Synthesis, Characterization, Cytotoxicity, and Antibacterial Studies of Persea americana Mill. (Avocado) Seed Husk Mediated Hydronium Jarosite Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. TEM and SAED and SEM and EDX
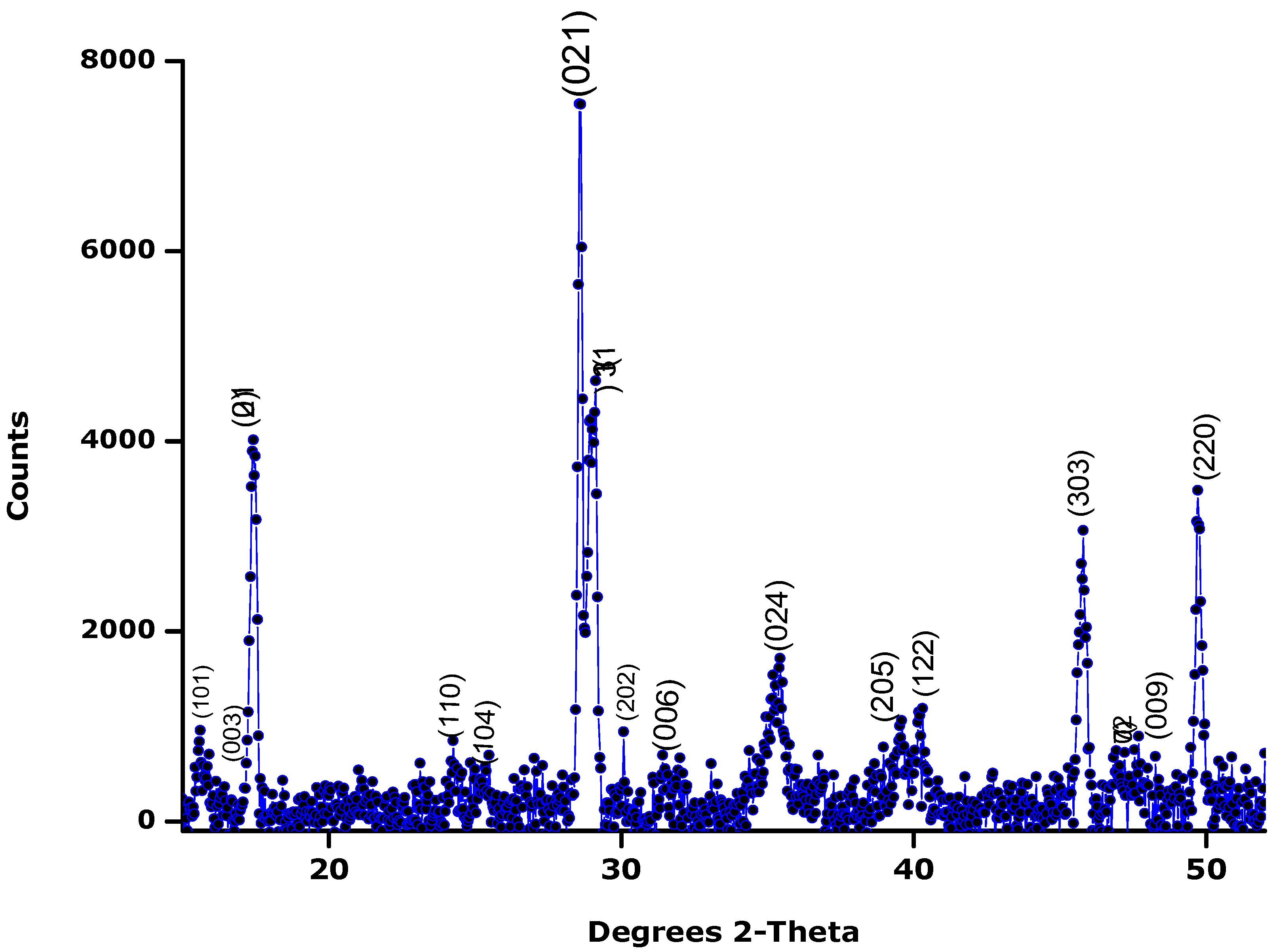
3.2. X-ray Diffraction
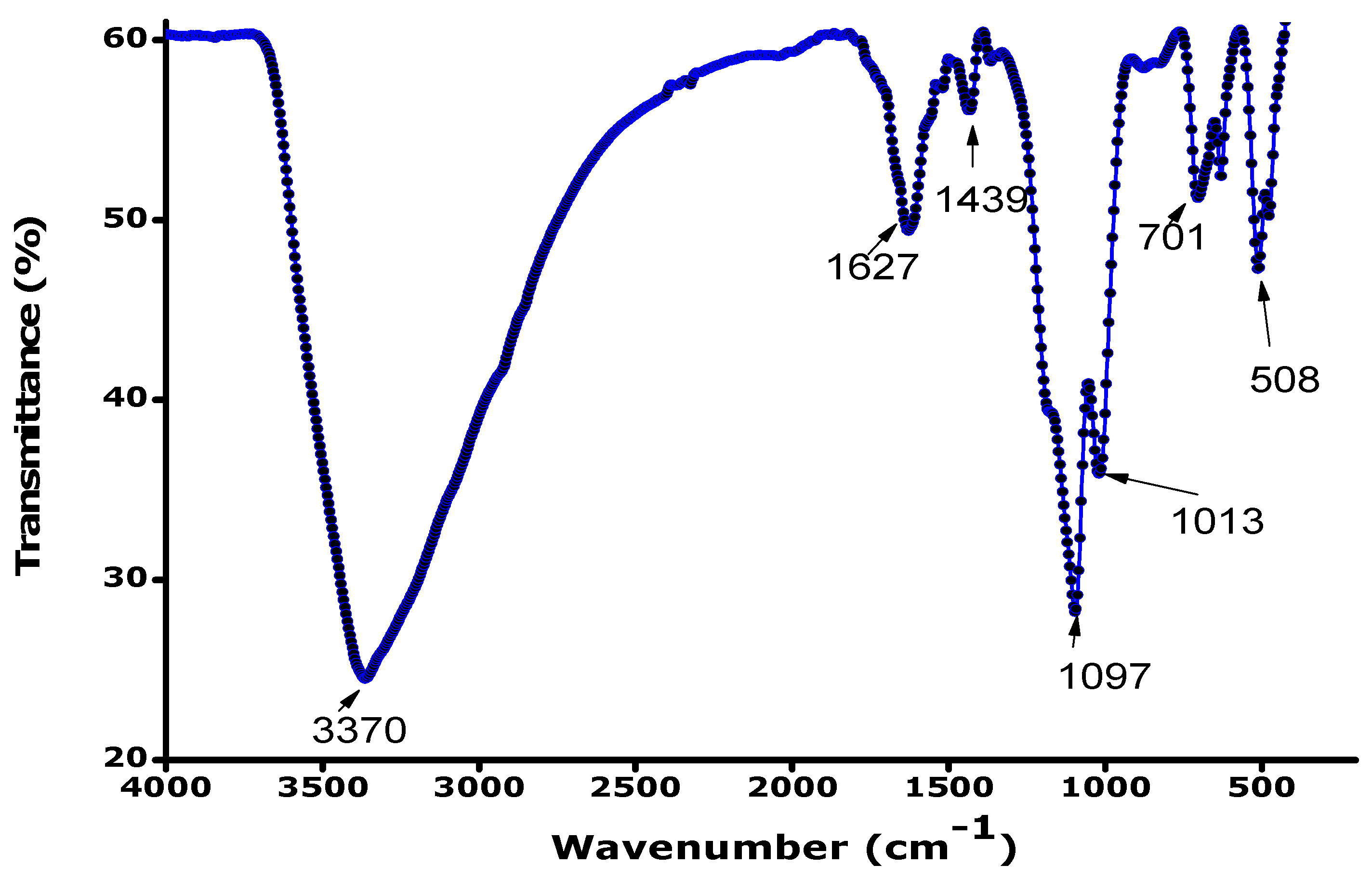
3.3. FTIR
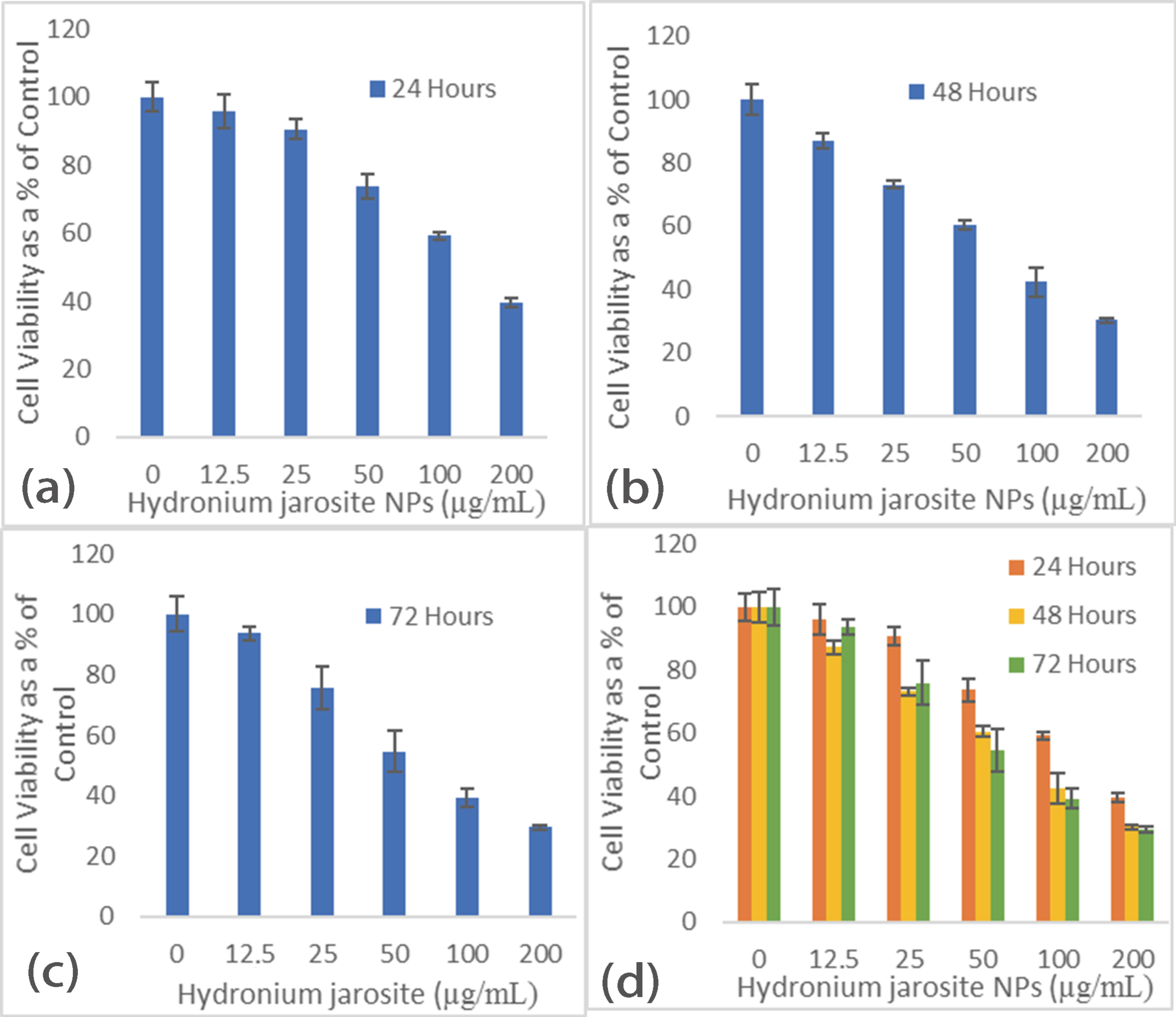
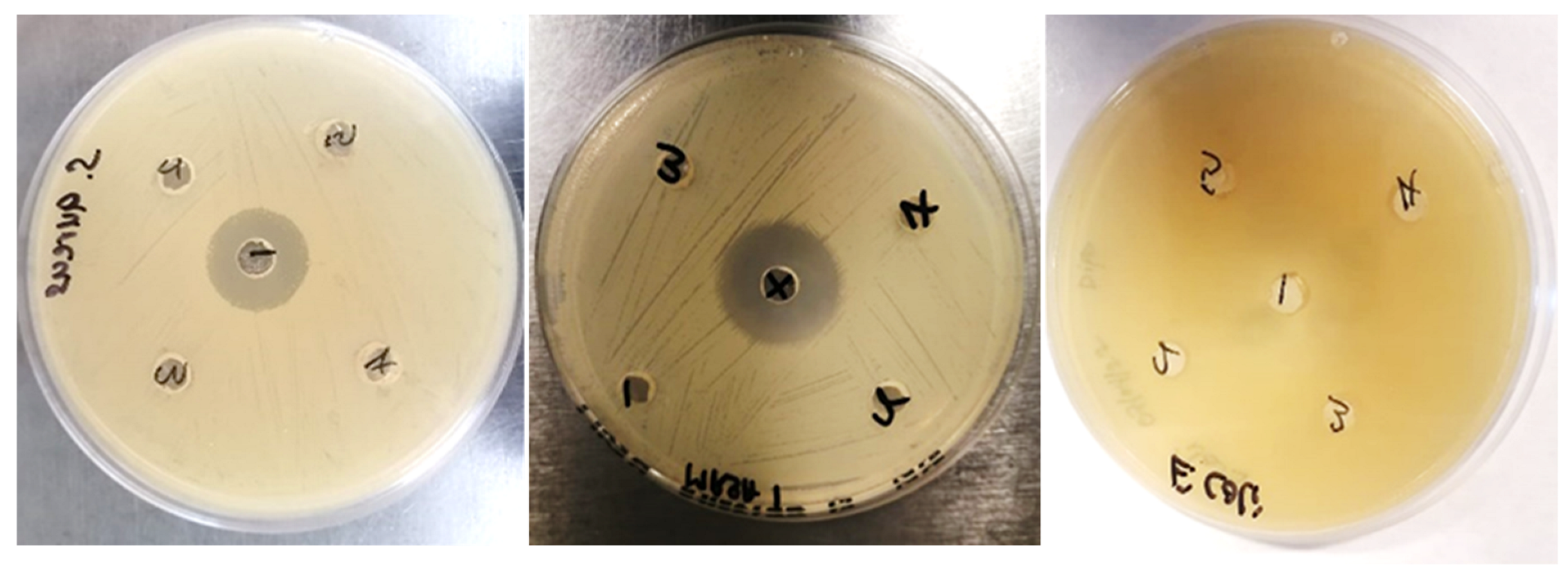
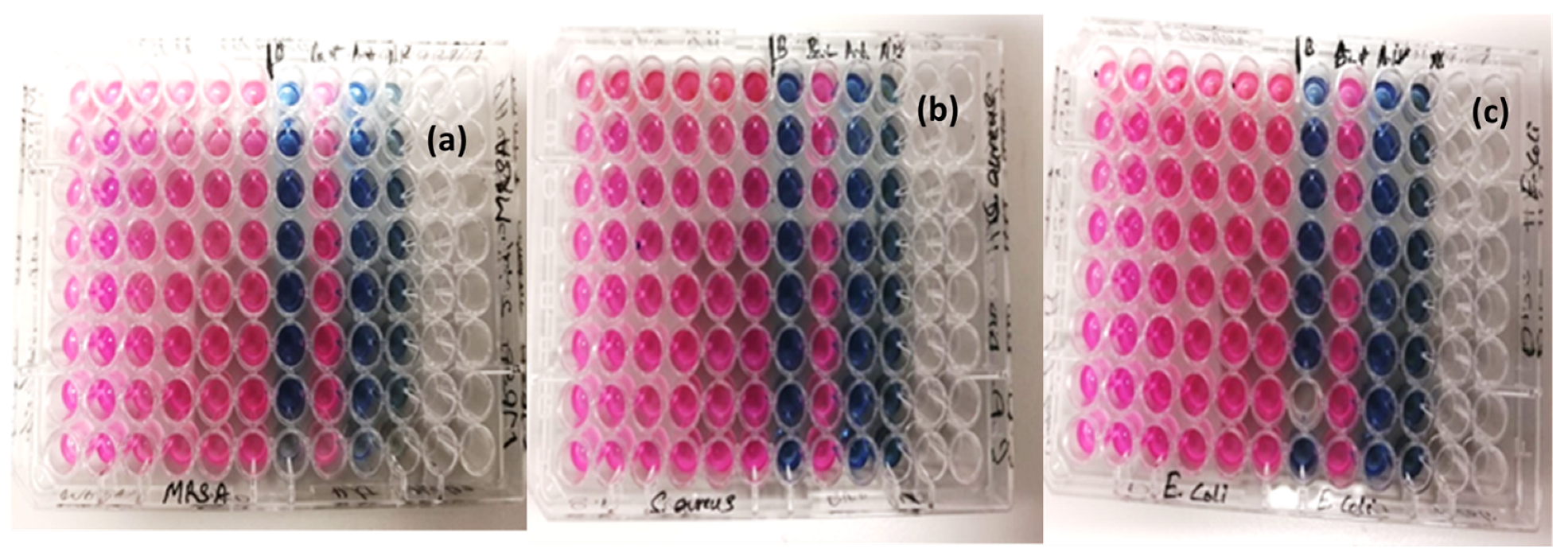
3.4. The Cytotoxicity and Antibacterial Effects
Micro-Plate Dilution Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayliss, P.; Kolitsch, U.; Nickel, E.H.; Pring, A. Alunite supergroup: Recommended nomenclature. Mineral Mag. 2010, 74, 919–927. [Google Scholar] [CrossRef]
- Plasil, J.; Skoda, R.; Fejfarova, K.; Cejka, J.; Kasatkin, A.V.; Dusek, M.; Talla, D.; Lapcak, L.; Machovic, V.; Dini, M. Hydroniumjarosite, (H3O)+ Fe3(SO4)2(OH)6, from Cerros Pintados, Chile: Single-crystal X-ray diffraction and vibrational spectroscopic study. Mineral. Mag. 2014, 78, 535–547. [Google Scholar] [CrossRef]
- Majzlan, J.; Stevens, R.; Boerio-Goates, J.; Woodfield, B.F.; Navrotsky, A.; Burns, P.C.; Crawford, M.K.; Amos, T.G. Thermodynamic properties, low-temperature heat-capacity anomalies and single crystal X-ray refinement of hydronium jarosite, (H3O)Fe3(SO4)2(OH)6. Phys. Chem. Miner. 2004, 31, 518531. [Google Scholar] [CrossRef]
