The Potential Therapeutic Role of Green-Synthesized Selenium Nanoparticles Using Carvacrol in Human Breast Cancer MCF-7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis and Characterization of SeNPs-CV
2.3. Cell Line and Culture Condition
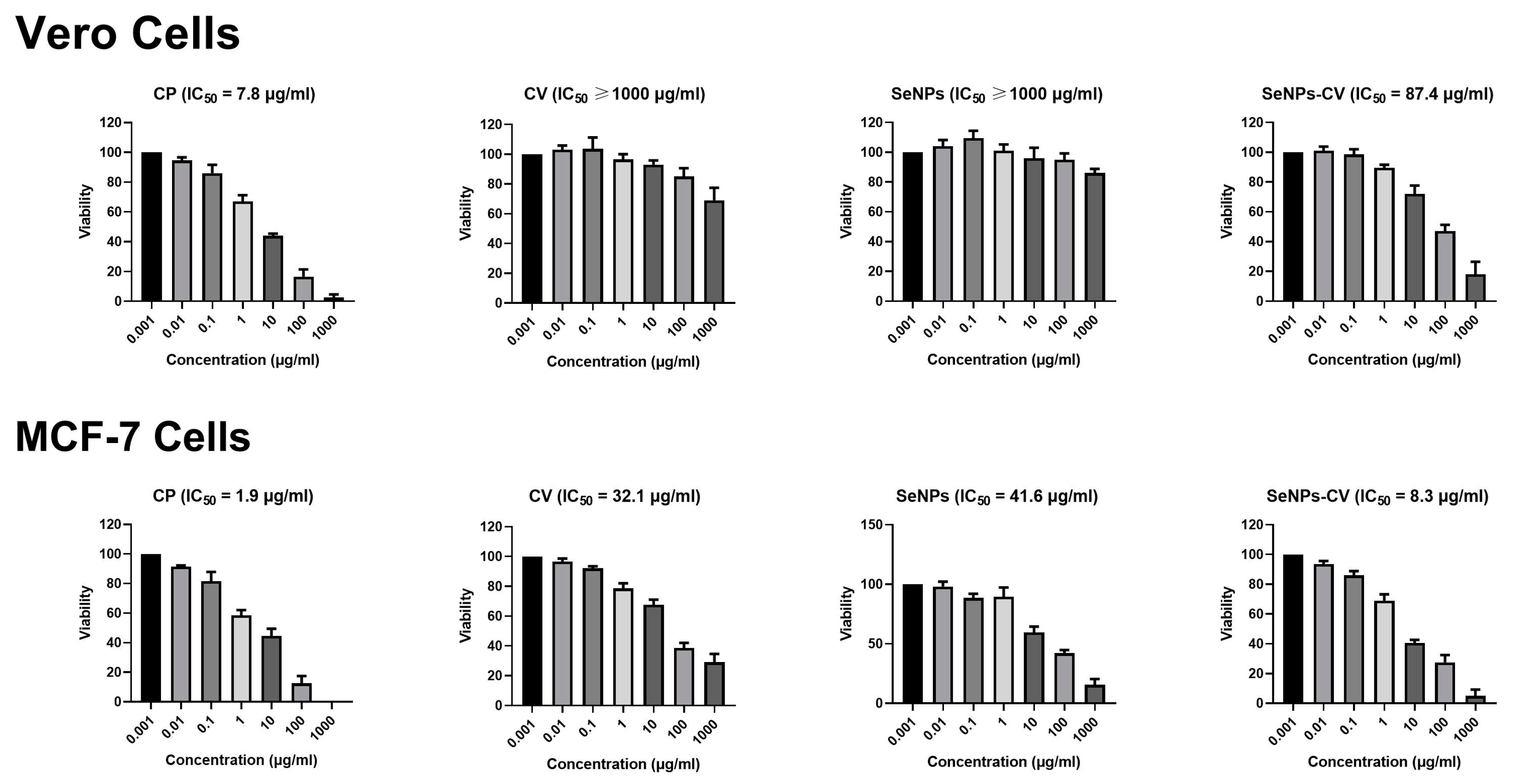
2.4. MTT Assay
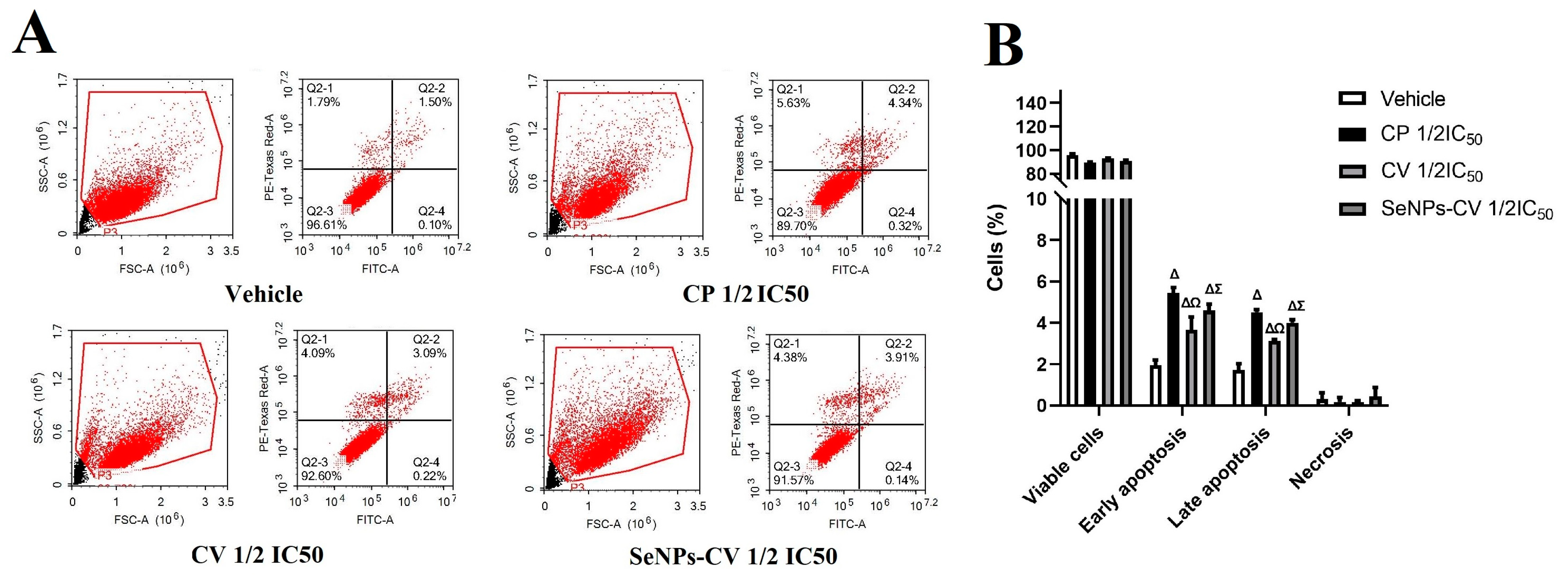
2.5. Determination of Cell Death by Flow Cytometry
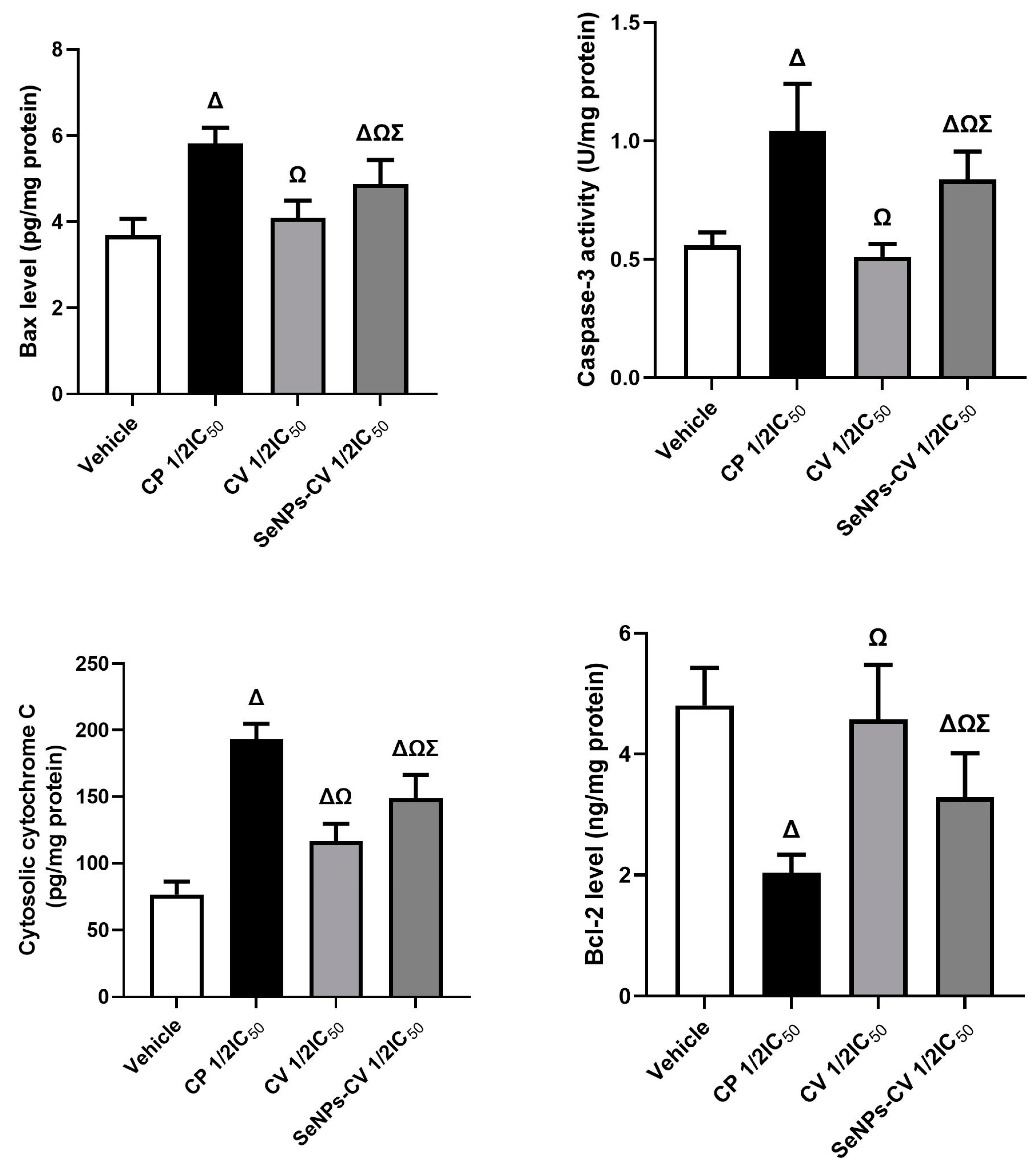
2.6. Apoptosis Markers Determination
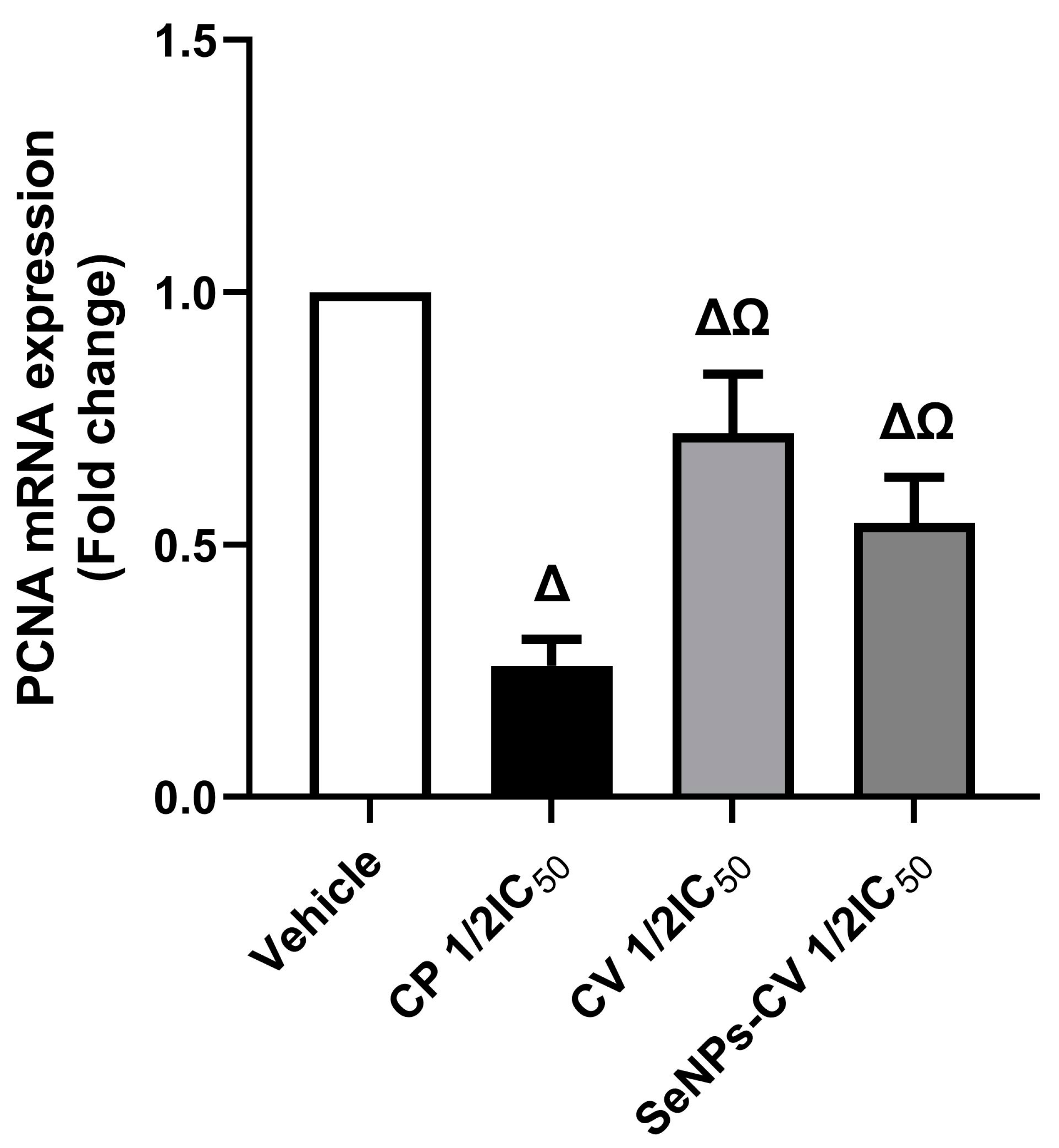
2.7. Molecular Assay for Assessing the Expression Level of Proliferating Cell Nuclear Antigen (PCNA)
- PCNA F: 5′-GCCAGAGCTCTTCCCTTACG-3′,
- R: 5′-TAGCTGGTTTCGGCTTCAGG-3′.
- β-actin F: 5’-GTCATTCCAAATATGAGATGCGT-3’
- R: 5’-GCTATCACCTCCCCTGTGTG-3’.
2.8. Assessment of Oxidative Stress Markers
2.9. Determination of the Antioxidant Markers
2.10. Evaluation of Interleukin-1 Beta (IL-1β)
2.11. Statistical Analysis
3. Results
3.1. SeNPs-CV Particles’ Characterization
3.2. Cytotoxic Effect and Growth Inhibition of SeNPs-CV
3.3. SeNPs-CV Treatment-Induced Apoptosis in MCF-7 Cells
3.4. Effect of SeNPs-CV in the Expression Rate of Proliferating Cell Nuclear Antigen (PCNA)
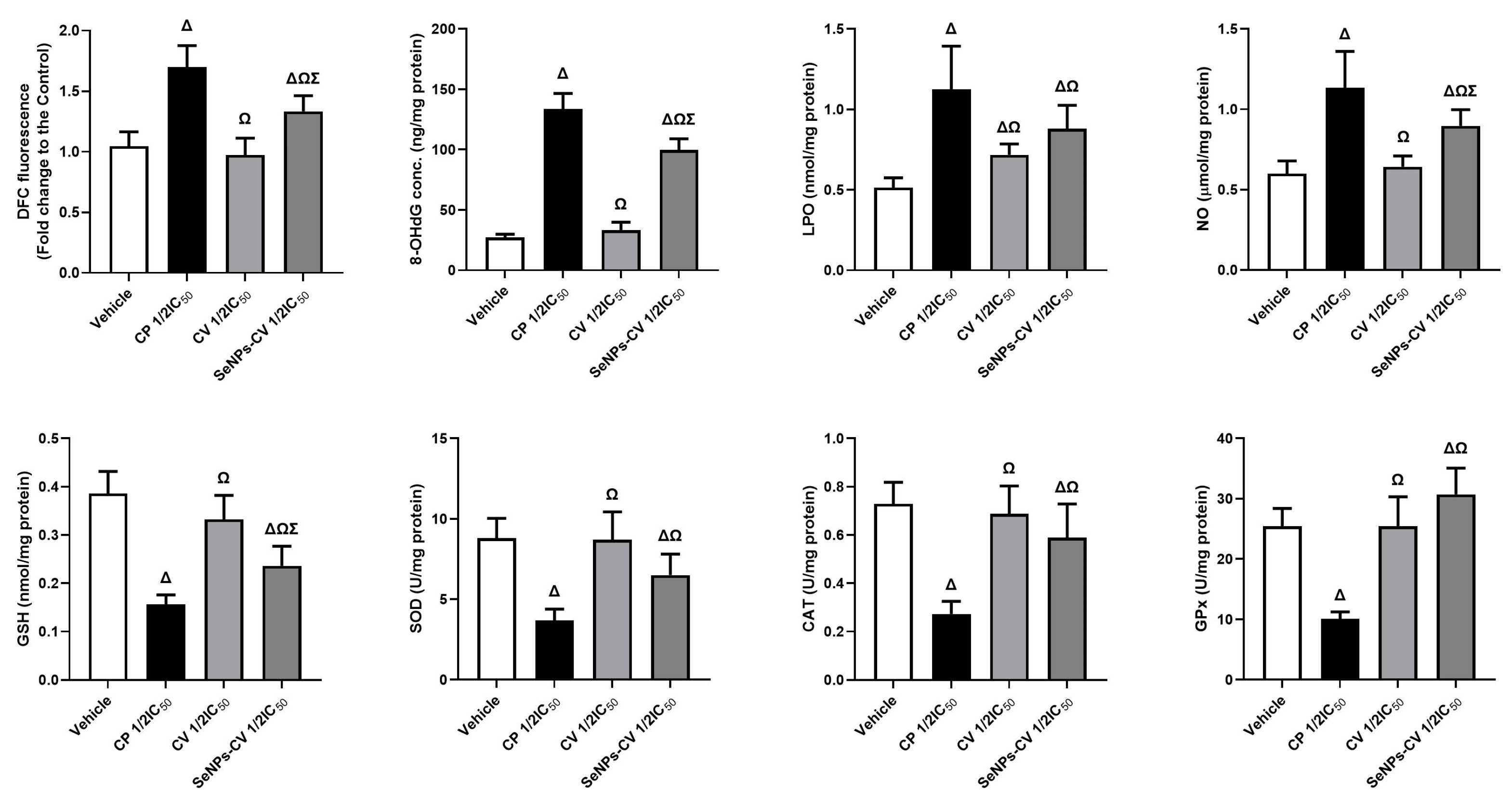
3.5. SeNPs-CV Treatment Enhanced the Oxidative Stress in MCF-7 Cells
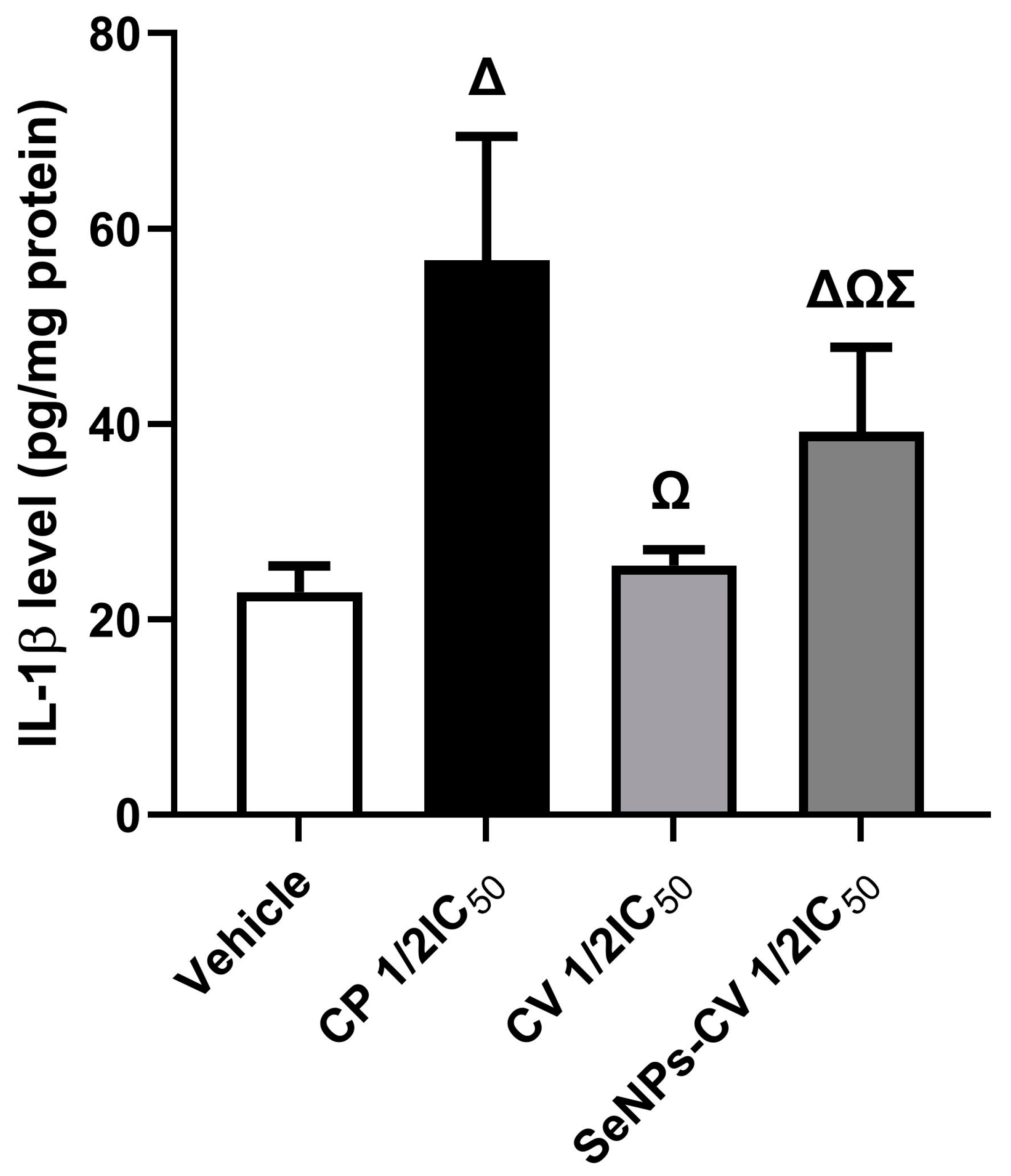
