The Effect of Tool Rotation Speed on the Formation of Eutectic Structure during Friction Stir Welding of Aluminum to Magnesium
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- The tool rotation speed plays a major role in the joint quality and intermetallic compounds;
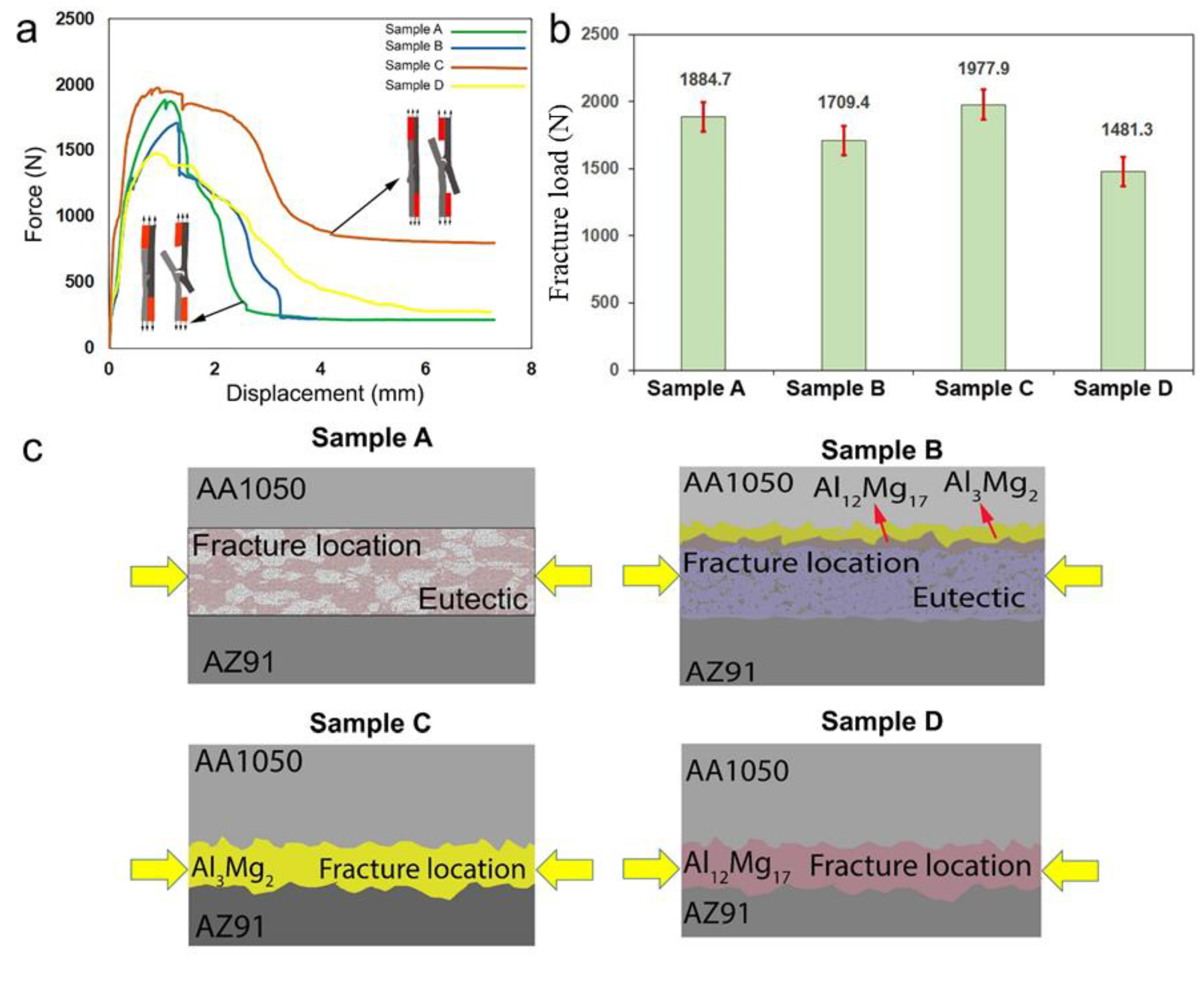
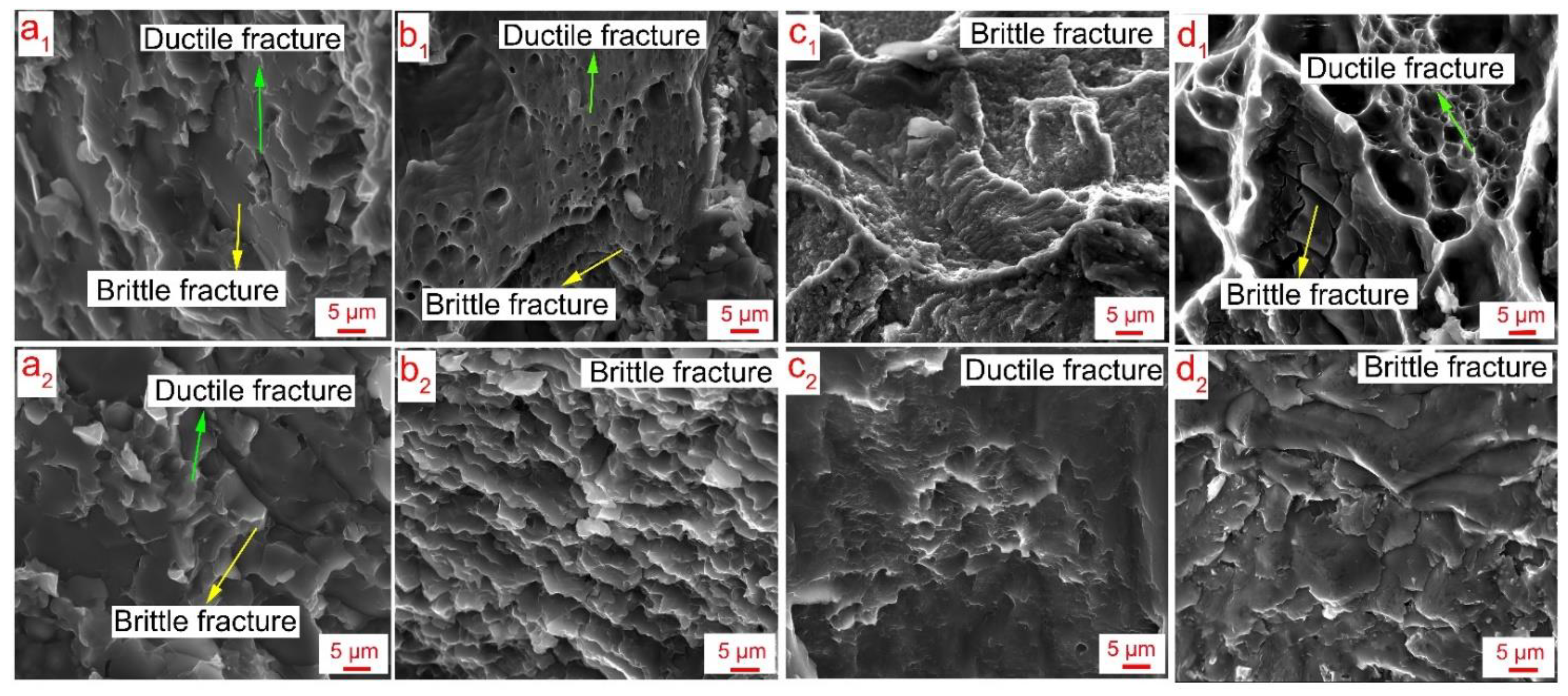
- At low rotation speed of the tool, a thin intermetallic layer forms at the interface, and the joint strength is the highest among all the parameters. The fracture propagates through the AZ91 layer;
- At high rotation speed, liquation takes place in the weld nugget which is composed of a eutectic compound. This liquation causes the slippage of the tool and lowers the heat generation during welding. Moreover, the diffusion rate of elements decreases due to the existence of the eutectic layer. Hence, a thin layer of intermetallic compound forms at the interface. The fracture propagates through the eutectic compound;
