Abstract
Fish is considered a highly nutritious food that constitutes the human diet, produced through fishing and aquaculture activities, to be marketed and consumed around the world in different presentations and culinary preparations. Fish is also very susceptible to spoilage and contamination by microorganisms throughout the food chain, which may be part of the usual microbiota or incorporated into food derived from inadequate hygiene practices in the food industry. Fish has been associated worldwide with disease outbreaks derived from consumption, where various bacteria and/or metabolites (biogenic amines) are some of the main casual agents. Citrobacter sp. is considered a pathogen in fish, as well as in humans, derived from the consumption of contaminated food, generating infections or histamine poisoning as it is part of the generating microbiota. Therefore, the objective of this document is to provide information on Citrobacter sp. through a general perspective on animal health and the safety of fish and fish products. Diseases derived from Citrobacter sp. contamination are included, as well as control, prevention, and sanitary legislation actions to promote animal health and the safety of foods of aquatic origin and the protection of public health.
1. Introduction
Fish is defined as any food that can be extracted from oceanic or continental waters (fresh or brackish) intended for human or animal feeding, being a generic term that includes fish, crustaceans, mollusks, algae, etc. [1].
Fish is considered a traditional and popular food in different regions around the world, and for certain countries, it constitutes the main contribution of protein of animal origin [2]. In addition, an increasing number of people choose fish as a healthy food option due not only to its source of protein of high biological value and digestibility, but also because of its content of polyunsaturated lipids, vitamins, and minerals [1,2,3].
The quality of fish for consumption regularly refers to different conditions, including aesthetic appearance, freshness, nutritional value, degree of deterioration, and safety, where the absence of pathogenic bacteria, viruses, parasites, toxins, or various chemical compounds is involved [1,2,3].
Fish and fish products are foods highly susceptible to contamination by various chemical hazards related to foodborne illnesses, including heavy metals, polychlorinated biphenyls, dioxins, insecticides, nitrosamines, antibiotics, hormones, biotoxins, biogenic amines (histamine), and biological hazards, such as parasites, viruses, and bacteria [2,4,5,6]. They have also been related to numerous outbreaks of infections or poisoning in humans around the world, derived from consumption of microorganisms, in which bacteria are the most causative-related agents [2,6,7,8].
Microorganisms can inhabit and survive in many natural environments, being transferred to food and contaminating it at different stages of the food chain through soil, air, water, insects, animals, and humans [9]. Throughout the food chain, from capture or harvest to consumption, fish undergo various changes that alter their sensory, chemical, and microbiological features. During those various phases, a series of critical points, such as time, temperature, conditions, and hygiene practices in handling, among others, must be monitored because if they are not adequately controlled, they impact the sanitary quality of the product, transforming it into a high-risk food in terms of health intended for human consumption [10,11]. In addition to this, the presence of hazards that put people’s health and perception at risk has implications for the acceptability of fish for consumption, as well as the contribution of this food to human nutrition and health [12].
Citrobacter sp. has been related to agents of biological origin harmful to human and animal health. Therefore, the objective of this document is to provide a general informative perspective on the various contaminating hazards in fish, specifically those of biological origin such as Citrobacter sp.; their impact on human and animal health, in addition to detection, prevention actions; and/or control of these hazards throughout the food chain, such as the implementation of good practices, the Hazard Analysis and Critical Control Points (HACCP) system, microbiological analysis, and sanitary regulation in fish and fish products. All of this in promotion of the safety of foods of aquatic origin destined for human consumption and the protection of public health.
2. Foodborne Diseases
Food safety refers to the guarantee that it will not cause harm or illness to the person who consumes it. This attribute is part of food’s basic characteristics, along with nutritional, sensory, and commercial features, which make up the total quality of food. There exists correspondence between the safety and health of consumers, and its obtention is fundamental and indisputable in terms of public health issues around the world [11,13].
Foodborne diseases (FDs) are syndromes derived from the consumption of food or beverages accidentally, incidentally, and/or intentionally contaminated at any stage of the food chain (from the farm to consumption) by pathogenic microorganisms or toxic substances, affecting consumer health, individually or collectively [14,15].
FDs constitute an important health problem worldwide due to their incidence, sequelae, mortality, new forms of transmission, vulnerable population groups, increased resistance of causative agents to antimicrobial compounds, loss of productivity, costs associated with health services, implementation, and monitoring of food safety policies, in addition to their effects on trade and tourism [14,16,17,18]. It is estimated that every year, around 600 million people worldwide become sick, from which 420,000 die [14,18]. These diseases present a variety of gastrointestinal symptoms, including nausea, vomiting, diarrhea, abdominal pain, and fever, with occasional severe complications, such as sepsis, meningitis, abortions, Reiter’s syndrome, Guillan–Barré syndrome, or death [15]. Its most affected population groups are children, pregnant women, immunocompromised people, the elderly, and the general population, especially those in a state of poverty [14,15,17].
It has been established that climate change; modification in society’s eating habits, such as the consumption of packaged foods, consumption of food outside home, sale of prepared meals and fast foods; incorporation of new technologies in food production; travel; commerce international level; vulnerability; susceptibility of the human being; and the inability of clinical and/or environmental laboratories to perform timely diagnostic analyses are factors that have favored the increase in FDs [16,19,20].
Approximately 250 FD-causing agents have been reported, which may be chemicals released in nature (toxic inorganic compounds, antimicrobials, growth promoters, toxic food additives, lubricants, dyes, natural toxins, disinfectants, heavy metals, pesticides, and substances used in the cleaning industry), as well as those of physical origin (fragments of glass, metal, wood, or others) and biological origin (bacteria, parasites, viruses, fungi, and prions) [16,17].
The most frequent FD, in cases and outbreaks, are those derived from biological contamination [15,17]. Generally, foods of animal or vegetable origin are rarely sterile, containing microbial associations whose composition depends on the organisms that reach it and how they multiply, survive, and interact in the food over time [18]. The microorganisms responsible for diseases through food are numerous, with bacterial agents being the main culprits [15,17], among which are adenovirus, rotavirus, norovirus, hepatitis A and E viruses, Citrobacter freundii, Salmonella spp., Vibrio sp., E. coli, Clostridium sp., Cronobacter sp., L. monocytogenes, Campylobacter sp., S. aureus, Aeromonas sp., Y. enterocolitica, B. cereus, Shigella spp., Cryptosporidium parvum, Entamoeba histolytica, Anisakis sp., Giardia lamblia, Trichinella sp., and Toxoplasma sp., among others [5,6,14,15,20,21,22].
