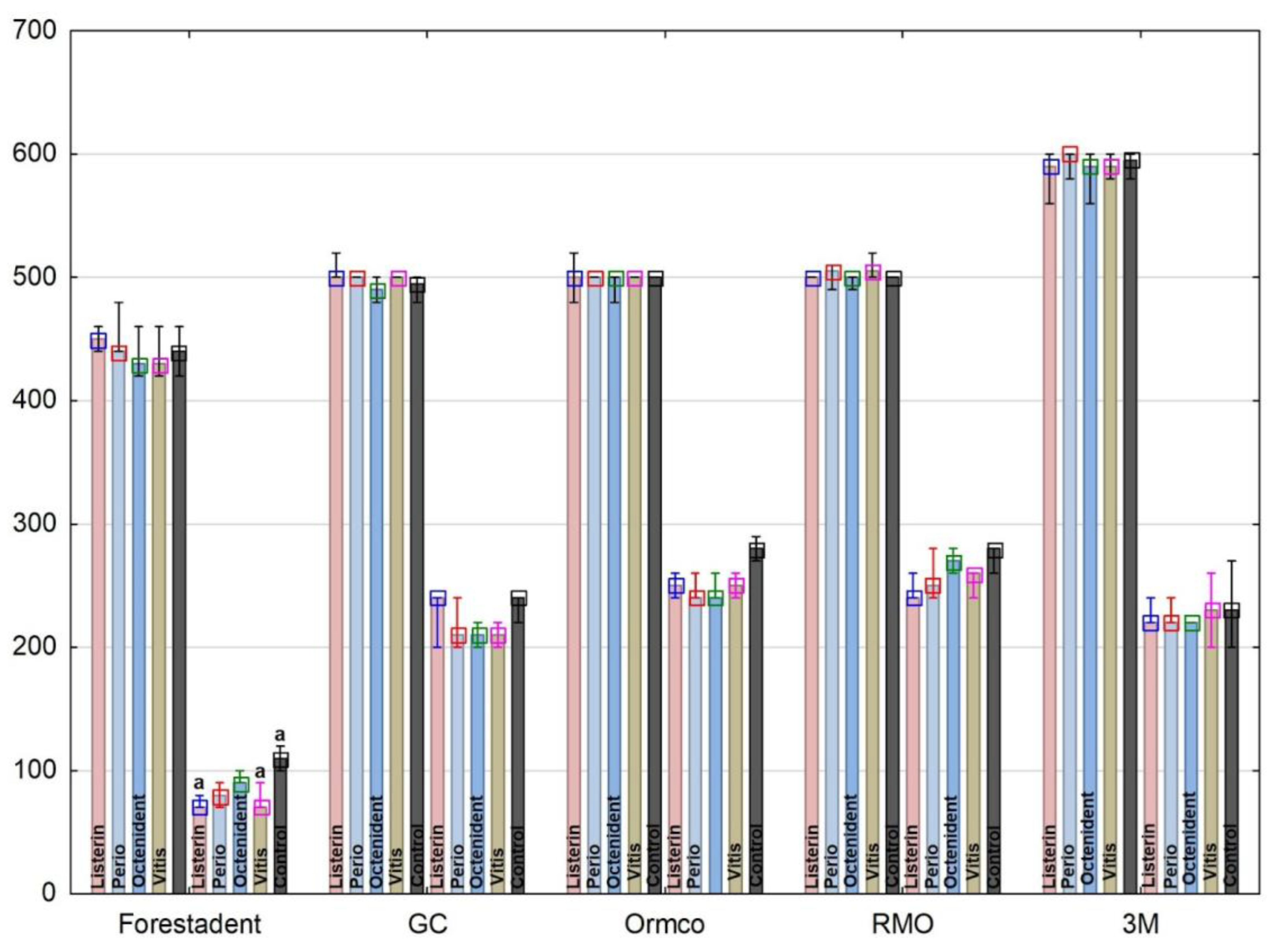
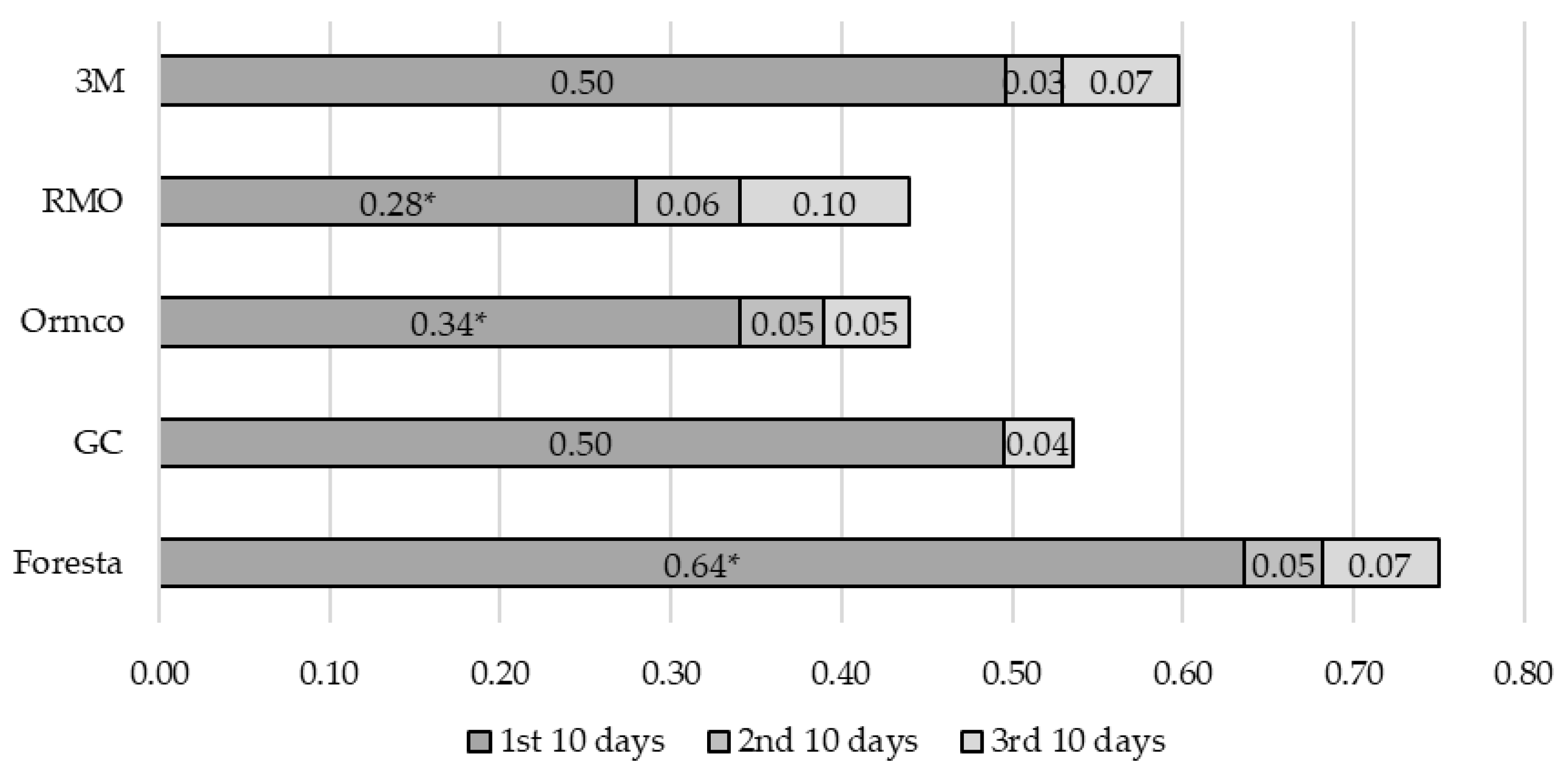

| Oral Antiseptic (OA) | Manufacturer | Active Substance |
|---|---|---|
| Octenident | Schülke & Mayr GmbH, Norderstedt, Germany | Octenidine dihydrochloride |
| Vitis Orthodontic | DENTAID S.L, Cerdanyola, Spain | Cetylpyridinium chloride (CPC) |
| Perio Plus+ | Curaden Germany GmbH, Stutenseeu, Germany | Chlorhexidine |
| Listerine Total Care | Johnson & Johnson, New Brunswick, NJ, USA | herbal active ingredients |
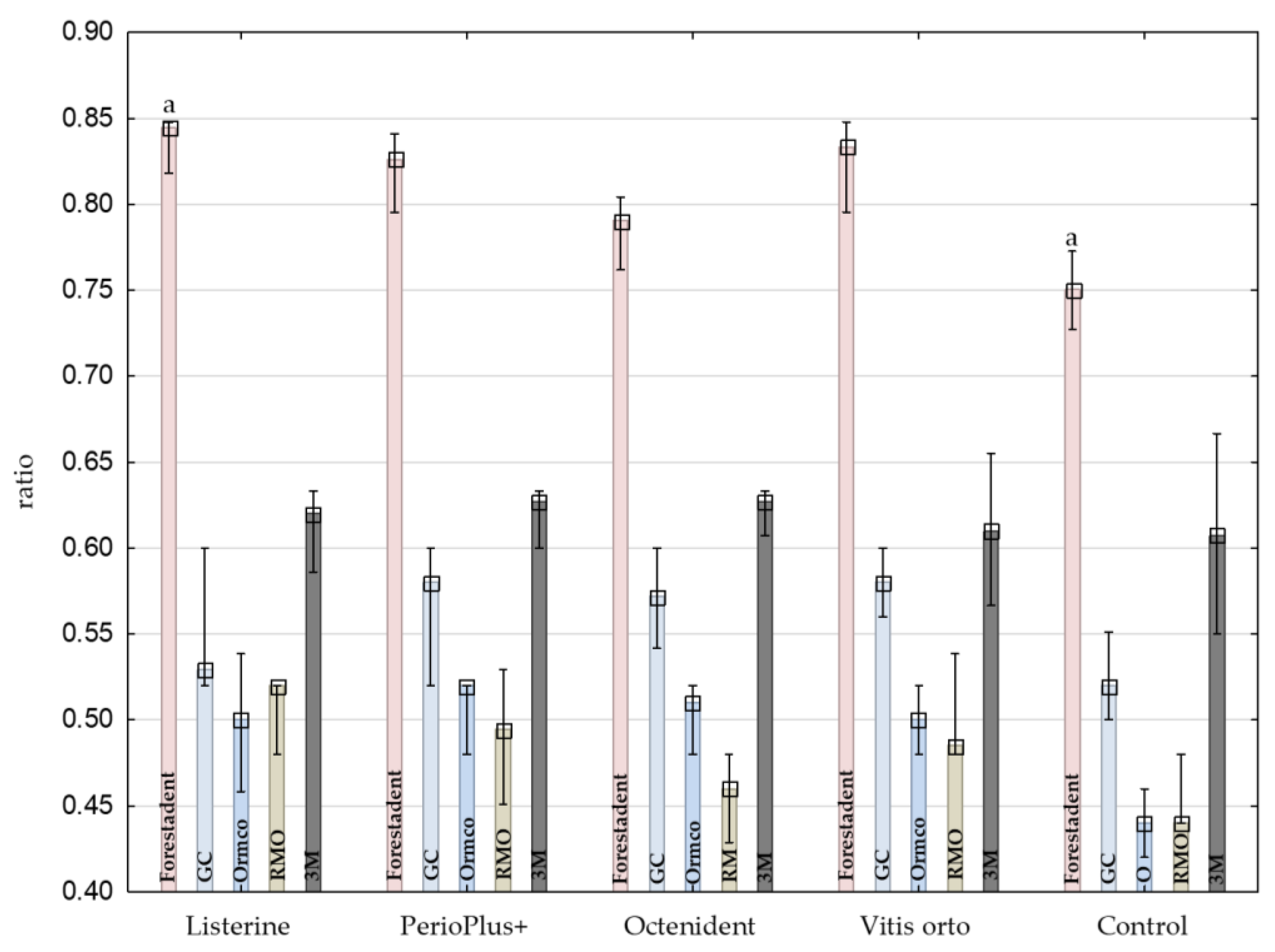
| OA | Brand | Q25 | Median | Q75 | |
|---|---|---|---|---|---|
| Listerine | Forestadent | 0.82955 | 0.84437 | 0.84783 | a |
| GC | 0.52 | 0.52923 | 0.56923 | ||
| Ormco | 0.46917 | 0.5 | 0.52923 | a | |
| RMO | 0.5 | 0.52 | 0.52 | a | |
| 3M | 0.59668 | 0.62024 | 0.63333 | ||