- At a higher welding traverse speed with a lower rotational speed, insufficient frictional local heat input and lower exposure time lead to inadequate metallurgical bonding at the AA1050-AZ91 interface. Some defects are observed in this sample due to insufficient material flow;
- The results suggested that rotation rate affects the formation of eutectic structures at the interface;
- The formation of eutectic compounds is influenced by the rotation speed, which is not related to the advanced speed at higher rotation speeds;
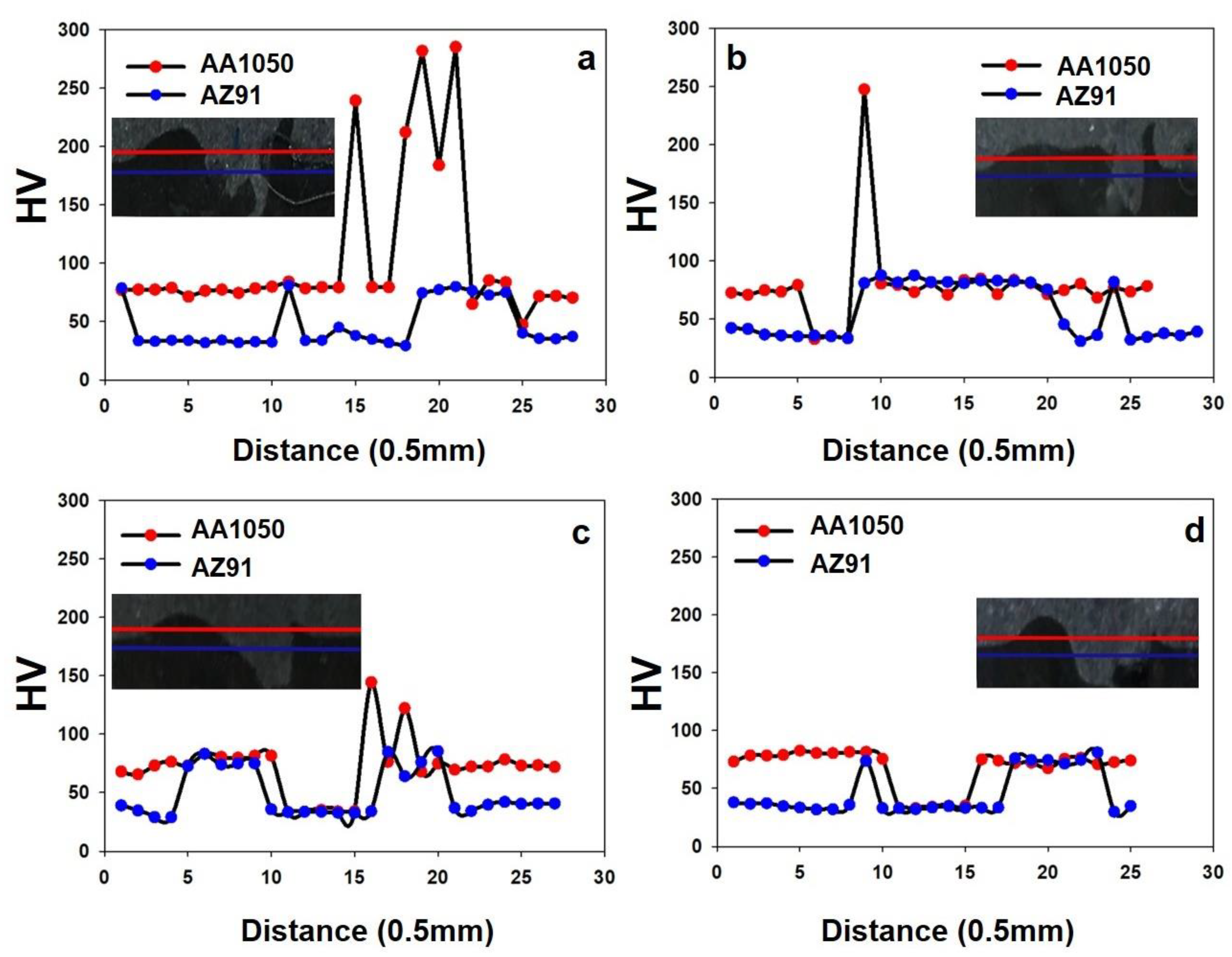
- When aluminum penetrates magnesium, it enhances the hardness of the magnesium, whereas magnesium infiltration in aluminum decreases the hardness of the aluminum;
- The formation of eutectic compositions plays a significant role in ensuring consistent hardness levels.
