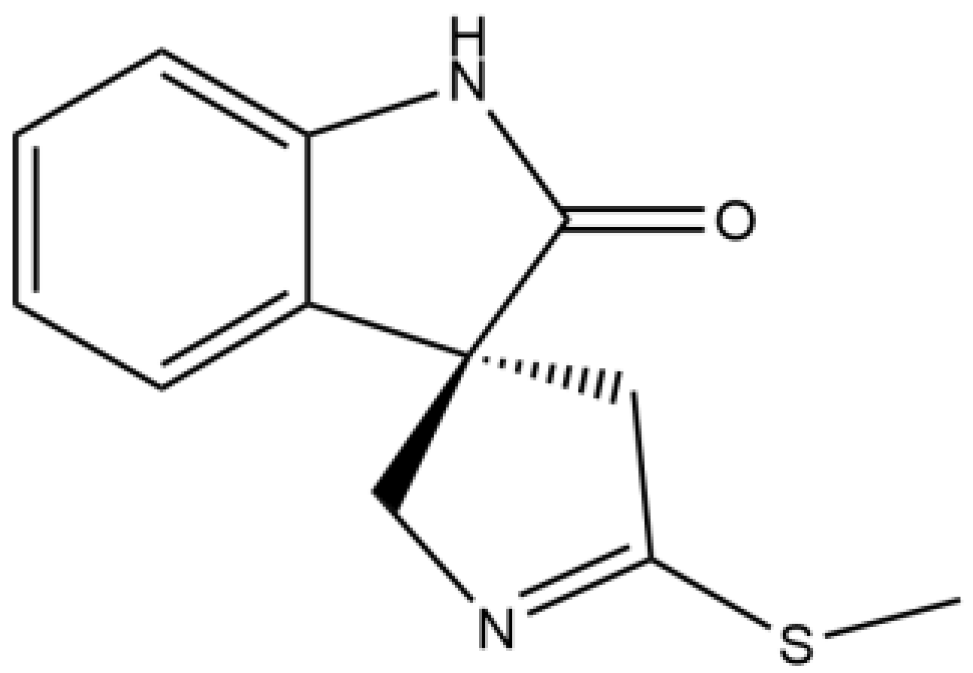
Investigation into the Binding Site of (-)-Spirobrassinin for Herbicidal Activity Using Molecular Docking and Molecular Dynamics Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking
2.2. Quantification of Phytohormones Content
2.3. Docking Results Verification Experiments
2.4. Cultivation and Bioassay of C. vulgaris
2.5. Preparation of PSBD1 and (-)-Spirobrassinin Structure
2.6. Molecular Dynamics Simulations
2.7. Binding Free Energy Calculation
2.8. Statistical Analysis
3. Results
3.1. Molecular Docking of (-)-Spirobrassinin with Herbicidal Target Sites and Plant Growth Regulator Target Sites
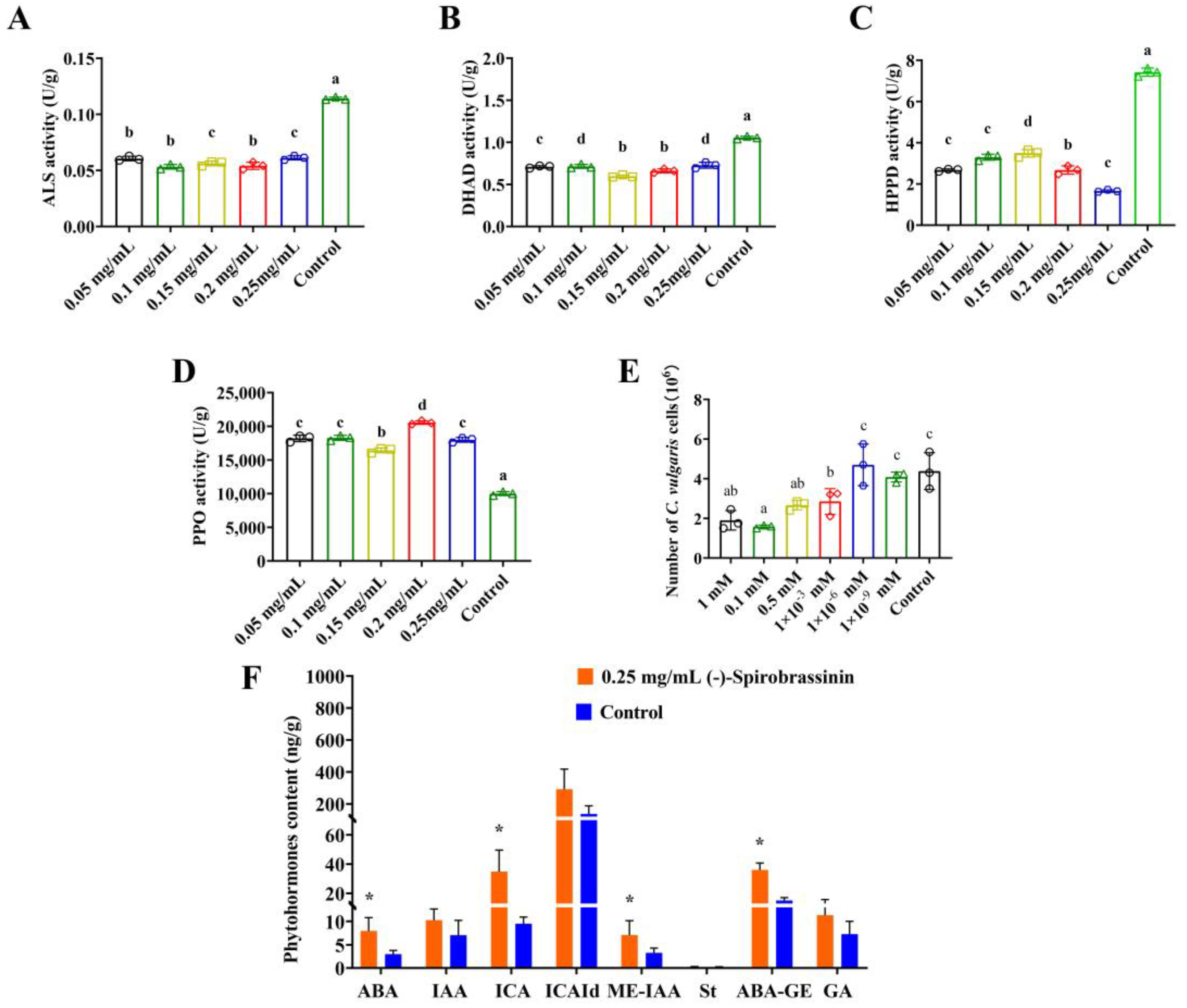
3.2. The Effects of (-)-Spirobrassinin on HPPD, ALS, DHAD, PPO, and Phytohormones
3.3. The Stable Binding Mode between (-)-Spirobrassinin and PSBD1
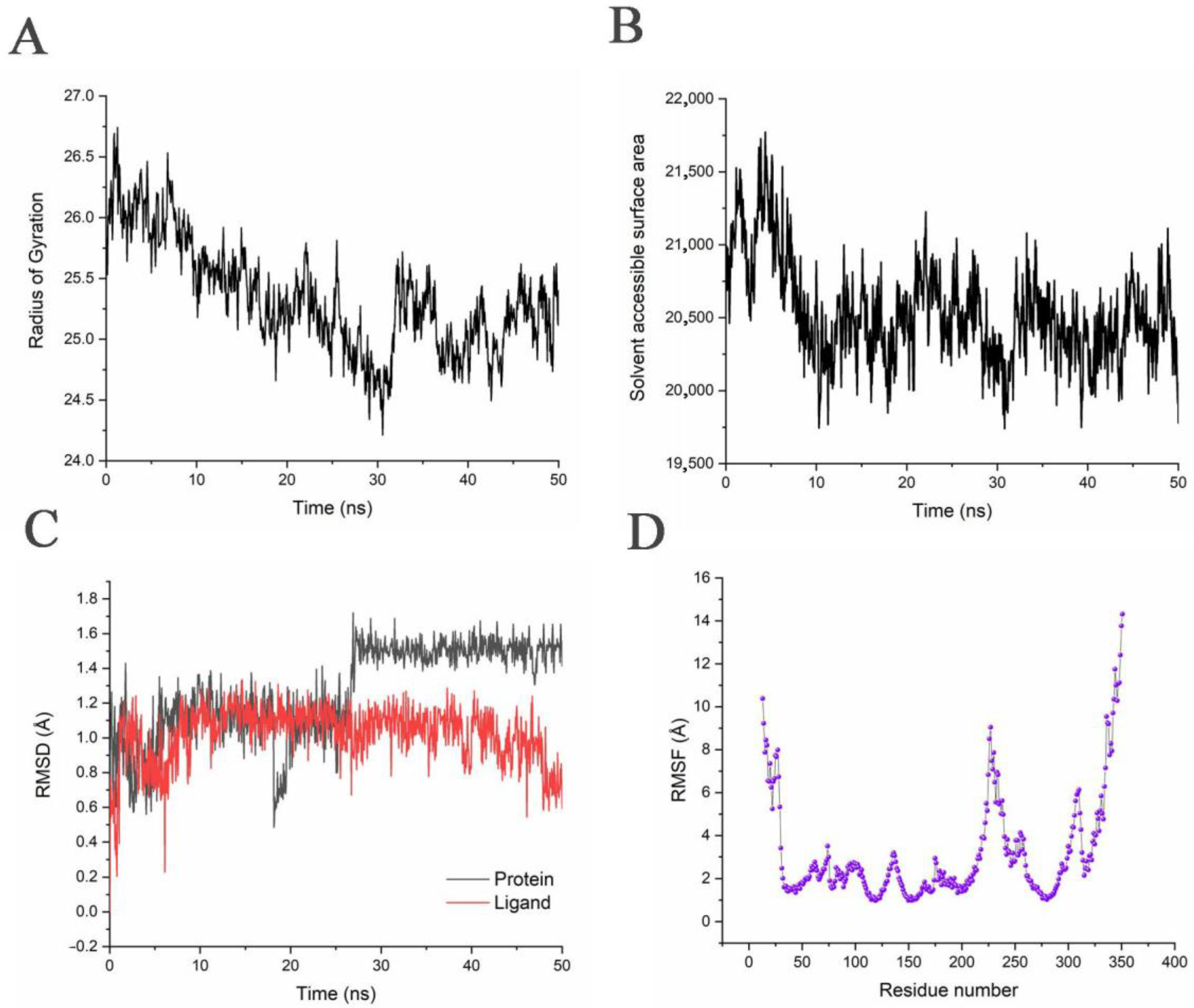
3.4. Stability of the System during the Simulation Process
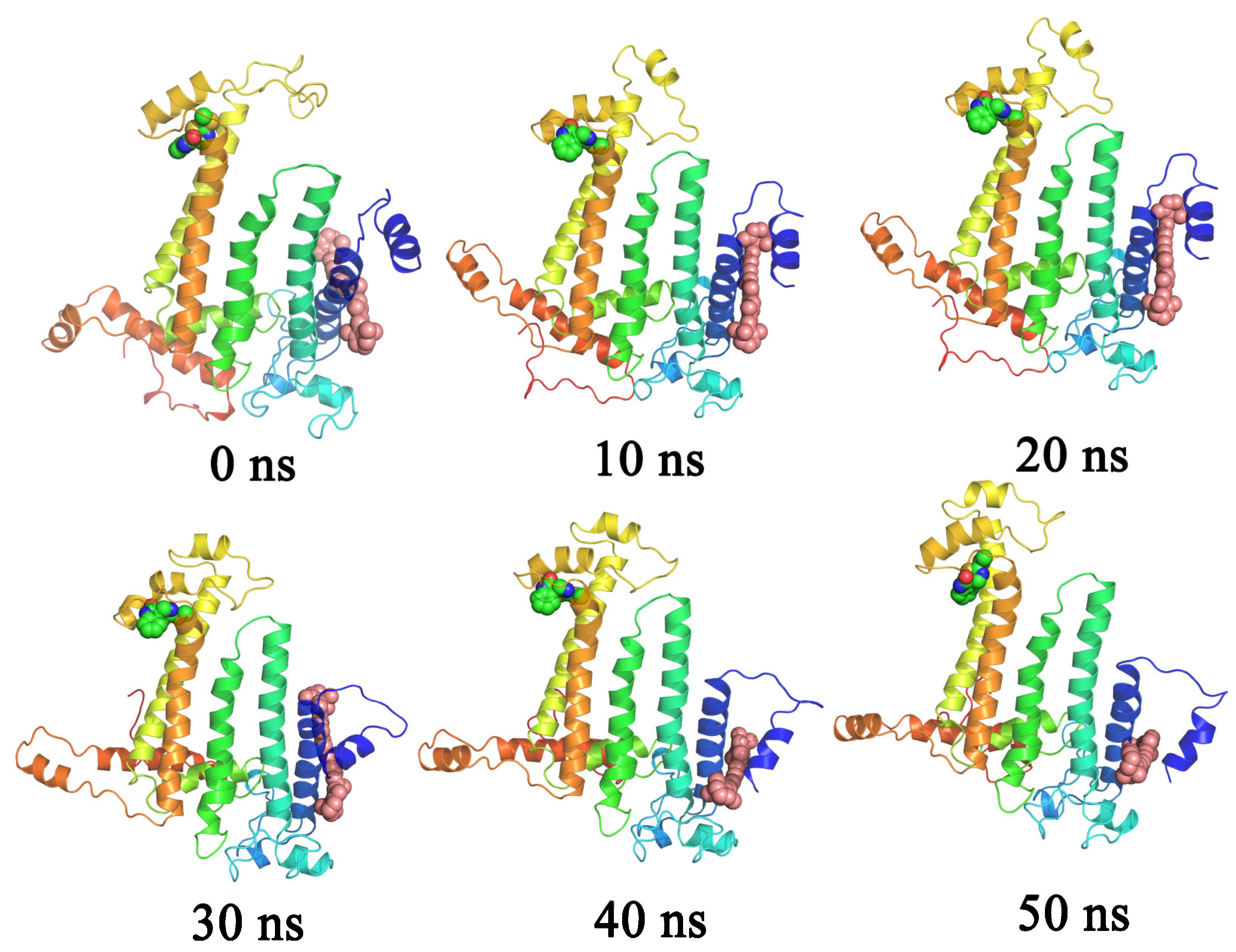
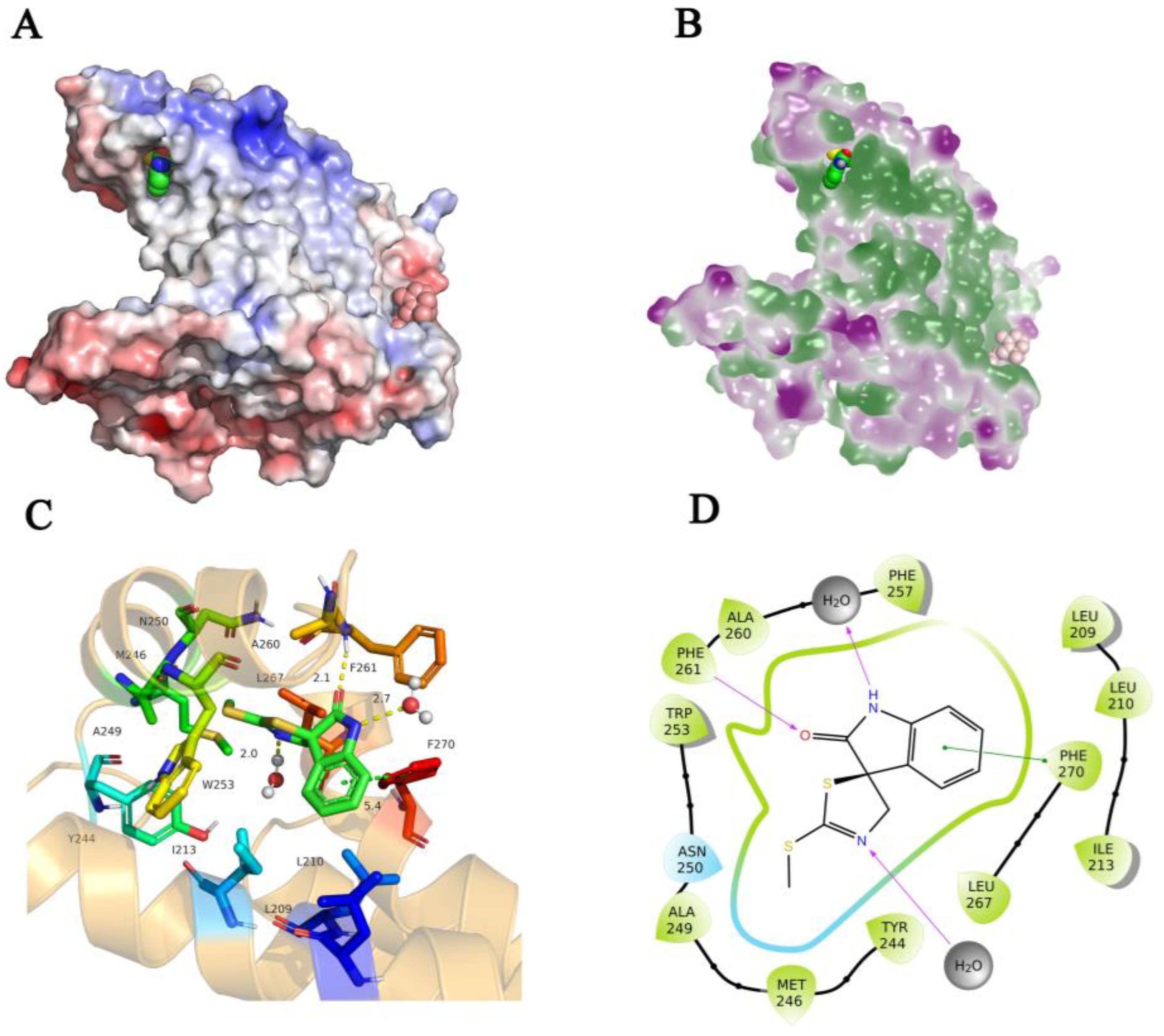
3.5. Binding Mode of the (-)-Spirobrassinin with the PSBD1
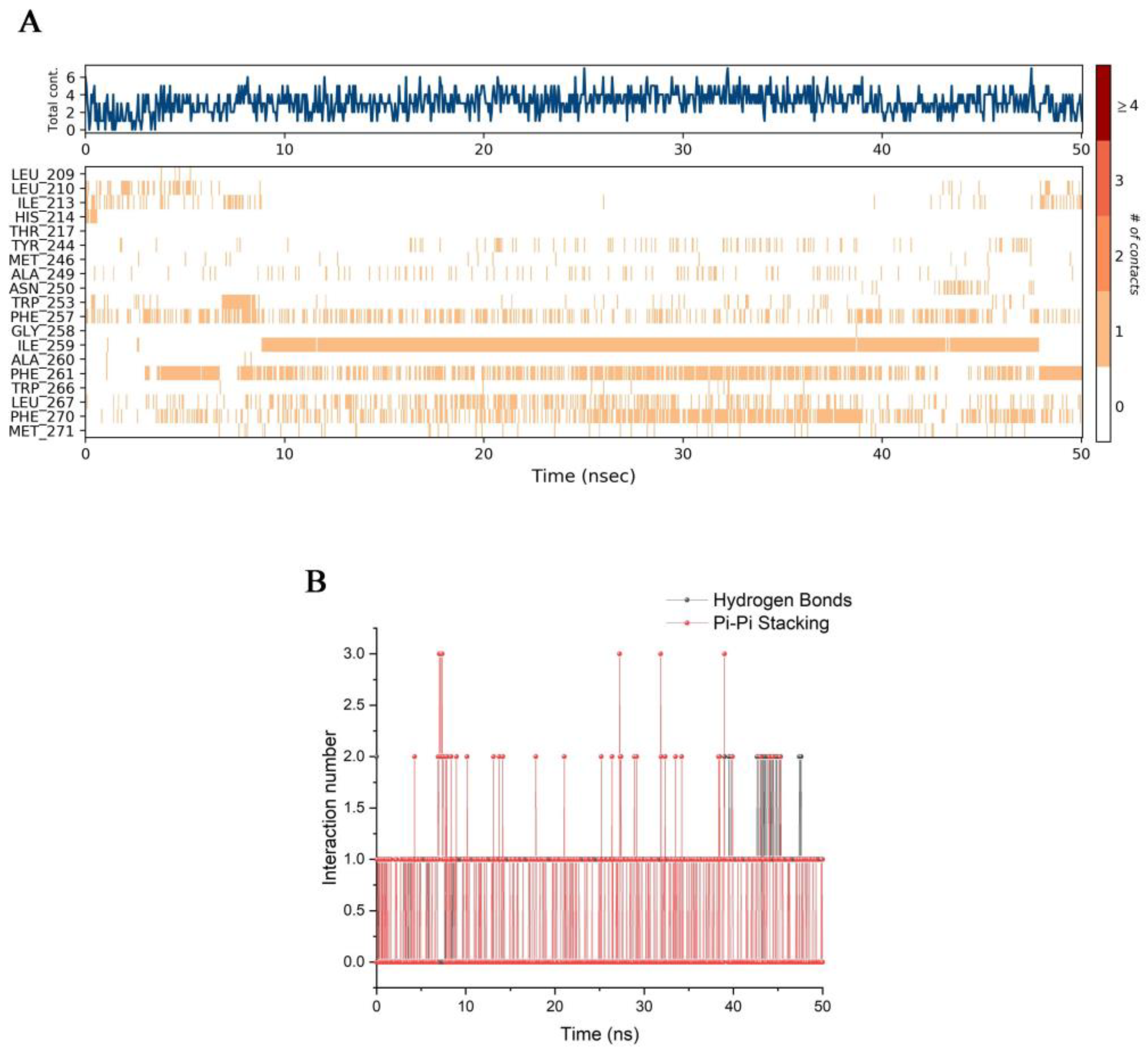
3.6. The Analysis of the (-)-Spirobrassinin-PSBD1 Contacts Revealed the Types and Quantities of Amino Acids Involved
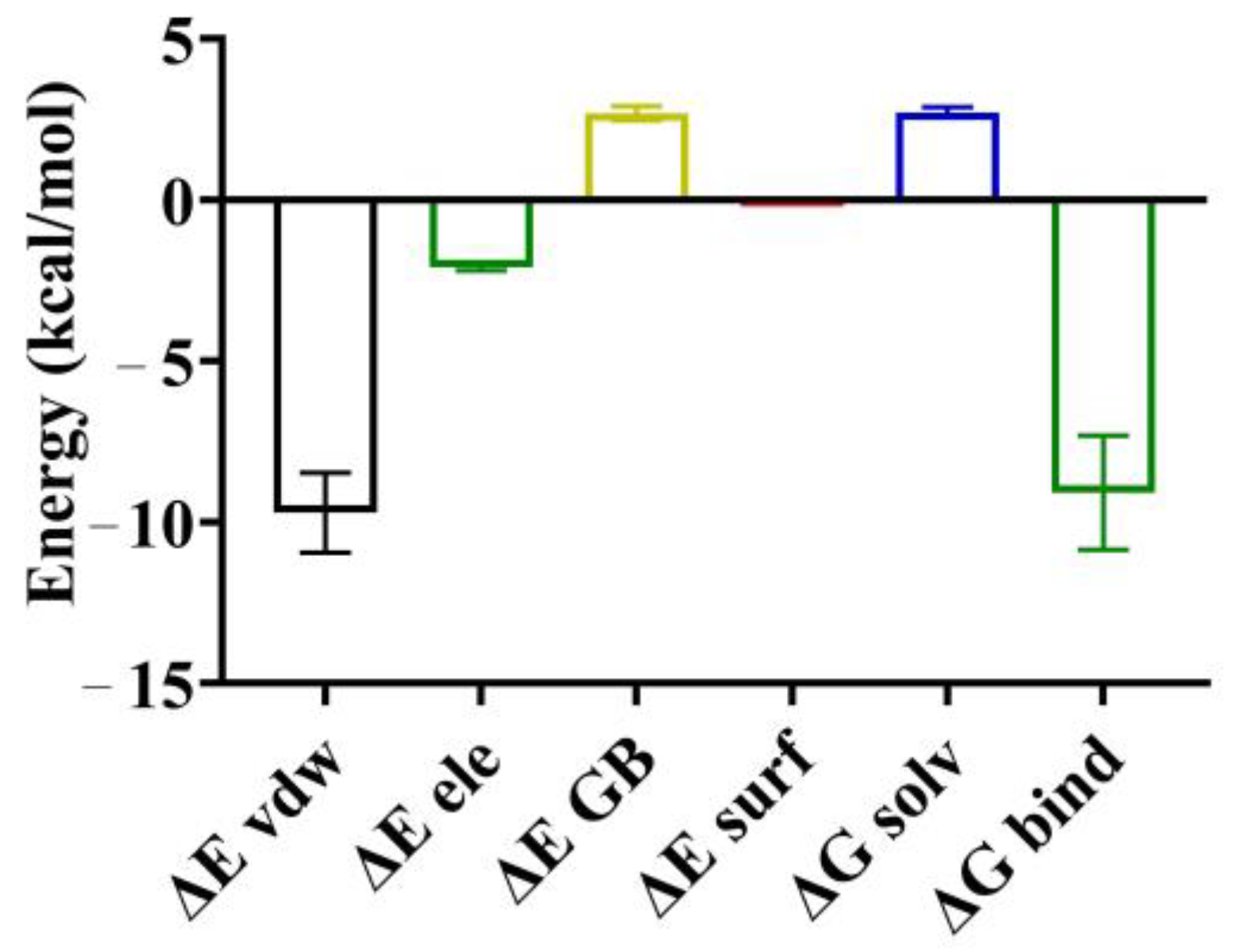