In FDs caused by microorganisms or toxins, symptoms can manifest hours to days after the consumption of the contaminated food. This has generated its classification in (A) foodborne infections that occur when a self-established pathogen is present in the food and multiplies itself in the consumer, with two variants: (I) invasive infections, which are recognized by the pathogen’s colonization of host tissues and organs. Some causative microorganisms are viruses, protozoa, and bacteria, such as Salmonella spp., Aeromonas spp., Campylobacter spp., Shigella, Vibrio parahaemolyticus, Yersinia spp., and entero-invasive Escherichia coli; (II) toxin infections, which are produced by non-invasive bacteria, capable of colonizing and reproducing in the intestinal tract of the host, where they excrete toxins such as Vibrio cholerae, Bacillus cereus (enterotoxin producer), Clostridium botulinum, Clostridium perfringens, and enteropathogenic variants of E. coli, producing enterotoxins and/or verotoxins. Symptoms are also classified by (B) food poisoning, which frequently manifests faster than infections. They are caused by toxins produced by microorganisms that multiple to a certain concentration in the food, an aspect controlled by mechanisms such as quorum sensing. Some causative microorganisms include Clostridium botulinum, Bacillus cereus (producer of emetic toxin), and Staphylococcus aureus [17].
3. Fish
Fish is a fundamental part of a healthy diet, being a source of proteins, lipids, vitamins, and minerals, contributing to nutritional needs through food by consumers [1,23]. Fish for human consumption comes from capture fishing and aquaculture activities, estimating a worldwide production in both activities, in 2018, of around 178.5 million tons, allocating 156.4 million tons for human consumption with a per capita consumption of 20.5 kg [24]. The largest producers are Asian countries, such as China, India, Indonesia, and Vietnam, among others [24].
The function of food in humans, including fish, is to promote and sustain growth, bodily functions, replace or repair tissue, supply energy, and meet nutritional needs. However, to achieve these functions, not only is availability required, but so is innocuity, during their production, handling, conservation, and consumption [19]. Fish contributes substantially to food and nutrition security mainly in low- and middle-income countries; nevertheless, they are prone to contamination with a variety of chemical and/or biological hazards (Table 1) [12].
Fish and fish products are highly nutritious, but they are also susceptible to deterioration and contamination derived from autolysis, oxidation, and microbial activity due to high water activity, tissues’ neutral pH, and a high proportion of nutrients easily utilizable by microorganisms [1,2,11], where the nutritional value and quality of the fish is related to factors such as species, age, their environment, food, capture conditions, handling, storage, transportation, and distribution [11].
Human contact with and/or consumption of fish represents health hazards due to a variety of potentially causative agents of zoonotic diseases caused by bacteria, including Clostridium spp., Aeromonas spp., Plesiomonas spp., Vibrio spp., Streptococcus spp., Staphylococcus spp., Edwardsiella spp., Salmonella spp., and Pseudomonas spp., among others [25]. Fish and fish products have been related to outbreaks of foodborne diseases around the world where different causative agents, including bacteria, have been identified [7,8]. These diseases generally occur due to the ingestion of raw fish or because the fish have subjected to inadequate heat-treatment conditions, contaminated products after or during processing, inadequate or absent hygienic conditions and/or bad handling, conservation, and/or sale practices [7,8,18,26].

Table 1.
Hazards associated with diseases in humans from the consumption of fish and fish products [9,26,27,28].
Table 1.
Hazards associated with diseases in humans from the consumption of fish and fish products [9,26,27,28].
| Hazard Type | Disease Causal Agent | Example |
|---|---|---|
| Biological | Bacteria | Vibrio spp., Salmonella spp., Shigella spp., Plesiomonas shigelloides, Edwardsiella tarda, Listeria monocytogenes, Staphylococcus aureus, Escherichia coli, Clostridium sp., Bacillus cereus, Campylobacter jejuni, Aeromonas spp., Citrobacter freundii, and Citrobacter braakii. |
| Parasites | Gnathostoma spp., Pseudoterranova spp., Anisakis spp., Phocanema spp., Angiostrongylus spp., Contracaecum spp., Diphyllobothrium spp., Phagicola spp., Clonorchis spp., Paragonimus spp., Heterophyes spp., and Cryptosporidium spp. | |
| Virus | Hepatitis A, hepatitis E, adenovirus, norovirus, astrovirus, rotavirus, and enterovirus. | |
| Fungi | Fusarium spp., Aspergillus spp., Penicillium spp. | |
| Chemical | Biotoxins | Tetrodotoxin, ciguatera (ciguatoxin, scaritoxin, maitotoxin, palytoxin, and okadaic acid), Gempilotoxin, and mycotoxins. |
| Heavy Metals | Lead, arsenic, cadmium, copper, mercury | |
| Organic Compounds | Polycyclic aromatic hydrocarbons, polychlorinated biphenyls, polybrominated diphenyl ethers, dioxins, pesticides, microplastics, antibiotics, and hormones. | |
| Biogenic Amines | Histamine, putrescine, and cadaverine. |
Microbiology of Fish
In living or recently caught fish, the microorganisms are located on the skin, gills, and intestines. The bacterial population is variable, with a normal range of 102–107 CFU/cm2 on the surface of the skin and gills; meanwhile in the intestines, they range between 103 and 109 CFU/g [3,29], and in fresh muscle, they range from 102 to 106 CFU/g [30]. Fish and other aquatic organisms have a microbial population that is dependent on that present in the aquatic environment where they live [29,31].
Bacteria on the skin and the gastrointestinal content of freshly caught, living fish do not invade the muscular tissue and internal organs, being considered sterile, due to the action of their natural defenses [29,31]. Although Buras et al. [29] reported that fish rearing in treated domestic sewage presented bacteria in muscle, where the number of bacteria causing their appearance in fish muscle has been termed the “threshold concentration”, concluding that for aspects of public health, fish can be raised in treated wastewater if the bacteriological quality of the water is compatible with the “threshold concentration” levels of the fish grown in ponds. When the fish dies, the bacteria present can penetrate inside, giving rise to spoilage, meaning that if the conditions and practices in capture and processing procedures are inadequate, contamination can be generated, accelerating spoilage and a risk to the health of consumers [31,32].
Health risks can be caused by different pathogenic bacteria, which can be classified as follows: (1) autochthonous, which are those whose natural habitat is water (C. botulinum, Listeria spp., Vibrio spp., Plesiomonas shigelloides, Edwardsiella tarda, Pseudomonas spp., and Aeromonas spp., among others) and are widely distributed in the aquatic environments of different places in the world, where temperature and salinity have a selective effect. They are also considered part of the natural microbiota of the fish [2,4,33]; and (2) non-native, which includes bacteria present in water with fecal contamination and/or associated with inadequate hygiene practices and conditions during capture, transportation, processing, and handling phases, identifying various enterobacteria, such as E. coli, Shigella spp., Salmonella spp., Enterobacter cloacae, Citrobacter sp., Serratia sp., P. vulgaris, Listeria monocytogenes, S. agalactiae, Staphylococcus epidermidis, and Staphylococcus aureus, among others [2,4,30,31,34].