3.6. SeNPs-CV Stimulated IL-1β Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Otaibi, A.M.; Al-Gebaly, A.S.; Almeer, R.; Albasher, G.; Al-Qahtani, W.S.; Abdel Moneim, A.E. Potential of green-synthesized selenium nanoparticles using apigenin in human breast cancer MCF-7 cells. Environ. Sci. Pollut. Res. Int. 2022, 29, 47539–47548. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Obeidat, S.; Al-Bagawi, A.; Fareid, M.; El-Borady, O.; Kassab, R.; Abdel Moneim, A. Evaluation of the Potential Role of Silver Nanoparticles Loaded with Berberine in Improving Anti-Tumor Efficiency. Pharm. Sci. 2022, 28, 86–93. [Google Scholar] [CrossRef]
- Othman, M.S.; Obeidat, S.T.; Aleid, G.M.; Al-Bagawi, A.H.; Fareid, M.A.; Hameed, R.A.; Mohamed, K.M.; Abdelfattah, M.S.; Fehaid, A.; Hussein, M.M.; et al. Green Synthetized Selenium Nanoparticles Using Syzygium aromaticum (Clove) Extract Reduce Pentylenetetrazol-Induced Epilepsy and Associated Cortical Damage in Rats. Appl. Sci. 2023, 13, 1050. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Abdelfattah, M.S.; El-khadragy, M.; Al-Megrin, W.A.; Fehaid, A.; Kassab, R.B.; Abdel Moneim, A.E. Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice. Green. Process. Synth. 2023, 12, 20230010. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Wu, Y.; Zhang, Y. Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation. Life Sci. 2021, 266, 118894. [Google Scholar] [CrossRef]
- Carqueijeiro, I.; Langley, C.; Grzech, D.; Koudounas, K.; Papon, N.; O’Connor, S.E.; Courdavault, V. Beyond the semi-synthetic artemisinin: Metabolic engineering of plant-derived anti-cancer drugs. Curr. Opin. Biotechnol. 2020, 65, 17–24. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Javed, H.; Meeran, M.F.N.; Jha, N.K.; Ojha, S. Carvacrol, a Plant Metabolite Targeting Viral Protease (M(pro)) and ACE2 in Host Cells Can Be a Possible Candidate for COVID-19. Front. Plant. Sci. 2020, 11, 601335. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Ozgul Onal, M.; Ozturk, F. Comparison of ultrastructural changes and the anticarcinogenic effects of thymol and carvacrol on ovarian cancer cells: Which is more effective? Ultrastruct. Pathol. 2020, 44, 193–202. [Google Scholar] [CrossRef]
- Mastelic, J.; Jerkovic, I.; Blazevic, I.; Poljak-Blazi, M.; Borovic, S.; Ivancic-Bace, I.; Smrecki, V.; Zarkovic, N.; Brcic-Kostic, K.; Vikic-Topic, D.; et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.D.S.; Guimaraes, A.G. Antitumor Effects of Carvacrol and Thymol: A Systematic Review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef]
- Chen, G.; Yang, F.; Fan, S.; Jin, H.; Liao, K.; Li, X.; Liu, G.B.; Liang, J.; Zhang, J.; Xu, J.F.; et al. Immunomodulatory roles of selenium nanoparticles: Novel arts for potential immunotherapy strategy development. Front. Immunol. 2022, 13, 956181. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Alsharif, K.F.; Alzahrani, K.J.; Kabrah, S.; Al-Amer, O.; Oyouni, A.A.; Habotta, O.A.; Lokman, M.S.; Bauomy, A.A.; Kassab, R.B. Using green biosynthesized lycopene-coated selenium nanoparticles to rescue renal damage in glycerol-induced acute kidney injury in rats. Int. J. Nanomed. 2021, 16, 4335. [Google Scholar] [CrossRef]