| PerioPlus+ | Forestadent | 0.80682 | 0.82576 | 0.83712 | a |
| GC | 0.54 | 0.58 | 0.6 | ||
| Ormco | 0.5 | 0.52 | 0.52 | a | |
| RMO | 0.46018 | 0.49469 | 0.52471 | a | |
| 3M | 0.61035 | 0.62701 | 0.63333 | ||
| Octenident | Forestadent | 0.77381 | 0.79058 | 0.7999 | a |
| GC | 0.55083 | 0.57167 | 0.59167 | ||
| Ormco | 0.49 | 0.51 | 0.52 | a | |
| RMO | 0.43429 | 0.46 | 0.48 | a,b | |
| 3M | 0.61392 | 0.62701 | 0.63333 | b | |
| Vitis orto | Forestadent | 0.81439 | 0.83333 | 0.84058 | a |
| GC | 0.56 | 0.58 | 0.6 | ||
| Ormco | 0.48 | 0.5 | 0.52 | a | |
| RMO | 0.48 | 0.4851 | 0.51433 | a | |
| 3M | 0.57644 | 0.60977 | 0.64425 | ||
| Control | Forestadent | 0.7332 | 0.75052 | 0.76732 | a |
| GC | 0.51 | 0.52 | 0.53551 | ||
| Ormco | 0.43 | 0.44 | 0.45 | a | |
| RMO | 0.44 | 0.44 | 0.46 | a | |
| 3M | 0.55466 | 0.60725 | 0.66092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimunović, L.; Blagec, T.; Šutej, I.; Meštrović, S. Do Oral Antiseptics Affect the Force Degradation of Elastomeric Chains? Appl. Sci. 2023, 13, 7290. https://doi.org/10.3390/app13127290
Šimunović L, Blagec T, Šutej I, Meštrović S. Do Oral Antiseptics Affect the Force Degradation of Elastomeric Chains? Applied Sciences. 2023; 13(12):7290. https://doi.org/10.3390/app13127290
Chicago/Turabian StyleŠimunović, Luka, Tadeja Blagec, Ivana Šutej, and Senka Meštrović. 2023. "Do Oral Antiseptics Affect the Force Degradation of Elastomeric Chains?" Applied Sciences 13, no. 12: 7290. https://doi.org/10.3390/app13127290
APA StyleŠimunović, L., Blagec, T., Šutej, I., & Meštrović, S. (2023). Do Oral Antiseptics Affect the Force Degradation of Elastomeric Chains? Applied Sciences, 13(12), 7290. https://doi.org/10.3390/app13127290