Author Contributions
Funding
Conflicts of Interest
References
- Thirumoorthy, A.; Arjunan, T.; Kumar, K.S. Latest research development in aluminum matrix with particulate reinforcement composites—A review. Mater. Today Proc. 2018, 5, 1657–1665. [Google Scholar] [CrossRef]
- Ng, C.; Yahaya, S.; Majid, A. Reviews on aluminum alloy series and its applications. J. Acad. Ind. Res. 2017, 5, 708–716. [Google Scholar]
- Baghdadi, A.H.; Rajabi, A.; Selamat, N.F.M.; Sajuri, Z.; Omar, M.Z. Effect of post-weld heat treatment on the mechanical behavior and dislocation density of friction stir welded Al6061. Mater. Sci. Eng. A 2019, 754, 728–734. [Google Scholar] [CrossRef]
- He, L.; Pan, L.; Zhou, W.; Niu, Z.; Chen, X.; Chen, M.; Zhang, Q.; Pan, W.; Xiao, P.; Li, Y. Thermal corrosion behavior of Yb4Hf3O12 ceramics exposed to calcium-ferrum-alumina-silicate (CFAS) at 1400 °C. J. Eur. Ceram. Soc. 2023, 43, 4114–4123. [Google Scholar] [CrossRef]
- Pan, L.; He, L.; Niu, Z.; Xiao, P.; Zhou, W.; Li, Y. Corrosion behavior of ytterbium hafnate exposed to water-vapor with Al(OH)3 impurities. J. Eur. Ceram. Soc. 2023, 43, 612–620. [Google Scholar] [CrossRef]
- Beygi, R.; Talkhabi, A.A.; Mehrizi, M.Z.; Marques, E.A.; Carbas, R.J.; da Silva, L.F. A Novel Lap-Butt Joint Design for FSW of Aluminum to Steel in Tee-Configuration: Joining Mechanism, Intermetallic Formation, and Fracture Behavior. Metals 2023, 13, 1027. [Google Scholar] [CrossRef]
- Singh, V.P.; Patel, S.K.; Ranjan, A.; Kuriachen, B. Recent research progress in solid state friction-stir welding of aluminium–magnesium alloys: A critical review. J. Mater. Res. Technol. 2020, 9, 6217–6256. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, X.; Luan, G. Effect of root flaws on the fatigue property of friction stir welds in 2024-T3 aluminum alloys. Mater. Sci. Eng. A 2006, 418, 155–160. [Google Scholar] [CrossRef]
- Laska, A.; Szkodo, M.; Cavaliere, P.; Perrone, A. Influence of the Tool Rotational Speed on Physical and Chemical Properties of Dissimilar Friction-Stir-Welded AA5083/AA6060 Joints. Metals 2022, 12, 1658. [Google Scholar] [CrossRef]
- Shelley, T. Joining forces for multiple properties. Eureka 2003. [Google Scholar]
- Habibnia, M.; Shakeri, M.; Nourouzi, S.; Givi, M.B. Microstructural and mechanical properties of friction stir welded 5050 Al alloy and 304 stainless steel plates. Int. J. Adv. Manuf. Technol. 2015, 76, 819–829. [Google Scholar] [CrossRef]