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharm. 2021, 143, 112142. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Zrieq, R.; Al-Quraishy, S.; Abdel Moneim, A.E. Selenium Nanoparticles Attenuate Oxidative Stress and Testicular Damage in Streptozotocin-Induced Diabetic Rats. Molecules 2016, 21, 1517. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Othman, M.S.; Al-Bagawi, A.H.; Obeidat, S.T.; Fareid, M.A.; Habotta, O.A.; Moneim, A.E.A. Antitumor activity of zinc nanoparticles synthesized with berberine on human epithelial colorectal adenocarcinoma (Caco-2) cells through acting on Cox-2/NF-kB and p53 pathways. Anticancer Agents Med. Chem. 2021, 22, 2002–2010. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Tappel, A.L. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978, 52, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, T.P.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar] [CrossRef]
- Shahabadi, N.; Zendehcheshm, S.; Khademi, F. Selenium nanoparticles: Synthesis, in-vitro cytotoxicity, antioxidant activity and interaction studies with ct-DNA and HSA, HHb and Cyt c serum proteins. Biotechnol. Rep. 2021, 30, e00615. [Google Scholar] [CrossRef]
- El-Naa, M.M.; Othman, M.; Younes, S. Sildenafil potentiates the antitumor activity of cisplatin by induction of apoptosis and inhibition of proliferation and angiogenesis. Drug Des. Dev. Ther. 2016, 10, 3661–3672. [Google Scholar] [CrossRef]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anti-Cancer Drugs 2015, 26, 813–823. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, J.Y.; Lu, M.H.; Shi, Z.; Na, N.; Di, J.M. Carvacrol Alleviates Prostate Cancer Cell Proliferation, Migration, and Invasion through Regulation of PI3K/Akt and MAPK Signaling Pathways. Oxid. Med. Cell Longev. 2016, 2016, 1469693. [Google Scholar] [CrossRef]
- Koparal, A.T.; Zeytinoglu, M. Effects of Carvacrol on a Human Non-Small Cell Lung Cancer (NSCLC) Cell Line, A549. Cytotechnology 2003, 43, 149–154. [Google Scholar] [CrossRef]
- Melusova, M.; Jantova, S.; Horvathova, E. Carvacrol and rosemary oil at higher concentrations induce apoptosis in human hepatoma HepG2 cells. Interdiscip. Toxicol. 2014, 7, 189–194. [Google Scholar] [CrossRef]
- Othman, M.S.; Obeidat, S.T.; Al-Bagawi, A.H.; Fareid, M.A.; Fehaid, A.; Moneim, A.E.A. Green-synthetized selenium nanoparticles using berberine as a promising anticancer agent. J. Integr. Med. 2022, 20, 65–72. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Aindelis, G.; Pappa, A.; Chlichlia, K. Anticancer Activity of Biogenic Selenium Nanoparticles: Apoptotic and Immunogenic Cell Death Markers in Colon Cancer Cells. Cancers 2021, 13, 5335. [Google Scholar] [CrossRef]
- Soltani, L.; Darbemamieh, M. Anti-proliferative, apoptotic potential of synthesized selenium nanoparticles against breast cancer cell line (MCF7). Nucleosides Nucleotides Nucleic Acids 2021, 40, 926–941. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Lim, W.; Ham, J.; Bazer, F.W.; Song, G. Carvacrol induces mitochondria-mediated apoptosis via disruption of calcium homeostasis in human choriocarcinoma cells. J. Cell Physiol. 2019, 234, 1803–1815. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Bermúdez, J.M.; Aucejo, S.; Cameán, A.M. Cytotoxicity and morphological effects induced by carvacrol and thymol on the human cell line Caco-2. Food Chem. Toxicol. 2014, 64, 281–290. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, L.; Feng, L.; Wang, S.; Su, H.; Liu, H.; Liu, H.; Wu, Y. Mitochondrion-targeted selenium nanoparticles enhance reactive oxygen species-mediated cell death. Nanoscale 2020, 12, 1389–1396. [Google Scholar] [CrossRef]
- Moradipour, A.; Dariushnejad, H.; Ahmadizadeh, C.; Lashgarian, H.E. Dietary flavonoid carvacrol triggers the apoptosis of human breast cancer MCF-7 cells via the p53/Bax/Bcl-2 axis. Med. Oncol. 2022, 40, 46. [Google Scholar] [CrossRef]
- Dai, W.; Sun, C.; Huang, S.; Zhou, Q. Carvacrol suppresses proliferation and invasion in human oral squamous cell carcinoma. Onco. Targets. Ther. 2016, 9, 2297–2304. [Google Scholar] [CrossRef]
- Kirwale, S.; Pooladanda, V.; Thatikonda, S.; Murugappan, S.; Khurana, A.; Godugu, C. Selenium nanoparticles induce autophagy mediated cell death in human keratinocytes. Nanomedicine 2019, 14, 1991–2010. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Healthc. Mater. 2021, 10, e2100598. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xu, S.; Zhou, H.; Liu, H.; Jiang, W.; Hao, J.; Hu, Z. IL-1β induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Sci. Rep. 2017, 7, 41067. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sun, C.; Qiu, G.; Yao, L.; Yu, J.; Al Sberi, H.; Fouda, M.S.; Othman, M.S.; Lokman, M.S.; Kassab, R.B.; et al. Allicin mitigates hepatic injury following cyclophosphamide administration via activation of Nrf2/ARE pathways and through inhibition of inflammatory and apoptotic machinery. Environ. Sci. Pollut. Res. Int. 2021, 28, 39625–39636. [Google Scholar] [CrossRef]
- Ankarcrona, M.; Dypbukt, J.M.; Brüne, B.; Nicotera, P. Interleukin-1 beta-induced nitric oxide production activates apoptosis in pancreatic RINm5F cells. Exp. Cell Res. 1994, 213, 172–177. [Google Scholar] [CrossRef]
- Mathias, S.; Younes, A.; Kan, C.C.; Orlow, I.; Joseph, C.; Kolesnick, R.N. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1 beta. Science 1993, 259, 519–522. [Google Scholar] [CrossRef]
- Stoimenov, I.; Helleday, T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009, 37, 605–613. [Google Scholar] [CrossRef]
- Lu, E.M.; Ratnayake, J.; Rich, A.M. Assessment of proliferating cell nuclear antigen (PCNA) expression at the invading front of oral squamous cell carcinoma. BMC Oral. Health 2019, 19, 233. [Google Scholar] [CrossRef]
- Jurikova, M.; Danihel, L.; Polak, S.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Sinha, R. Molecular chemoprevention by selenium: A genomic approach. Mutat. Res. 2005, 591, 224–236. [Google Scholar] [CrossRef]