3.7. Binding Free Energy between the (-)-Spirobrassinin and PSBD1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, M.; Witt, A.; Winston, R. Weed biological control in low- and middle-income countries. Curr. Opin. Insect Sci. 2020, 38, 92–98. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Bhuiyan, T.F.; Anee, T.I.; Masud, A.A.C.; Nahar, K. Chapter 3—Phytotoxicity, environmental and health hazards of herbicides: Challenges and ways forward. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 55–99. [Google Scholar]
- Qu, T.; Du, X.; Peng, Y.; Guo, W.; Zhao, C.; Losapio, G. Invasive species allelopathy decreases plant growth and soil microbial activity. PLoS ONE 2021, 16, e0246685. [Google Scholar] [CrossRef]
- Khaledi, R.; Fayaz, F.; Kahrizi, D.; Talebi, R. PCR-based identification of point mutation mediating acetolactate synthase-inhibiting herbicide resistance in weed wild mustard (Sinapis arvensis). Mol. Biol. Rep. 2019, 46, 5113–5121. [Google Scholar] [CrossRef]
- Das, C.; Dey, A.; Bandyopadhyay, A. Allelochemicals: An Emerging Tool for Weed Management. In Evidence Based Validation of Traditional Medicines; Springer: Berlin/Heidelberg, Germany, 2021; pp. 249–259. [Google Scholar]