Therefore, the microbial load of freshly caught, living fish bacteria considerably influences their quality, safety, and useful life [4,10,30]. Factors such as food, season of the year, geographical area, age and species of the fish, capture system, conditions, and hygienic practices in the capture, harvesting, handling, conservation, storage, and transportation of fish qualitatively and quantitatively influence the spoilage and health risk for consumers of fish [4,10,30,31,33].
4. Citrobacter sp.
The Citrobacter genus is found within the so-called coliform bacilli, along with the Escherichia, Klebsiella, Enterobacter, and Serratia genera, which include overt and opportunistic pathogens responsible for a wide range of infections [35,36,37]. The genus Citrobacter spp. are bacilli belonging to the Enterobacteriaceae family and are Gram negative, 0.3–1.0 µm wide × 0.6–6 µm long, flagellated, aerobic, not sporulated, without capsule, facultative anaerobes, methyl red and catalase positive, fermenters (glucose and lactose), gas producers, citrate positive (due to their ability to use citrate as their only carbon source and to which they owe their name), capable of reducing nitrate to nitrite, lysine decarboxylase, Voges–Proskauer and oxidase negative. They also have an optimal growth temperature of 37 °C, are considered members of the normal intestinal microbiota of humans and animals, and can be isolated from various environmental sources (water, wastewater, soil, and food) [21,35,36,37,38,39,40,41,42,43]. These bacteria, like other enterobacteria, have specific differentiation antigens, such as “O”, which are lipopolysaccharides, or cell-wall proteins; “H” antigens, which are flagella proteins that can act as virulence factors, standing out lipopolysaccharide with endotoxin activity, in addition to α-hemolysin cytotoxins and enterotoxins [44].
The genus Citrobacter comprises 11 species: Citrobacter freundii; Citrobacter koseri; Citrobacter amalonaticus; Citrobacter braakii; Citrobacter farmeri; Citrobacter sedlakii; Citrobacter werkmanii; Citrobacter youngae; Citrobacter rodentium; Citrobacter gillenni; and Citrobacter murliniae [37,41,43]. From which Citrobacter freundii, C. diversus, and/or C. amalonaticus have been associated with various infections of the gastrointestinal system, urinary tract, respiratory tract, wounds, intra-abdominal area, bone, respiratory tract biliary (calculi or obstruction), surgical wounds, and central nervous system. They are also able to lodge in other organs and tissues [21,36,41,45].
Citrobacter is also associated with cases of septicemia that can occur in patients with multiple predisposing factors, such as being immunocompromised; they can also cause meningitis, septicemia, and lung infections in newborns and young children [35,44,46]. Infections by Citrobacter sp. can be fatal at an estimated 33–48% overall mortality rate, including a 30% mortality rate for children. The most prone population are children, people with immunodeficiency, and the elderly [21]. Therefore, these bacteria are considered an important issue in public health, labeled as opportunistic pathogens in the incidence of foodborne diseases [21,43].
4.1. Citrobacter, Fish, and Animal Health
Citrobacter species are generally known as opportunistic pathogens in human and veterinary medicine, including aquaculture activities, due to the presence of immunosuppressive factors (stress and pollution, among others) that favor the appearance of diseases. [39,42,47]. Clinical manifestations have been reported in patients who handled Nile tilapia (Oreochromis niloticus), leading to necrotizing fasciitis and osteomyelitis [39].
Intensive fish aquaculture activities are a profitable means to produce animal protein. The main condition to achieve this task is to maintain the vital and productive functions of the fish in adequate physiological conditions. However, changes in abiotic environmental factors, such as decreased oxygen, frequent changes in water temperature and pH, increased concentration of ammonia and carbon dioxide, and high stocking density, can favor animal stress and susceptibility to bacterial infections, which represent economic losses in aquaculture around the world [39,48].
Citrobacter sp. have been isolated from apparently healthy fish [39,42,49,50]. However, its negative effects on animal health in aquaculture activities have been reported, indicating most of the time its presence, along with the Aeromonas, Acinetobacter, Bacteroides, Flavobacterium, and Pseudomonas species in the intestine of fish, where the intestinal tract of fish seems to be involved in Citrobacter infection, being the first to become infected when exposed to contaminated water [50].
Regarding the animal health field, species such as C. freundii are common in eutrophic freshwater, from where it is possible to spread to fish [51], also causing infections in crustaceans, amphibians, reptiles, birds, and mammals, resulting in gastrointestinal diseases [39]. In fish, C. freundii has been predominantly isolated in mixed infections and, in some cases, it is pointed out as a secondary and opportunistic pathogen causing damage and high mortality [50,52]. Among the Citrobacter species reported as causing diseases in fish are Citrobacter braakii and C. freundii [47,50,52], the latter being associated with diseases in fish with episodes of hemorrhagic septicemia, severe enteritis, severe kidney disease, and gill lesions of catfish (Pseudoplatystoma spp.) [39,52]. In silver catfish (Rhamdia quelen), the infection presents anemia degenerative disease, protein loss, leukopenia with neutropenia, lymphocytosis and leucoblastosis, liver degeneration, a decrease in the amount of renal hematopoietic tissue, and the presence of melanomacrophage centers (MMCs) in the spleen and cephalic kidney of infected fish, contributing to the high mortality of affected fish [52]. In zebrafish (Danio rerio), the infection is associated with an increase in fish culture’s temperature [50,52]. In tilapia (Oreochromis mossambicus), tail necrosis, septicemia, hemorrhage, and reddening of the body have been reported. In Nile tilapia (O. niloticus), high mortality is present. In rainbow trout (Oncorhynchus mykiss), infection can produce gastroenteritis, dark pigmentation, incoordination, bilateral exophthalmia, lack of response to external stimuli, pale spleen, petechial hemorrhage in the intestine, and mortality [39,47,48,52]. In cyprinids or carp, such as Cyprinus carpio, infection produces hemorrhagic gastroenteritis, systemic infection [39,48,52], and for angelfish (Pter ophyllum scalare), eels (Anguilla japonica), ornamental fish, pacu (Piaractus mesopotamicus), shrimp (Litopenaeus vannamei), and freshwater rays (Potamotrygon motoro), its effects are bleeding and hepatic and splenic granulomas [52].
4.2. Citrobacter, Histamine, and Food Diseases by Fish Consumption
Fish, besides being a nutritious source of food, are susceptible to deterioration and endogenous or exogenous contamination by bacteria, particularly those that produce biogenic amines [23]. Biogenic amines are biologically active low-molecular-weight, organic, non-protein nitrogenous compounds present in numerous foods, such as cheese, wine, meat, vegetables, and fish [53,54].
Protein hydrolysis is carried out by the action of endoenzymes that release amino acids that, in turn, serve as a substrate for enzymes (amino acid decarboxylase) of microbial origin, resulting in the generation of biogenic amines (Figure 1) [39,54,55].