- Liu, Q.; Han, R.; Gao, Y.; Ke, L. Numerical investigation on thermo-mechanical and material flow characteristics in friction stir welding for aluminum profile joint. Int. J. Adv. Manuf. Technol. 2021, 114, 2457–2469. [Google Scholar] [CrossRef]
- Gaurav, S.; Mishra, R.; Zunaid, M. A critical review on mechanical and microstructural properties of dissimilar aluminum (Al)-magnesium (Mg) alloys. J. Adhes. Sci. Technol. 2022, 1–33. [Google Scholar] [CrossRef]
- Fu, B.; Qin, G.; Li, F.; Meng, X.; Zhang, J.; Wu, C. Friction stir welding process of dissimilar metals of 6061-T6 aluminum alloy to AZ31B magnesium alloy. J. Mater. Process. Technol. 2015, 218, 38–47. [Google Scholar] [CrossRef]
- Xu, Y.; Ke, L.; Mao, Y.; Yang, P.; Niu, P. Friction stir welding of aluminium to magnesium: A critical review. Mater. Sci. Technol. 2022, 38, 517–534. [Google Scholar] [CrossRef]
- Elangovan, K.; Balasubramanian, V.; Valliappan, M. Effect of tool pin profile and tool rotational speed on mechanical properties of friction stir welded AA6061 aluminium alloy. Mater. Manuf. Process. 2008, 23, 251–260. [Google Scholar] [CrossRef]
- Nonnenmann, T.; Beygi, R.; Carbas, R.J.; da Silva, L.F.; Öchsner, A. Feasibility study on hybrid weld-bonding between dissimilar material for automotive industry. Int. J. Adhes. Adhes. 2023, 121, 103316. [Google Scholar] [CrossRef]
- Safeen, M.W.; Russo Spena, P. Main issues in quality of friction stir welding joints of aluminum alloy and steel sheets. Metals 2019, 9, 610. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Shash, A.; Abd-Rabou, M.; ElSherbiny, M.G. Welding and processing of metallic materials by using friction stir technique: A review. J. Adv. Join. Process. 2021, 3, 100059. [Google Scholar] [CrossRef]
- Meschut, G.; Merklein, M.; Brosius, A.; Drummer, D.; Fratini, L.; Füssel, U.; Gude, M.; Homberg, W.; Martins, P.; Bobbert, M. Review on mechanical joining by plastic deformation. J. Adv. Join. Process. 2022, 5, 100113. [Google Scholar] [CrossRef]
- Mohammadi, J.; Behnamian, Y.; Mostafaei, A.; Izadi, H.; Saeid, T.; Kokabi, A.; Gerlich, A. Friction stir welding joint of dissimilar materials between AZ31B magnesium and 6061 aluminum alloys: Microstructure studies and mechanical characterizations. Mater. Charact. 2015, 101, 189–207. [Google Scholar] [CrossRef]