- Palanivel, H.; Tilaye, G.; Belliathan, S.; Benor, S.; Gebrie, S.; Murugesan, K. Allelochemicals as Natural Herbicides for Sustainable Agriculture to Promote a Cleaner Environment. In Strategies and Tools for Pollutant Mitigation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 93–116. [Google Scholar]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and its application as a weed management tool: A review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Rimando, A.M.; Schrader, K.K.; Aliotta, G.; Oliva, A.; Romagni, J.G. Chemicals from nature for weed management. Weed Sci. 2002, 50, 138–151. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Flint, D.H.; Emptage, M.H. Dihydroxy acid dehydratase from spinach contains a [2Fe-2S] cluster. J. Biol. Chem. 1988, 263, 3558–3564. [Google Scholar] [CrossRef]
- Flint, D.H.; Emptage, M.H.; Finnegan, M.G.; Fu, W.; Johnson, M.K. The role and properties of the iron-sulfur cluster in Escherichia coli dihydroxy-acid dehydratase. J. Biol. Chem. 1993, 268, 14732–14742. [Google Scholar] [CrossRef] [PubMed]
- The inhibitory activity of natural products on plant p-hydroxyphenylpyruvate dioxygenase. J. Phytochem. 2002, 60, 281–288. [CrossRef] [PubMed]
- Hao, G.F.; Zuo, Y.; Yang, S.G.; Chen, Q.; Zhang, Y.; Yin, C.Y.; Niu, C.W.; Xi, Z.; Yang, G.F. Computational Discovery of Potent and Bioselective Protoporphyrinogen IX Oxidase Inhibitor via Fragment Deconstruction Analysis. J. Agric. Food Chem. 2017, 65, 5581–5588. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.F.; Tan, Y.; Yang, S.G.; Wang, Z.F.; Zhan, C.G.; Xi, Z.; Yang, G.F. Computational and experimental insights into the mechanism of substrate recognition and feedback inhibition of protoporphyrinogen oxidase. PLoS ONE 2013, 8, e69198. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Ali, Z. Biochemical analysis, photosynthetic gene (psbA) down-regulation, and in silico receptor prediction in weeds in response to exogenous application of phenolic acids and their analogs. PLoS ONE 2023, 18, e0277146. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Kwiatkowski, M.; Chen, H.; Hoermayer, L.; Sinclair, S.; Zou, M.; Del Genio, C.I.; Kubeš, M.F.; Napier, R.; Jaworski, K.; et al. Adenylate cyclase activity of TIR1/AFB auxin receptors in plants. Nature 2022, 611, 133–138. [Google Scholar] [CrossRef]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Peterson, F.C.; Defries, A.; Park, S.Y.; Endo, A.; Nambara, E.; Volkman, B.F.; Cutler, S.R. Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc. Natl. Acad. Sci. USA 2013, 110, 12132–12137. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Yin, P.; Li, W.; Wang, L.; Yan, C.; Lin, Z.; Wu, J.Z.; Wang, J.; Yan, S.F.; Yan, N. The Molecular Basis of ABA-Independent Inhibition of PP2Cs by a Subclass of PYL