Histamine-producing microorganisms are related to the natural microbiota of fish, but they can also come from contamination in post-capture stages, such as processing, conservation, storage, or distribution systems [26,39,53,56]. Histamine-producing microorganisms in fish are varied, including species of the genera Streptococcus spp., Clostridium spp., Tetragenococcus spp., Bacillus spp., Acinetobacter spp., and Pseudomonas spp. Some enterobacteria considered indicators of food hygiene are Citrobacter spp., Serratia spp., Hafnia spp., Vibrio spp., Escherichia spp., Edwarsiella spp., Klepsiella spp., Salmonella spp., Shigella spp., Photobacterium spp., Enterobacter spp., Plesiomonas spp., Proteus spp., and Morganella, with the species M. morganii considered the main producer [26,32,53,57,58,59,60,61,62,63].
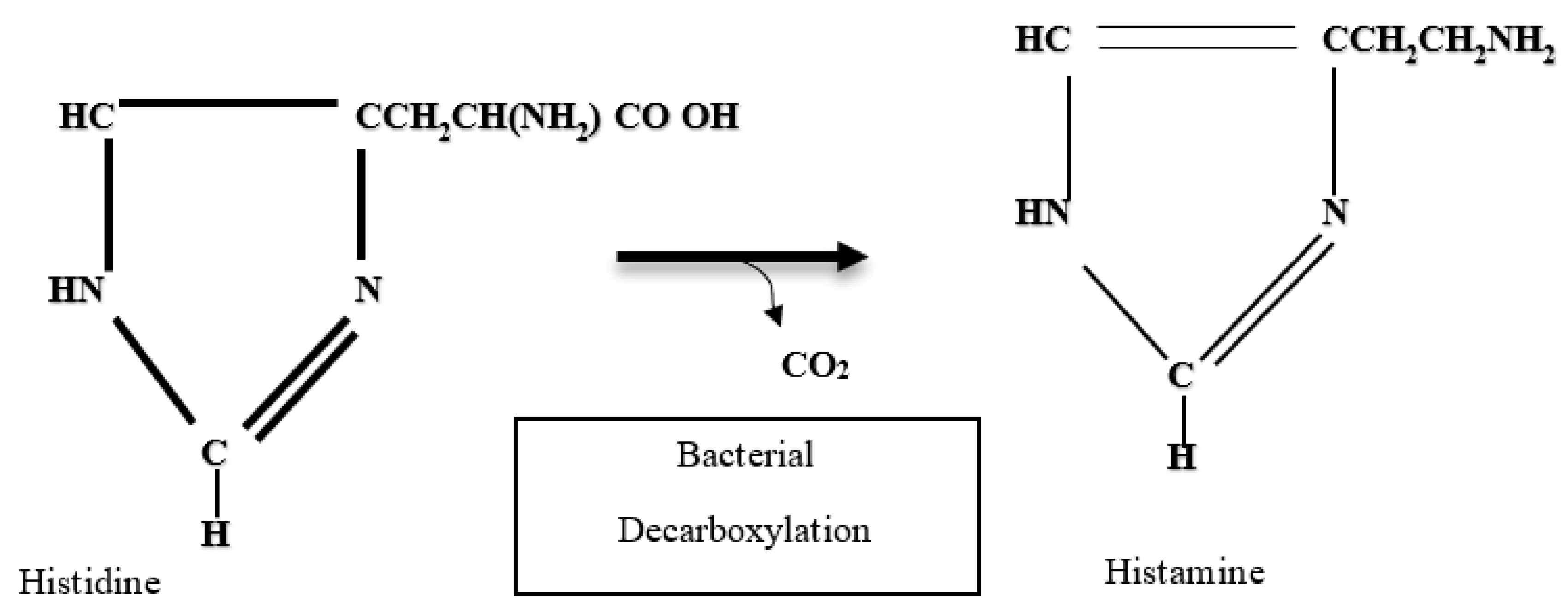
The amino acids that undergo decarboxylation reactions generate various biogenic amines, where histidine produces histamine, tryptophan produces tryptamine, lysine produces cadaverine, and where arginine gives rise to putrescine, spermidine, spermine and tyrosine gives rise to tyramine. These compounds have the particularity of presenting ammoniacal odors and of putrefaction, which are indicators of fish deterioration, and have good correlation with values of total volatile bases, trimethylamines, hypoxanthine, and pH [32,55].
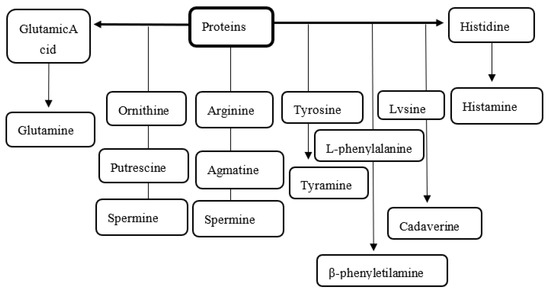
Figure 1.
Degradation of proteins and amino acids in postmortem fish and formation of biogenic amines [55,64].
Figure 1.
Degradation of proteins and amino acids in postmortem fish and formation of biogenic amines [55,64].
In fresh or recently caught fish, the content of biogenic amines is low or negligible and histamine levels are less than 0.01 mg/100g [65]. In fresh sardines, histamine is present at levels of less than 5 mg/100 g, cadaverine is present at levels of less than 15 mg/100 g, and putrescine is present at levels of less than 1 mg/100 g, but they increase as deterioration progresses [55,66]. It has been pointed out that the generation of biogenic amines results from the availability of free amino acids and microorganisms that produce decarboxylase enzymes, in addition to other factors such as temperature, salts, presence of fermentable carbohydrates, oxygen concentration, redox potential, and pH [32].
The consumption of foods with high levels of biogenic amines has been related to negative effects on the health of consumers, with gastrointestinal, neurological, and hemodynamic disorders with different symptoms, such as malaise, nausea, respiratory disorders, hot flashes, sweating, palpitations, migraines, headaches, head, itchy eyes, hyper and hypotension, stomach and intestinal problems, and pseudo-allergic reactions [54,56]. The presence of biogenic amines especially affects consumers with cardiovascular, respiratory, and gastrointestinal disorders; alcoholism problems; and vitamin B12 deficiency, among others [32].
Among the different biogenic amines, histamine is mainly related to cases of poisoning from fish consumption due to its high levels [54,66]. Histamine has a vasoactive and psychoactive action, and it is a mediator of allergic diseases, so the consumption of foods with histamine can present the same symptoms of allergic processes [54].
On the other hand, histamine is a thermostable derivative of the decarboxylation of free histidine in fish muscle and derived products through the activity of microbial enzymes (histidine-decarboxylase), whose activity can be affected by factors such as temperature, pH, concentration of histidine, oxygen tension, and the presence of vitamins and coenzymes, and the most determining factors are temperature and pH (Figure 2) [53,60].