- Jones, F.S.; Bigham, J.M.; Gramp, J.P.; Tuovinen, O.H. Synthesis and properties of ternary (K, NH4, H3O)-jarosites precipitated from Acidithiobacillus ferrooxidans cultures in simulated bioleaching solutions. Mater. Sci. Eng. C 2014, 44, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.F.; Rachael-Anne, W.; Kloprogge, J.T.; Martens, W.N. Thermal decomposition of hydronium jarosite (H3O)Fe3(SO4)2(OH)6. J. Therm. Anal. Calorim. 2006, 83, 213–218. [Google Scholar]
- Zhihui, X.; Jianru, L.; Lixiang, Z. Photo-Fenton-like degradation of azo dye methyl orange using synthetic ammonium and hydronium jarosite. J. Alloy. Compd. 2013, 546, 112–118. [Google Scholar]
- Bigham, J.M.; Jones, F.S.; Özkaya, B.; Sahinkaya, E.; Puhakka, J.A.; Tuovinen, O.H. Characterization of jarosites produced by chemical synthesis over a temperature gradient from 2 to 40 °C. Int. J. Miner. Process. 2010, 94, 121–128. [Google Scholar] [CrossRef]
- Labib, S.; Abdelaal, S.; Abdelhady, A.; Elmaghraby, E.K. Preparation and characterization of jarosite nanorods synthesized by microwave hydrothermal method. Mater. Chem. Phys. 2020, 256, 123654. [Google Scholar]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef]