Proteins. Mol. Cell 2011, 42, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Ueguchi-Tanaka, M.; Nakatsu, T.; Nakajima, M.; Naoe, Y.; Ohmiya, H.; Kato, H.; Matsuoka, M. Structural basis for gibberellin recognition by its receptor GID1. Nature 2008, 456, 520–523. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- Anandakrishnan, R.; Aguilar, B.; Onufriev, A.V. H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012, 40, W537–W541. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Maffucci, I.; Contini, A. An Updated Test of AMBER Force Fields and Implicit Solvent Models in Predicting the Secondary Structure of Helical, β-Hairpin, and Intrinsically Disordered Peptides. J. Chem. Theory Comput. 2016, 12, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Cheatham, T.E., 3rd; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, K.G.; Jaeger, V.W.; Pfaendtner, J. The general AMBER force field (GAFF) can accurately predict thermodynamic and transport properties of many ionic liquids. J. Phys. Chem. B 2015, 119, 5882–5895. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Miller, B.R., 3rd; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Gaillard, T. Evaluation of AutoDock and AutoDock Vina on the CASF-2013 Benchmark. J. Chem. Inf. Model. 2018, 58, 1697–1706. [Google Scholar] [CrossRef]
- Martina Chripkova, F.Z.a.J.M. Antiproliferative Effect of Indole Phytoalexins. Molecules 2016, 21, 1626. [Google Scholar] [CrossRef]
- Jan Petersen, R.B. Frank Walker, and Karl Hurle, Weed Suppression by Release of Isothiocyanates from Turnip-Rape Mulch. J. Agron. 2001, 93, 37–43. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Garcia, E.A.L.; Wang, Y.; Butch, C.J. Drug Chemical Space as a Guide for New Herbicide Development: A Cheminformatic Analysis. J. Agric. Food Chem. 2022, 70, 9625–9636. [Google Scholar] [CrossRef]
- Brzezowski, P.; Ksas, B.; Havaux, M.; Grimm, B.; Chazaux, M.; Peltier, G.; Johnson, X.; Alric, J. The function of protoporphyrinogen IX oxidase in chlorophyll biosynthesis requires oxidised plastoquinone in Chlamydomonas reinhardtii. Commun. Biol. 2019, 2, 159. [Google Scholar] [CrossRef]
- Koch, M.; Breithaupt, C.; Kiefersauer, R.; Freigang, J.; Huber, R.; Messerschmidt, A. Crystal structure of protoporphyrinogen IX oxidase: A key enzyme in haem and chlorophyll biosynthesis. EMBO J. 2004, 23, 1720–1728. [Google Scholar] [CrossRef]
- Steenackers, W.; Cesarino, I.; Klíma, P.; Quareshy, M.; Vanholme, R.; Corneillie, S.; Kumpf, R.P.; Van de Wouwer, D.; Ljung, K.; Goeminne, G.; et al. The Allelochemical MDCA Inhibits Lignification and Affects Auxin Homeostasis. Plant. Physiol. 2016, 172, 874–888. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.K. In vitro reconstitution of an abscisic acid signalling pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef]