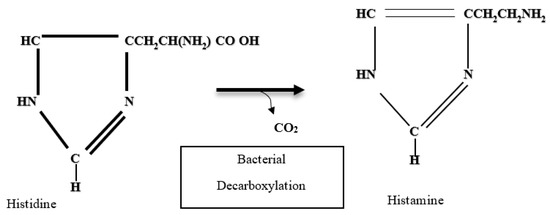
Figure 2.
Histamine formation by decarboxylation of histidine [55].
The fish and fish products associated with high proportions of histidine in muscle and susceptible to decarboxylase activity are especially those of the Scombridae family, such as tuna (Thunnus thynnus), mahi-mahi (Coryphaena hippurus), oily fish (Pomatomus saltatrix), sardines (Sardine pilchardus), mackerel (Scomber scombrus), amberjack (Seriola zonata), and abalone (Haliotis spp.) [26,32,53,60], as well as other species, such as dorado (Coryphaena spp.), anchoveta (Engraulis spp.), smooth (Mugil curema), and snook (Centropomus undecimalis) [23,59]. This amine is considered a health risk in the processing of fish for human or animal consumption. Once histamine is generated in fish, the potential to generate poisoning is high because it is thermostable, meaning it is not destroyed by any thermal process, whether it is cooking or sterilization, as well as storage temperatures, such as refrigeration and freezing [26,32,67]. As such, its detection is necessary to comply with tolerance levels, sanitary control, and protection of public health [23,59]. The presence of histamine in a food is an indicator of poor microbiological quality and hygienic management of fish [56], as well as an indicator of the low quality of the raw material of products preserved by heat treatment, and an indicator of quality in fishmeal, where the content of biogenic amines reflects the state of freshness of the raw material from which the product was obtained [55].
Poisoning from consumption of histamine-contaminated fish can occur within a few minutes to a few hours after ingestion, and the duration of symptoms varies from a few hours up to 24 h and, in some cases, the toxic effects persist for several days [26,68]. The levels to produce intoxication effects are 50 mg of histamine/100 g of fish [65]. In most cases of poisoning illness, histamine levels in fish exceed 200 ppm, frequently reaching above 500 ppm [68].
The symptoms of poisoning can be nausea, dyspnea, diarrhea, pruritus, urticaria, heart palpitations, hyper or hypotension, headache, respiratory distress, and in severe cases, bronchospasms [26,32,65,66,68]. In addition, the effect of histamine can be enhanced by the presence of other amines, such as tyramine, putrescine and cadaverine, in which the latter has no biological activity, but they inhibit enzymes such as diamine oxidase and histamine N-methyltransferase in histamine metabolism [32,55,66]. Given the benign development and the tendency for spontaneous resolution, patients often do not require treatment for poisoning, but if required, this can be gradual as symptoms appear. Treatments include gastric lavage, administration of cathartics, bronchodilators, and antihistamines, either orally or intravenously [32,65]. Patients being treated with isoniazid or monoamine oxidase inhibitors are more vulnerable to histamine poisoning and have more severe and prolonged symptoms because these drugs inhibit histamine metabolism. On the other hand, patients undergoing treatment with antihistamines block the effect of histamine, and it can be said that they are protected in a certain way; however, it has been pointed out that the prophylactic use of antihistamines is not justified [65].
5. Isolation and Detection of Enterobacteria in Food Case: Citrobacter sp.
Commonly for the isolation and detection of microorganisms, such as enterobacteria in various samples, when the culture is viable, phenotypic identification is used, which includes the visible characteristics of bacteria, such as morphology, serological, biochemical, and metabolic properties using various simple, enriched media that are differential and/or selective [69].
For the isolation and growth of Citrobacter sp., different culture media can be used through culture, such as Luria–Bertani (LB) agar, tryptic soy agar (TSA), blood agar (BA), MacConkey agar, cystine–lactose–electrolyte-deficient (CLED) agar, Salmonella-Shigella (SS) agar, xylose lysine deoxycholate (XLD) agar, Eosin Methylene Blue (EMB) agar, and Violet Red Bile Glucose agar (VRBG), among others [21,38,40,41,47,50]. The identification or confirmation of other enterobacteria, even at the species level, can be achieved by analysis of enzymatic activities, metabolic capacity, antigenic determinants, or susceptibility to bactericidal agents [21,35,37,40,41,47,70]. Within the biochemical identification tests, all the species were identified as Citrobacter ferment glucose with the production of gas (Table 2). The susceptibility pattern of the isolates helps to identify C. koseri, which has a susceptibility pattern to antibiotics such as Klebsiella (resistant to ampicillin and ticarcillin) and is sensitive to ciprofloxacin, carbapenems, third-generation cephalosporins, piperacillin-tazobactam, aminoglycosides, and trimethoprim sulfamethoxazole [37].

Table 2.
Identification tests or biochemical confirmation of the genus Citrobacter spp. of other enterobacteria [21,41,71,72].
Bacteria belonging to the genus Citrobacter spp. are closely related to Salmonella. Therefore, some similarities in cell surface antigens and biochemical properties may occur between them [73]. Species such as Citrobacter freundii, in its isolation and identification, can result in the false identification of these bacteria, such as Salmonella sp. or Proteus sp., so it can lead to more tests (Table 3) and require more time to obtain the most detailed results with the increase in the cost of analysis [41].

Table 3.
Biochemical identification tests for the differentiation of Citrobacter freundii and C. koseri with other enterobacteria [41,72].
Various generalized methods have been developed in liquid and/or solid media for the isolation and enumeration of enterobacteria in food, which are generally used as indicators of food contamination, inadequate hygiene practices during and after processing, and health risks from the consumption of contaminated food [38], as well as the identification of where Citrobacter species can be found and identified through the use of several differential and selective media. Among the existing methods are the ISO 21528-1:2017 method for detection and enumeration by the most probable number (NMP) technique, and ISO 21528-2:2017 for detection and enumeration by colony count. Jimenez et al. [74] proposed a method for detection and enumeration by colony count which involves the dilution of the sample in peptone water and subsequent inoculation in a selective culture medium (Red Violet Bile agar) and incubation at 35 °C/48 h and 44.5 °C/24 h for subsequent quantification of total coliforms and fecal coliforms, respectively, as well as subsequent identification of colonies through different biochemical tests. In addition, the methods proposed by Pascual and Calderón [38] present the phases of pre-enrichment, enrichment, and sample dilution in different nutritive culture media, such as peptone water or tryptone soy broth (TSB), Mossel EE broth for enterobacteria and subsequent growth and identification in differential culture medium, such as Violet Red Bile Glucose Agar, and confirmation through various biochemical tests for enterobacteria, such as the oxidase test and Klieger’s Iron Agar (KIA) test.
On the other hand, sometimes the absence of a relationship between the phenotypic characteristics of the isolate under study and those corresponding to bacterial strains of the type species cause the phenotypic methods to make the most probable but not definitive identification. To face these drawbacks, modern or genotypic and/or proteomic identification methods have been developed and implemented as complementary or alternative procedures that are faster and more sensitive in obtaining results [69,73].