- Shi, L.; Wu, C. Transient model of heat transfer and material flow at different stages of friction stir welding process. J. Manuf. Process. 2017, 25, 323–339. [Google Scholar] [CrossRef]
- Husain, M.M.; Sarkar, R.; Pal, T.; Prabhu, N.; Ghosh, M. Friction stir welding of steel: Heat input, microstructure, and mechanical property co-relation. J. Mater. Eng. Perform. 2015, 24, 3673–3683. [Google Scholar] [CrossRef]
- Krishnan, M.; Subramaniam, S.K. Investigation of mechanical and metallurgical properties of friction stir corner welded dissimilar thickness AA5086-AA6061 aluminium alloys. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]
- Liu, F.; Fu, L.; Chen, H. Effect of high rotational speed on temperature distribution, microstructure evolution, and mechanical properties of friction stir welded 6061-T6 thin plate joints. Int. J. Adv. Manuf. Technol. 2018, 96, 1823–1833. [Google Scholar] [CrossRef]
- Salih, O.S.; Ou, H.; Wei, X.; Sun, W. Microstructure and mechanical properties of friction stir welded AA6092/SiC metal matrix composite. Mater. Sci. Eng. A 2019, 742, 78–88. [Google Scholar] [CrossRef]
- Buffa, G.; Baffari, D.; Di Caro, A.; Fratini, L. Friction stir welding of dissimilar aluminium–magnesium joints: Sheet mutual position effects. Sci. Technol. Weld. Join. 2015, 20, 271–279. [Google Scholar] [CrossRef]
- Liu, H.; Fujii, H.; Maeda, M.; Nogi, K. Mechanical properties of friction stir welded joints of 1050–H24 aluminium alloy. Sci. Technol. Weld. Join. 2003, 8, 450–454. [Google Scholar] [CrossRef]
- Beygi, R.; Akhavan-Safar, A.; Carbas, R.; Barbosa, A.; Marques, E.; da Silva, L. Utilizing a ductile damage criterion for fracture analysis of a dissimilar aluminum/steel joint made by friction stir welding. Eng. Fract. Mech. 2022, 274, 108775. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, S.; Yang, S.; Lu, Z.; Yan, K. Influence of cooling conditions on joint properties and microstructures of aluminum and magnesium dissimilar alloys by friction stir welding. Int. J. Adv. Manuf. Technol. 2016, 83, 673–679. [Google Scholar] [CrossRef]
- Lv, X.; Wu, C.; Padhy, G. Diminishing intermetallic compound layer in ultrasonic vibration enhanced friction stir welding of aluminum alloy to magnesium alloy. Mater. Lett. 2017, 203, 81–84. [Google Scholar] [CrossRef]