- Botha, N.L.; Cloete, K.J.; Welegergs, G.G.; Akbari, M.; Morad, R.; Kotsedi, L.; Matinise, N.; Bucher, R.; Azizi, S.; Maaza, M. Physical properties of computationally informed phyto-engineered 2-D nanoscaled hydronium jarosite. Sci. Rep. 2023, 13, 2442. [Google Scholar]
- Ji-Yun, Y.; Chung-Hyun, L.; So-Young, P. Antioxidant Effects of Avocado Seeds and Seed Husks as a Potential Natural Preservative. Kor. J. Pharmacogn. 2021, 52, 49–54. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Tong, W.; Hu, W.; Wang, Y.; Zhang, Y. Photochemical degradation of chloramphenicol over jarosite/oxalate system: Performance and mechanism investigation. J. Environ. Chem. Eng. 2021, 9, 104570. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Habib, F.; Darwish, N.; Shanableh, A. Iron sulfide nanoparticles prepared using date seed extract: Green synthesis, characterization and potential application for removal of ciprofloxacin and chromium. Powder Technol. 2021, 380, 219–228. [Google Scholar]
- Spratt, H.; Rintoul, L.; Avdeev, M.; Martens, W. The thermal decomposition of hydronium jarosite and ammoniojarosite. J. Therm. Anal. Calorim. 2014, 115, 101–109. [Google Scholar] [CrossRef]