Therefore, modern technologies for the detection and identification of Citrobacter species in laboratory samples have been developed, which through molecular analysis of the subunit ribosomal RNA gene (16S rRNA) by polymerase chain reaction (PCR) in its different variants, have reported good utility and are even being used for epidemiological studies [21,35,40,42,70]. Meanwhile, proteome analysis using matrix-assisted laser-desorption ionization and time-of-flight mass spectroscopy (MALDI-TOF MS) to identify strains in culture is also an alternative to traditional methods for greater sensitivity, specificity, and speed in obtaining results [40,75,76,77].
Finally, for the microbiological analysis of a phenotypic nature to determine the presence and quantification of histidine-decarboxylating and histamine-producing bacteria in food samples (including fish), a method has been developed by preparing decimal dilutions of the sample and using a culture medium consisting of 0.5% tryptone, 0.5% yeast extract, 2.7% L-histidine-2HCl, 0.5% NaCl, 0.1% CaCO3, 2.0% agar, and 0.006% bromocresol purple (pH 5.3), incubating the plates at 37 °C/72 h. After these procedures, the presence of histamine-forming colonies make the medium color vary from yellow to purple, indicating an increase in pH and a positive reaction due to histamine accumulation [23,56,57].
Determination of Biogenic Amines (Histamine) in Fish and Fish Products
Histamine, once generated in food, cannot be eliminated by any heat treatment, so to guarantee the safety of fish and fish products, in relation to histamine content, various colorimetric, immunoenzymatical, and chromatographic methods have been developed and proposed, such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and thin-layer chromatography (TLC) of qualitative, semiquantitative, and quantitative natures [62,78,79,80,81]. The choice of method to be used considers its efficiency, sensitivity, simplicity, speed, cost of materials, and used reagents [82]. Among the different methods are fluorometric-AOAC 977.13, which is the most used procedure for fish samples. It is sensitive and reproducible, but it is also complex and consumes a lot of analysis time; this methodology presents extraction with methanol and passage through an ion exchange column, as well as derivatization and quantification with external standards [55,68].
In the spectrophotometric method, the sample is subjected to processes of homogenization, centrifugation, and extraction with saline and butanol solutions, measuring the absorbance of extracts at 496 nm. The histamine concentration in the sample is calculated by means of a calibration curve using a histamine standard [23]. Immunoenzymatic methods are useful for the detection of histamine in fish and are used for studies in case of surveillance of conditions and adequate hygienic practices for product preservation, suspected intoxication, and applications in HACCP systems; the method can be qualitative or semiquantitative [80,83].
The basic principle of an immunoenzymatic study, such as the enzyme-linked immunosorbent assay (ELISA), is to use an enzyme to detect the binding of antigen to antibody. The enzyme converts a colorless (chromogenic) substrate to a colored product, indicating the presence of an antigen-binding antibody. Therefore, the ELISA can be used to detect the presence of antigens or antibodies in the sample, depending on its design [84]. The commercial kits available to detect histamine in fresh fish products and meals can only discriminate products with less than 50 ppm, but for health regulation purposes, such as the European Union Regulation 2073/2005 that considers several detection levels (100, 200, and 400 mg/kg) of histamine content in fishery products, further confirmation of the positive sample is needed with higher-precision analytical techniques based on HPLC [80].
In recent years, various methods based on high-performance liquid chromatography (HPLC) and/or gas chromatography (GC) have been developed for the determination of histamine in fish, which require chemical derivatization (dansyl chloride) or O-phthalaldehyde (OPA) to improve the detection of biogenic amine by UV–vis absorption or fluorescence, and where the derivatization step can be pre-column, column, or post-column, and quantification can be represented by a standard curve. These methods are fast, sensitive, and reproducible for the determination of one or more biogenic amines [55,56,80,85,86,87].
Regardless of the method carried out for the determination of histamine in fish and fish products, when they are sent to the laboratory, both the suspicious fish and/or residues of the same batch must be transported in a cold chain (frozen) to avoid the new formation of histamine during the transfer process for analysis. In addition, it must be considered that histamine levels can vary enormously between the different parts of the fish body, so different samples of the same specimen or lot must always be selected and sent for analysis, in the case of diagnosis of poisonings [65].
6. Control and Prevention: Animal Health and Food Diseases by Consumption of Fish (Microbiology Contamination and Biogenic Amines)
The presence of various microorganisms, including enterobacteria, such as Citrobacter sp., in food production systems (aquaculture and fishing) is considered hazardous to animal health and a potential risk of contamination in the different phases of the food chain that can compromise food safety.
For the control and prevention of diseases in animals, related to aquaculture activities and due to various causative agents, including bacteria, it is recommended to implement good hygiene practices, including personal hygiene, cleaning and filling of ponds, the management of the quantity and quality of water (microbiological conditions, temperature, dissolved oxygen concentration, pH, turbidity), appropriate fish population density in ponds, removal and daily elimination of sick or dead fish, avoiding the use of chemical substances, favoring a nutritious diet adequate in quantity and quality for farmed fish, and the cleanliness of capture equipment and containers [88,89].
Meanwhile, in the post-capture or harvest stages, the implementation of different procedures, practices, and/or food-safety-management systems, such as good manufacturing practices, Sanitation Standard Operating Procedures (SSOPs), and the Hazard Analysis Critical Control Points (HACCP) System, are a fundamental part of reducing the presence of pathogens and achieving the safety of fish and fish products intended for human consumption [89,90,91,92,93].
The histamine-producing microbial population is related to the natural microbiota of fish and the aquatic environment, as well as bacterial contamination of fish from poor hygienic practices and conditions during capture, handling, processing, and storage (including failures in the cold chain) that favor the acceleration of postmortem changes and the early appearance of signs of decomposition due to autolytic and microbial activity with sensory changes, composition, and pH, as well as the formation of biogenic amines (histamine, tyrosine, putrescine, and cadaverine, among others) that can affect the health of consumers [14,23,32,53,56,59,94]. For the control and prevention of the risk of histamine poisoning derived from fish consumption and considering that once the poison is generated, there is no treatment that inactivates it, it is necessary to apply actions to minimize the presence and activity of histamine-producing bacteria [67].
The presence and activity of biogenic amine-generating microorganisms in fish can be reduced by applying good manufacturing hygiene practices, effective cleaning, and disinfection procedures in processing areas, control of preservation, and storage temperatures with the use of low temperatures (0 °C or close) from the catch and throughout the food chain, as well as through the application of the HACCP system and control and prevention measures in the formation of biogenic amines in fish at dangerous levels [23,26,32,56,62,67].