- Kumar, N.; Yuan, W.; Mishra, R. Challenges and opportunities for friction stir welding of dissimilar alloys and materials (Chapter 7). In Friction Stir Welding of Dissimilar Alloys and Materials; Elsevier Butterworth-Heinemann: Oxford, UK, 2015; pp. 123–126. [Google Scholar]
- Paul, A.R.; Mukherjee, M.; Singh, D. A critical review on the properties of intermetallic compounds and their application in the modern manufacturing. Cryst. Res. Technol. 2022, 57, 2100159. [Google Scholar] [CrossRef]
- Shah, L.; Othman, N.; Gerlich, A. Review of research progress on aluminium–magnesium dissimilar friction stir welding. Sci. Technol. Weld. Join. 2018, 23, 256–270. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, H.; Kou, S. Liquation tendency and liquid-film formation in friction stir spot welding. Weld. J. 2008, 87, 202S–211S. [Google Scholar]
- Yamamoto, N.; Liao, J.; Watanabe, S.; Nakata, K. Effect of intermetallic compound layer on tensile strength of dissimilar friction-stir weld of a high strength Mg alloy and Al alloy. Mater. Trans. 2009, 50, 2833–2838. [Google Scholar] [CrossRef]
- Firouzdor, V.; Kou, S. Al-to-Mg friction stir welding: Effect of positions of Al and Mg with respect to the welding tool. Weld. J. 2009, 88, 213–224. [Google Scholar]
- Prabha, K.A.; Putha, P.K.; Prasad, B.S. Effect of tool rotational speed on mechanical properties of aluminium alloy 5083 weldments in friction stir welding. Mater. Today Proc. 2018, 5, 18535–18543. [Google Scholar] [CrossRef]
- Ji, S.; Meng, X.; Liu, Z.; Huang, R.; Li, Z. Dissimilar friction stir welding of 6061 aluminum alloy and AZ31 magnesium alloy assisted with ultrasonic. Mater. Lett. 2017, 201, 173–176. [Google Scholar] [CrossRef]
- Rao, H.; Yuan, W.; Badarinarayan, H. Effect of process parameters on mechanical properties of friction stir spot welded magnesium to aluminum alloys. Mater. Des. 2015, 66, 235–245. [Google Scholar] [CrossRef]
- Firouzdor, V.; Kou, S. Al-to-Mg friction stir welding: Effect of material position, travel speed, and rotation speed. Metall. Mater. Trans. A 2010, 41, 2914–2935. [Google Scholar] [CrossRef]
- Beygi, R.; Zarezadeh Mehrizi, M.; Akhavan-Safar, A.; Mohammadi, S.; da Silva, L.F. A Parametric Study on the Effect of FSW Parameters and the Tool Geometry on the Tensile Strength of AA2024–AA7075 Joints: Microstructure and Fracture. Lubricants 2023, 11, 59. [Google Scholar] [CrossRef]