- McCarty, D. Genetic Control and Integration of Maturation and Germination Pathways in Seed Development. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2003, 46, 71–93. [Google Scholar] [CrossRef]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar] [CrossRef] [PubMed]
- Battaglino, B.; Grinzato, A.; Pagliano, C. Binding Properties of Photosynthetic Herbicides with the Q(B) Site of the D1 Protein in Plant Photosystem II: A Combined Functional and Molecular Docking Study. Plants 2021, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
| Herbicidal Target Sites/Plant Growth Regulator Target Sites | Binding Energy (Kcal/mol) |
|---|---|
| ALS | −5.22 |
| DHAD | −5.56 |
| HPPD | −5.89 |
| PPO | −6.43 |
| ACC | −4.27 |
| PSBD1 | −7.3 |
| DAD2 | −4.97 |
| GIDI | −5.94 |
| TIR1 | −4.49 |
| D14−D3−ASK1 | 11.93 |
| COLI−ASK1 | −3.92 |
| PLY2 | −6.15 |
| Residue Name | RMSF(Å) |
|---|---|
| ALA | 14.468 |
| ASN | 14.033 |
| GLU | 12.360 |
| GLY | 12.329 |
| ARG | 11.412 |
| PRO | 11.190 |
| VAL | 10.923 |
| HIS | 10.758 |
| GLU | 10.574 |
| GLU | 10.338 |
| LEU | 10.268 |
| GLY | 10.096 |
| ASN | 9.928 |
| PRO | 9.707 |