It is estimated that the optimum temperature for fish conservation, to avoid the generation of histamine, is close to 0 °C and no higher than 4 °C from the moment of capture, maintaining a constant temperature during all stages of the food chain [68,79,95,96]. The production of histamine begins at temperatures of around 7 °C, with increased production from 20 °C. As such, the inadequate storage of fish, generally above 20 °C, seems to be the most important predisposing factor for the generation of high histamine levels [26,96]. However, it has been shown that histamine can accumulate in fish and reach toxic levels even at temperatures as low as 5 °C. At these temperatures, and after exposure to brief periods of storage at high temperatures ranging from 15 to 20 °C, they form large amounts of histamine even though bacterial growth ceases; this is because the decarboxylase enzyme synthesized during the brief periods of permanence of the fish at high temperatures is enough for subsequent prolonged production of histamine [96].
The toxicity of biogenic amines is related to factors associated with consumers, including the foods consumed, individual susceptibility, health status, drug use, etc., which makes it difficult to indicate the levels of toxicity in food [54]. Sanitary regulations established around the world have been developed to guarantee the safety of fishing products by controlling and reducing various contaminating hazards, such as histamine.
Therefore, in terms of food safety regulation, the European Union presents Regulation (EC) 852/2004 on the hygiene of food products that establishes the hygiene conditions and practices applied to all stages of production, transformation, distribution, and exports of food. Regulation (EC) 853/2004 establishes specific hygiene standards for food of animal origin, including the application of procedures based on the principles of hazard analysis and critical control points, as well as conservation requirements for products of fishing, considering the use of temperatures between 0 and 4 °C. The council directive 91/493/EEC and regulation (EC) 2073/2005 that establish the sampling and analysis of products indicate that when nine samples are taken from a lot of fish for histamine analysis, the average value should not be greater than 100 ppm in fish, scombrids, and cupleids families, marketed during their useful life [54,66,68]. The Food and Drug Administration (FDA) has established regulatory limits considering that fish with histamine ratios of ≥50 ppm are in a state of decomposition, and fish containing histamine at ≥500 ppm are a danger to humans and public health [54,68]. The Indian Food Safety and Standards Authority has proposed the maximum acceptable limit for histamine as 100 mg/kg [62]. Meanwhile, the sanitary regulations for processed, fresh, refrigerated, and frozen fish products in Latin American countries, such as Mexico, include official Mexican standard NOM-242-SSA1-2009, which establishes a maximum limit of 100 mg/kg of histamine in fish of the Clupeidae, Scombridae, Scombresociidae, Pomatomidae, and Coryphaenidae families.
On the other hand, regarding the contamination of fishery products by Citrobacter sp., several countries around the world, including Mexico, do not have sanitary regulations or specific limits for the presence of this microorganism associated with gastrointestinal diseases caused by food consumption and part of the histamine-generating microbiota in post-harvest fish. However, being part of the enterobacteria family, it has been pointed out that fecal coliforms, E. coli, and enteric pathogens such as Salmonella spp. are considered some of the microbiological indicators to determine contamination and health risk in various fresh, refrigerated, and frozen products intended for commercialization and human consumption [79,97,98,99].
This may have its advantages in terms of investment in time to obtain results, equipment, and materials for use in a microbiological analysis, in addition to the fact that analysis of these indicators can be associated with a greater risk of disease due to the consumption of these products contaminated with their respective causal agents. On the other hand, disadvantages may be that analysis of indicators such as coliforms involves the detection and generalized proportion of a diverse group of microorganisms in which Citrobacter may or may not be present in samples.
7. Conclusions
Fish are a source of highly nutritious food that is part of the human diet, widely traded and consumed around the world. Fish, in addition to being a source of nutritious food, are also very susceptible to deterioration and contamination at any stage of the food chain, compromising the safety and health of consumers.
Among the biological hazards generally associated with outbreaks of diseases transmitted by fish consumption are bacteria and toxic metabolites (biogenic amines). These microorganisms, several of them enterobacteria that are pathogenic to humans, can be autochthonous to fish or exogenous due to contamination of the product when it is captured in water contaminated with fecal matter or in post-capture phases (handling, transformation, conservation, distribution, commercialization, and preparation prior to consumption) under inadequate hygiene conditions and practices, including failures in thermal processes.
Citrobacter sp. has been associated both with being part of autochthonous fish microorganisms and as products of contamination in post-capture stages. Species of the genus have been considered fish pathogens as they are related to cases of infection, causing mortality and affectation in animal production, and in the generation of gastrointestinal infections in humans because of the consumption of contaminated food. In addition, Citrobacter spp. has been associated with being part of the histamine-generating microbiota in fish (biogenic amine linked to cases of food poisoning from fish consumption) regularly subjected to inadequate storage temperatures.
The demand for healthy and nutritious food by a constantly growing population will continue in the coming years, so the food industry must continue to focus on the control, reduction, or prevention of the various hazards that cause diseases derived from fish consumption contaminated by enterobacteria, including Citrobacter sp. and/or metabolites, which will always imply the implementation of adequate hygiene and conservation practices and conditions throughout the food chain (from the farm to the consumer’s table) in order to promote the availability of food and safeguard public health.
Author Contributions
A.D.J.C.-S. conceptualized and conducted the general activities of the study. M.d.l.P.S.-C., M.D.-R., E.T.-O., L.D.E.-C. information search and analysis o. All authors have read and agreed to the published version of the manuscript.
Funding
The development of this manuscript was supported by the National Council of Humanities, Sciences and Technologies of Mexico (CONAHCYT) and researchers for Mexico program-CONAHCYT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Alejandro De Jesús Cortés Sánchez and Luis Daniel Espinosa-Chaurand acknowledge financial support given by the National Council of Humanities, Sciences and Technologies of Mexico (CONAHCYT) to “researcher” position at UAML and CIBNOR. The development of this manuscript was supported by the National Council of Humanities, Sciences and Technologies of Mexico (CONAHCYT) and researchers for Mexico program-CONAHCYT.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Paiva Soares, K.M.; Gonçalves, A.A. Qualidade e segurança do pescado. Rev. Inst. Adolfo Lutz. 2012, 71, 1–10. Available online: http://www.ial.sp.gov.br/resources/insituto-adolfo-lutz/publicacoes/rial/10/rial71_1_completa/1426.pdf (accessed on 5 June 2023).
- Huss, H.H. Aseguramiento de la Calidad de los Productos Pesqueros. FAO Documento Técnico de Pesca 334. Laboratorio Tecnológico. Ministerio de Pesca. Dinamarca. Organización de las Naciones Unidas para la Agricultura y la Alimentación Roma. 1997. Available online: http://higiene.unex.es/Bibliogr/Libros/pescaFAO/indice.htm (accessed on 20 February 2023).
- Huss, H.H. El Pescado Fresco: Su Calidad y Cambios de su Calidad. FAO Documento Técnico de Pesca 348. Organización de las Naciones Unidas para la Agricultura y la Alimentación. Laboratorio Tecnológico. Ministerio de Pesca. Dinamarca. 1998. Available online: https://www.fao.org/3/v7180s/v7180s00.htm (accessed on 3 March 2023).