- Kumar, U.; Acharya, U.; Saha, S.C.; Saha Roy, B. Microstructure and mechanical property of friction stir welded Al-Mg joints by adopting modified joint configuration technique. Mater. Today Proc. 2020, 26, 2083–2088. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.-C.; Strong, D.; Prangnell, P. Stationary shoulder FSW for joining high strength aluminum alloys. J. Mater. Process. Technol. 2015, 221, 187–196. [Google Scholar] [CrossRef]
- Beygi, R.; Pouraliakbar, H.; Torabi, K.; Fallah, V.; Kim, S.; Shi, R.; da Silva, L. The inhibitory effect of stir zone liquefaction and eutectic-phase formation on the growth of γ/β intermetallics during dissimilar FSW of Al/Mg alloys. J. Manuf. Process. 2021, 70, 152–162. [Google Scholar] [CrossRef]
- Ji, S.; Li, Z.; Zhang, L.; Zhou, Z.; Chai, P. Effect of lap configuration on magnesium to aluminum friction stir lap welding assisted by external stationary shoulder. Mater. Des. 2016, 103, 160–170. [Google Scholar] [CrossRef]
- Ghiasvand, A.; Ranjbarnodeh, E.; Mirsalehi, S.E. The microstructure and mechanical properties of single-pass and double-pass lap joint of Al 5754H-11 and Mg AZ31-O alloys by friction stir welding. J. Mater. Res. Technol. 2023, 23, 6023–6038. [Google Scholar] [CrossRef]
- Akbari, M.; Aliha, M.; Keshavarz, S.; Bonyadi, A. Effect of tool parameters on mechanical properties, temperature, and force generation during FSW. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 1033–1043. [Google Scholar] [CrossRef]
- Mao, Y.; Yang, P.; Zhang, W.; Li, N.; Nie, H.; Lin, D.; Ke, L. Improving tensile-shear properties of friction stir lap welded dissimilar Al/Mg joints by eliminating hook defect and controlling interfacial reaction. Chin. J. Aeronaut. 2022, in press. [Google Scholar] [CrossRef]
- Lakshminarayanan, A.; Annamalai, V. Fabrication and performance evaluation of dissimilar magnesium–aluminium alloy multi-seam friction stir clad joints. Trans. Nonferrous Met. Soc. China 2017, 27, 25–35. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Liu, Z.; Zhai, X.; Bian, G.; Zhang, T.; Dong, P. Improvement in tensile strength of Mg/Al alloy dissimilar friction stir welding joints by reducing intermetallic compounds. J. Alloys Compd. 2021, 861, 157942. [Google Scholar] [CrossRef]
- Sato, Y.S.; Park, S.H.C.; Michiuchi, M.; Kokawa, H. Constitutional liquation during dissimilar friction stir welding of Al and Mg alloys. Scr. Mater. 2004, 50, 1233–1236. [Google Scholar] [CrossRef]