| GLU | 9.427 |
| ASP | 8.934 |
| PHE | 8.914 |
| TRP | 8.766 |
| ARG | 8.653 |
| VAL | 8.521 |
| ILE | 8.452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dong, B.; Wang, D.; Jia, X.; Zhang, Q.; Liu, W.; Zhou, H. Investigation into the Binding Site of (-)-Spirobrassinin for Herbicidal Activity Using Molecular Docking and Molecular Dynamics Simulations. Appl. Sci. 2023, 13, 7287. https://doi.org/10.3390/app13127287
Wang Y, Dong B, Wang D, Jia X, Zhang Q, Liu W, Zhou H. Investigation into the Binding Site of (-)-Spirobrassinin for Herbicidal Activity Using Molecular Docking and Molecular Dynamics Simulations. Applied Sciences. 2023; 13(12):7287. https://doi.org/10.3390/app13127287
Chicago/Turabian StyleWang, Yu, Baozhu Dong, Dong Wang, Xinyu Jia, Qian Zhang, Wanyou Liu, and Hongyou Zhou. 2023. "Investigation into the Binding Site of (-)-Spirobrassinin for Herbicidal Activity Using Molecular Docking and Molecular Dynamics Simulations" Applied Sciences 13, no. 12: 7287. https://doi.org/10.3390/app13127287
APA StyleWang, Y., Dong, B., Wang, D., Jia, X., Zhang, Q., Liu, W., & Zhou, H. (2023). Investigation into the Binding Site of (-)-Spirobrassinin for Herbicidal Activity Using Molecular Docking and Molecular Dynamics Simulations. Applied Sciences, 13(12), 7287. https://doi.org/10.3390/app13127287