- Sklute, E.C.; Glotch, T.D.; Piatek, J.L.; Woerner, W.R.; Martone, A.A.; Kraner, M.L. Optical constants of synthetic potassium, sodium, and hydronium jarosite. Am. Miner. 2015, 100, 1110–1122. [Google Scholar] [CrossRef]
- Forray, F.L.; Navrotsky, A.; Hudson-Edwards, K.A. Synthesis, Characterization and Thermochemistry of Synthetic Pb–As, Pb–Cu and Pb–Zn. Jarosites Geochim. Et Cosmochim. Acta 2014, 127, 107–119. [Google Scholar] [CrossRef]
- Aguilar-Carrillo, J.; Villalobos, M.; Pi-Puig, T.; Escobar-Quiroz, I.N.; Romero, F.M. Synergistic Arsenic (V) and Lead (II) retention on synthetic jarosite. I. Simultaneous structural incorporation behaviour and mechanism. Electronic Supplementary Material (ESI) for Environmental Science: Processes & Impacts. R. Soc. Chem. 2017, 20, 354–369. [Google Scholar]
- Sapana, J.; Rizwan, A.; Nirmala, K.J.; Rajesh, K.M. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar]
- Ankamwar, B.; Chaudhary, M.; Sastry, M. Gold Nanotriangles Biologically Synthesized Using Tamarind Leaf Extract and Potential Application in Vapor Sensing. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2005, 35, 19. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid Synthesis of Au, Ag, and Bimetallic Au Core-Ag Shell Nanoparticles Using Neem (Azadirachta Indica) Leaf Broth. J. Colloid Interface Sci. 2004, 275, 496. [Google Scholar] [CrossRef] [PubMed]