- Jagadeesha, C. Dissimilar friction stir welding between aluminum alloy and magnesium alloy at a low rotational speed. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2014, 616, 55–62. [Google Scholar] [CrossRef]
- Shi, H.; Chen, K.; Liang, Z.; Dong, F.; Yu, T.; Dong, X.; Zhang, L.; Shan, A. Intermetallic compounds in the banded structure and their effect on mechanical properties of Al/Mg dissimilar friction stir welding joints. J. Mater. Sci. Technol. 2017, 33, 359–366. [Google Scholar] [CrossRef]

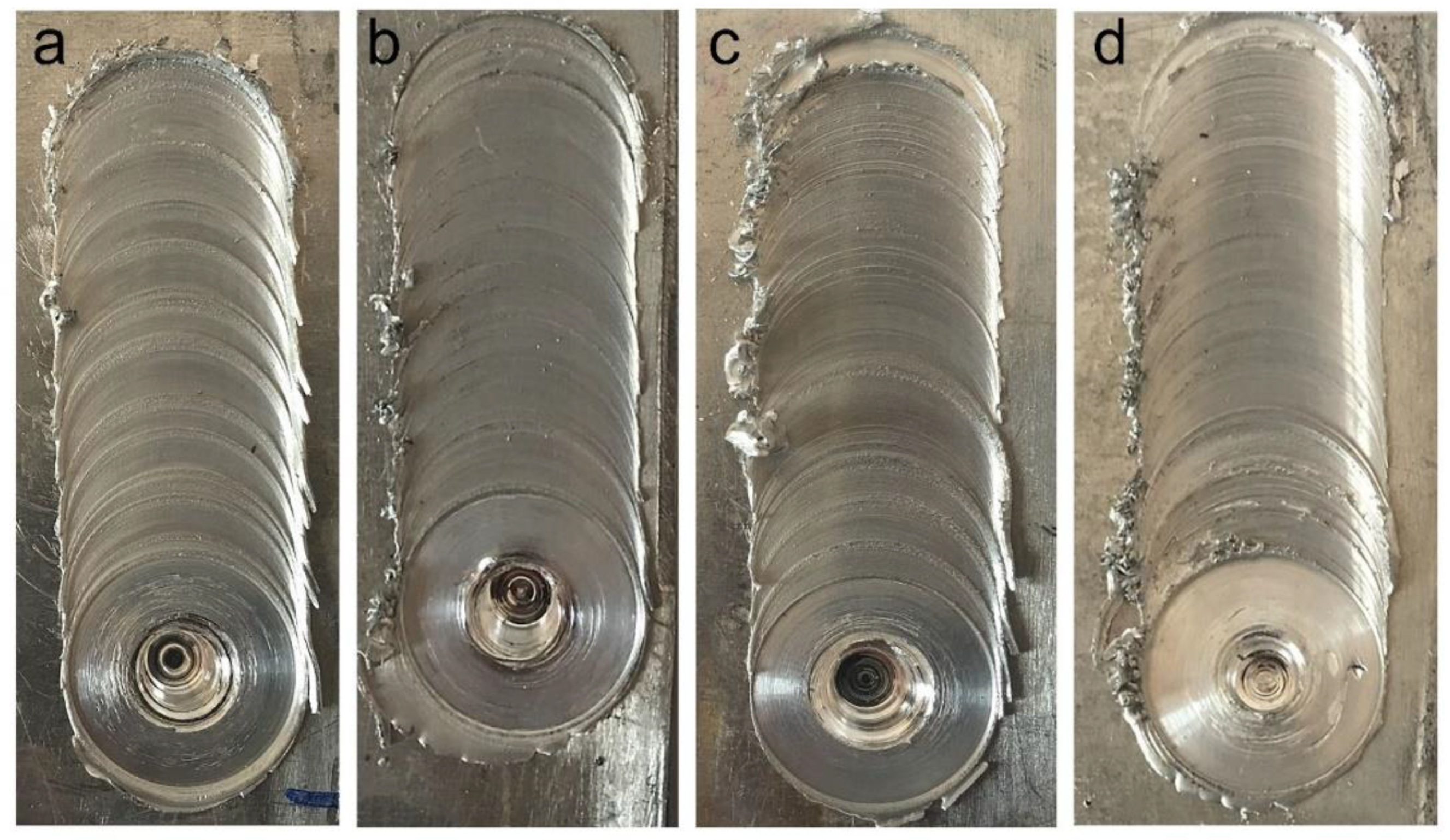
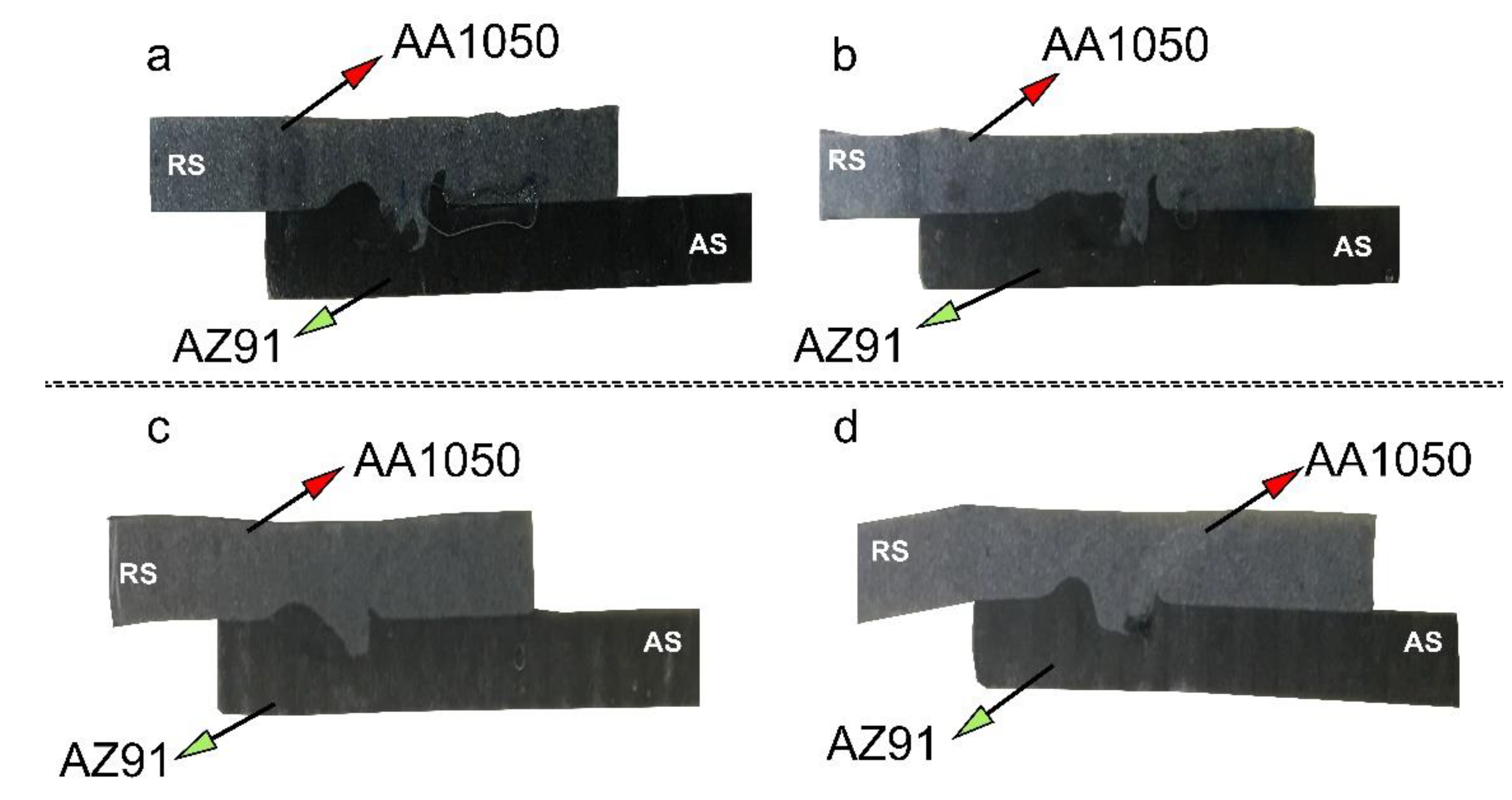

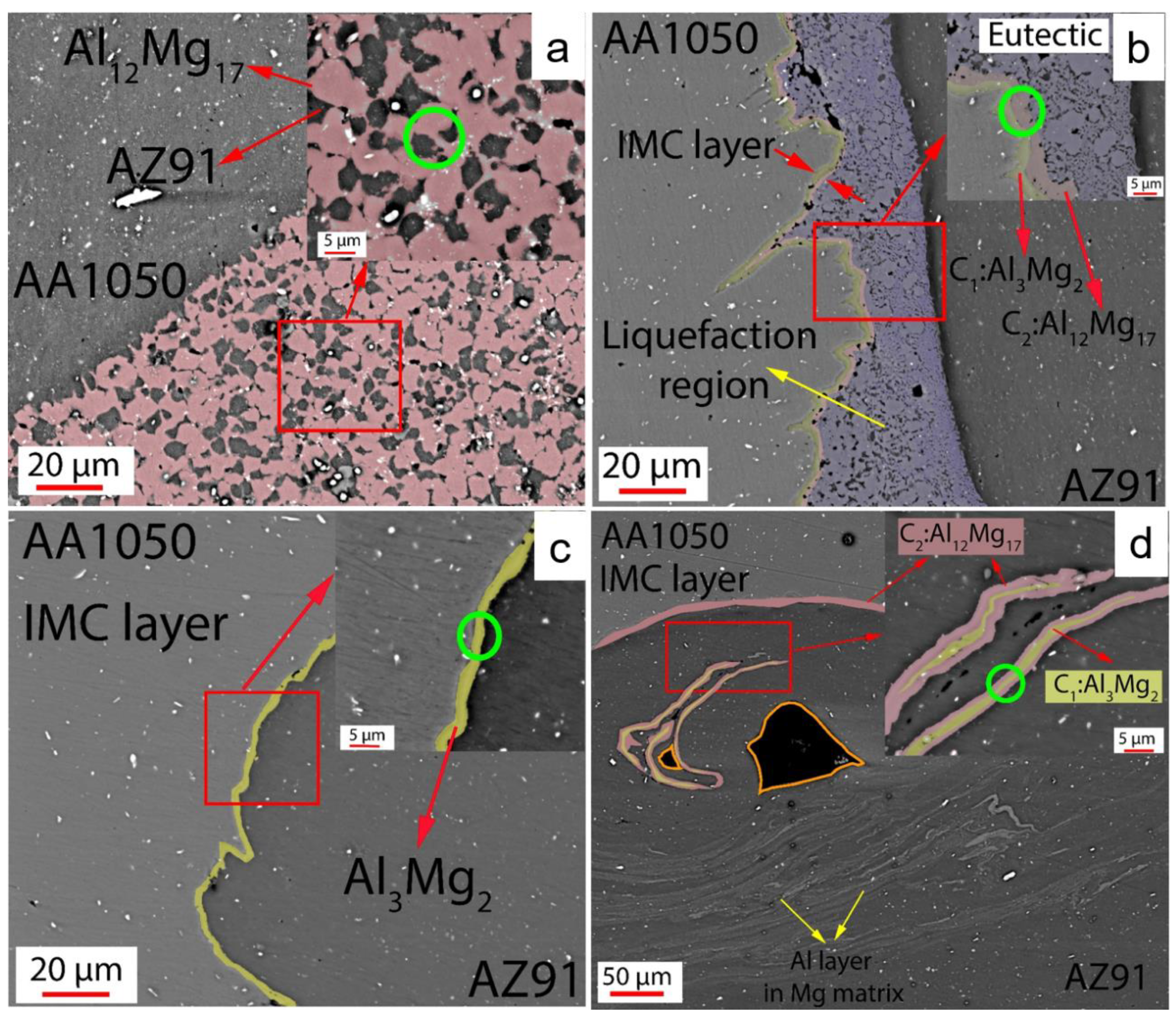
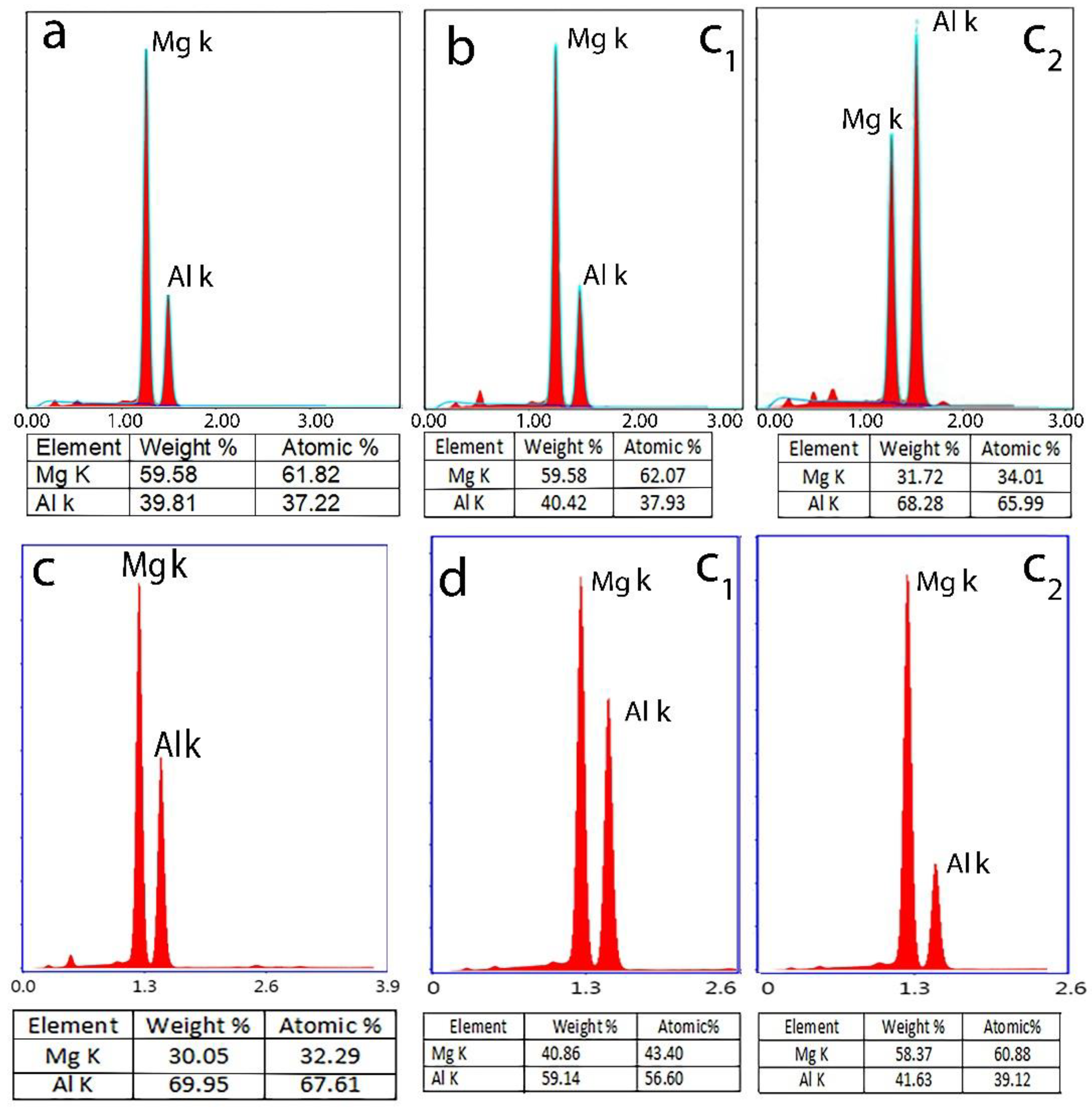
| Al | V | Zn | Mn | Fe | Si | Cu |
|---|---|---|---|---|---|---|
| Balance | 0.010 | 0.050 | 0.050 | 0.42 | 0.2 | 0.08 |
| Mg | Fe | Si | Ni | Cu | Mn | Zn | Al | Ca |
|---|---|---|---|---|---|---|---|---|
| Balance | 0.005 | 0.3 | 0.005 | 0.051 | 0.202 | 0.403 | 8.14 | 0.577 |
| Sample | Welding Speed (mm/min) | Tool Rotation Speed (rpm) |
|---|---|---|
| A | 23.5 | 950 |
| B | 37.5 | 950 |
| C | 23.5 | 600 |
| D | 37.5 | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torabi, K.; Beygi, R.; Eisaabadi Bozchaloei, G.; da Silva, L.F.M. The Effect of Tool Rotation Speed on the Formation of Eutectic Structure during Friction Stir Welding of Aluminum to Magnesium. Appl. Sci. 2023, 13, 7133. https://doi.org/10.3390/app13127133
Torabi K, Beygi R, Eisaabadi Bozchaloei G, da Silva LFM. The Effect of Tool Rotation Speed on the Formation of Eutectic Structure during Friction Stir Welding of Aluminum to Magnesium. Applied Sciences. 2023; 13(12):7133. https://doi.org/10.3390/app13127133
Chicago/Turabian StyleTorabi, Kiarash, Reza Beygi, Ghasem Eisaabadi Bozchaloei, and Lucas F. M. da Silva. 2023. "The Effect of Tool Rotation Speed on the Formation of Eutectic Structure during Friction Stir Welding of Aluminum to Magnesium" Applied Sciences 13, no. 12: 7133. https://doi.org/10.3390/app13127133
APA StyleTorabi, K., Beygi, R., Eisaabadi Bozchaloei, G., & da Silva, L. F. M. (2023). The Effect of Tool Rotation Speed on the Formation of Eutectic Structure during Friction Stir Welding of Aluminum to Magnesium. Applied Sciences, 13(12), 7133. https://doi.org/10.3390/app13127133