- Subramaniyan, J.; Krishnan, G.; Balan, R.; Mgj, D.; Ramasamy, E.; Ramalingam, S.; Veerabathiran, R.; Thandavamoorthy, P.; Mani, G.K.; Thiruvengadam, D. Carvacrol modulates instability of xenobiotic metabolizing enzymes and downregulates the expressions of PCNA, MMP-2, and MMP-9 during diethylnitrosamine-induced hepatocarcinogenesis in rats. Mol. Cell Biochem. 2014, 395, 65–76. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, M.S.; Aboelnaga, S.M.; Habotta, O.A.; Moneim, A.E.A.; Hussein, M.M. The Potential Therapeutic Role of Green-Synthesized Selenium Nanoparticles Using Carvacrol in Human Breast Cancer MCF-7 Cells. Appl. Sci. 2023, 13, 7039. https://doi.org/10.3390/app13127039
Othman MS, Aboelnaga SM, Habotta OA, Moneim AEA, Hussein MM. The Potential Therapeutic Role of Green-Synthesized Selenium Nanoparticles Using Carvacrol in Human Breast Cancer MCF-7 Cells. Applied Sciences. 2023; 13(12):7039. https://doi.org/10.3390/app13127039
Chicago/Turabian StyleOthman, Mohamed S., Shimaa M. Aboelnaga, Ola A. Habotta, Ahmed E. Abdel Moneim, and Manal M. Hussein. 2023. "The Potential Therapeutic Role of Green-Synthesized Selenium Nanoparticles Using Carvacrol in Human Breast Cancer MCF-7 Cells" Applied Sciences 13, no. 12: 7039. https://doi.org/10.3390/app13127039
APA StyleOthman, M. S., Aboelnaga, S. M., Habotta, O. A., Moneim, A. E. A., & Hussein, M. M. (2023). The Potential Therapeutic Role of Green-Synthesized Selenium Nanoparticles Using Carvacrol in Human Breast Cancer MCF-7 Cells. Applied Sciences, 13(12), 7039. https://doi.org/10.3390/app13127039