- Armendariz, V.; Herrera, I.; Peralta-Videa, J.R.; Jose-Yacaman, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size Controlled Gold Nanopartilce Formation by Avena Sativa Biomass: Use of Plants in Nanobiotechnology. J. Nanopart. Res. 2004, 6, 377. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of Silver and Gold Nanoparticles by Noval Sundried Cinnamomum Camphora Leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Aqarbeh, M.M.; Abdulaziz, F.M. Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem. Int. 2020, 6, 42–48. [Google Scholar]
- Ramazanli, V.N.; Ahmadov, I.S. Synthesis of silver nanoparticles by using extract of olive leaves. Adv. Biol. Earth Sci. 2022, 7, 238–244. [Google Scholar]
- Baran, A.; Fırat Baran, M.; Keskin, C.; Hatipoğlu, A.; Yavuz, Ö.; İrtegün Kandemir, S.; Eftekhari, A. Investigation of antimicrobial and cytotoxic properties and specification of silver nanoparticles (AgNPs) derived from Cicer arietinum L. green leaf extract. Front. Bioeng. Biotechnol. 2022, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Setyawan, H.Y.; Sukardi, S.; Puriwangi, C.A. Phytochemicals properties of avocado seed: A review. IOP Conf. Series Earth Environ. Sci. 2021, 733, 012090. [Google Scholar] [CrossRef]
- Priya, N.; Kamalji, K.; Sidhu, A.K. Green Synthesis: An Eco-friendly Route for the Synthesis of Iron Oxide Nanoparticles. Front. Nanotechnol. 2021, 3, 655062. [Google Scholar] [CrossRef]
- Buarki, F.; AbuHassan, H.; Al Hannan, F.; Henari, F.Z. Green Synthesis of Iron Oxide Nanoparticles Using Hibiscus rosa sinensis Flowers and Their Antibacterial Activity. J. Nanotechnol. 2022, 1–6. [Google Scholar] [CrossRef]
- Guo, H.; Wu, S.; Wu, H.; Li, G.; Sun, Z.; Wei, Z. Jarosite-induced cytotoxicity via the generation of reactive oxygen species and apoptosis in human hepatoma HepG2 cells. J. Hazard. Mater. 2016, 304, 142–150. [Google Scholar]
- Ramírez-Hernández, M.J.; Valera-Zaragoza, M.; Viñas-Bravo, O.; Huerta-Heredia, A.A.; Peña-Rico, M.A.; Juarez-Arellano, E.A.; Paniagua-Vega, D.; Ramírez-Vargas, E.; Sánchez-Valdes, S. In search of cytotoxic selectivity on cancer cells with biogenically synthesized Ag/AgCl nanoparticles. Beilstein J. Nanotechnol. 2022, 13, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, R.; Wang, X.; Yin, N.; Cao, Y. Toxicity of jarosite to Daphnia magna and the role of oxidative stress. Environ. Pollut. 2019, 250, 741–748. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botha, N.L.; Cloete, K.J.; Matinise, N.; David, O.M.; Dube, A.; Maaza, M. Synthesis, Characterization, Cytotoxicity, and Antibacterial Studies of Persea americana Mill. (Avocado) Seed Husk Mediated Hydronium Jarosite Nanoparticles. Appl. Sci. 2023, 13, 8963. https://doi.org/10.3390/app13158963
Botha NL, Cloete KJ, Matinise N, David OM, Dube A, Maaza M. Synthesis, Characterization, Cytotoxicity, and Antibacterial Studies of Persea americana Mill. (Avocado) Seed Husk Mediated Hydronium Jarosite Nanoparticles. Applied Sciences. 2023; 13(15):8963. https://doi.org/10.3390/app13158963
Chicago/Turabian StyleBotha, Nandipha L., Karen J. Cloete, Nolubabalo Matinise, Oladipupo M. David, Admire Dube, and Malik Maaza. 2023. "Synthesis, Characterization, Cytotoxicity, and Antibacterial Studies of Persea americana Mill. (Avocado) Seed Husk Mediated Hydronium Jarosite Nanoparticles" Applied Sciences 13, no. 15: 8963. https://doi.org/10.3390/app13158963
APA StyleBotha, N. L., Cloete, K. J., Matinise, N., David, O. M., Dube, A., & Maaza, M. (2023). Synthesis, Characterization, Cytotoxicity, and Antibacterial Studies of Persea americana Mill. (Avocado) Seed Husk Mediated Hydronium Jarosite Nanoparticles. Applied Sciences, 13(15), 8963. https://doi.org/10.3390/app13158963