- Herrera Arias, F.C.; Santos Buelga, J.A. Prevalencia de Salmonella spp. en pescado fresco expendido en Pamplona (Norte de Santander). Bistua Rev. Fac. Cienc. Básicas 2005, 3, 34–42. Available online: https://www.redalyc.org/pdf/903/90330205.pdf (accessed on 2 February 2023).
- Chong, A.; Peñuelas, M.; Guerrero, M.; Cabezas, C.; Díaz, O.; Martín, C.; Varela, C. Brotes de transmisión alimentaria. Red Nacional de Vigilancia Epidemiológica. 2012–2020. Bol. Epidemiológico Semin. 2021, 29, 53–63. Available online: https://revista.isciii.es/index.php/bes/article/view/1157 (accessed on 6 February 2023).
- Friesema, I.H.M.; Slegers-Fitz-James, I.A.; Wit, B.; Franz, E. Surveillance and characteristics of food-borne outbreaks in the Netherlands, 2006 to 2019. Euro Surveill. 2022, 27, 2100071. [Google Scholar] [CrossRef] [PubMed]
- Alerte, V.; Cortés, S.; Díaz, J.; Vollaire, J.; Espinoza, M.E.; Solari, V.; Torres, M. Brotes de enfermedades transmitidas por alimentos y agua en la Región Metropolitana, Chile (2005–2010). Rev. Chilena Infectol. 2012, 29, 26–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Espinosa, L.; Varela, C.; Martínez, E.V.; Cano, R. Brotes de enfermedades transmitidas por alimentos. España, 2008-2011 (excluye brotes hídricos). Bol. Epidemiológico Semin. 2014, 22, 130–145. Available online: https://repisalud.isciii.es/bitstream/handle/20.500.12105/14377/BES_22_11_1.pdf?sequence=1 (accessed on 20 January 2023).
- Cortés Sánchez, A.D.J.; Espinosa Chaurand, L.D.; Díaz Ramírez, M.; Torres Ochoa, E. Plesiomonas: A review on food safety, fish-borne diseases, and tilapia. Sci. World J. 2021, 2021, 3119958. [Google Scholar] [CrossRef]
- Corrales Ramírez, L.C.; Alvarado Ospina, M.A.; Castillo Fonseca, L.A.; Camacho Beltran, Y.C. Estudio bacteriológico de la calidad del pescado fresco, Bagre (Pseudoplatystoma sp.) y Mojarra Roja (Oreochromis sp.) comercializado en el municipio de El Colegio, Cundinamarca (Colombia). Nova 2011, 9, 149–157. Available online: https://hemeroteca.unad.edu.co/index.php/nova/article/download/497/1070 (accessed on 10 January 2023). [CrossRef]
- Fuertes Vicente, H.G.; Paredes López, F.; Saavedra Gálvez, D.I. Buenas prácticas de manufactura y preservación a bordo: Pescado inocuo. Big Bang Faustiniano. 2018, 3, 41–45. Available online: http://datos.unjfsc.edu.pe/index.php/BIGBANG/article/view/234 (accessed on 5 January 2023).
- Eltholth, M.; Fornace, K.; Grace, D.; Rushton, J.; Häsler, B. Assessing the chemical and microbiological quality of farmed tilapia in Egyptian fresh fish markets. Glob. Food Sec. 2018, 17, 14–20. [Google Scholar] [CrossRef]
- De la Fuente Salcido, N.M.; Corona, J.E.B. Inocuidad y bioconservación de alimentos. Acta Univ. 2010, 20, 43–52. Available online: https://www.redalyc.org/pdf/416/41613084005.pdf (accessed on 20 January 2023). [CrossRef]
- Guzmán, C.A.; Rodríguez-Rodríguez, V.C.; Calderón-Rangel, A. Contaminantes microbiológicos en un mercado del sur de Montería: Un riesgo para la salud pública. Cienc. Agric. 2017, 14, 89–97. [Google Scholar] [CrossRef]
- Soto Varela, Z.; Pérez Lavalle, L.; Estrada Alvarado, D. Bacterias causantes de enfermedades transmitidas por alimentos: Una mirada en Colombia. Rev. Salud Uninorte 2016, 32, 105–122. [Google Scholar] [CrossRef]
- Olea, A.; Díaz, J.; Fuentes, R.; Vaquero, A.; García, M. Vigilancia de brotes de enfermedades transmitidas por alimentos en Chile. Rev. Chilena Infectol. 2012, 29, 504–510. [Google Scholar] [CrossRef]
- Torrens, H.R.; Argilagos, G.B.; Cabrera, M.S.; Valdés, J.B.; Sáez, S.M.; Viera, G.G. Las enfermedades transmitidas por alimentos, un problema sanitario que hereda e incrementa el nuevo milenio. Rev. Electrónica De Vet. 2015, 16, 1–27. Available online: https://www.redalyc.org/pdf/636/63641401002.pdf (accessed on 30 January 2023).
- Mendez, M.M.; Rodríguez, J.; Arístides, R.; Minier Pouyou, L.; Zayas Tamayo, E.; Soler Santana, R. Caracterización de agentes bacterianos aislados en brotes de enfermedades transmitidas por alimentos. MEDISAN 2020, 24, 235–251. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1029-30192020000200235&lng=es&tlng=pt (accessed on 30 January 2023).
- López Aday, D.; Rivero Álvarez, E.; Martínez Torres, A.; Alegret Rodríguez, M. Enfermedades transmitidas por alimentos en Villa Clara. Rev. Cubana Hig. Epidemiol. 2013, 51, 203–213. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1561-30032013000200009&lng=es&tlng=es (accessed on 27 February 2023).
- Aminharati, F.; Ehrampoush, M.H.; Dallal, M.M.S.; Yaseri, M.; Tafti, A.A.D.; Rajabi, Z. Citrobacter freundii foodborne disease outbreaks related to environmental conditions in Yazd Province, Iran. Iran. J. Public Health 2019, 48, 1099. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6635343/pdf/IJPH-48-1099.pdf (accessed on 27 February 2023). [CrossRef]
- Hashim, M.H.; Al Khafaji, M.H. Isolation and identification of Citrobacter freundii from chicken meat samples using cultural and molecular techniques. Iraqi J. Sci. 2018, 59, 1216–1224. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 1–324. [Google Scholar] [CrossRef]
- Barba Quintero, G.; Ramírez De León, J.A.; Cortés Ruiz, J.A.; Sánchez Humaran, I.L.; Ruelas Inzunza, J.R.; Moreno Hernández, J.M. Contenido de histamina y calidad microbiológica de pescado comercializado en Mazatlán, Sinaloa. BIOtecnia 2012, 14, 3–12. Available online: https://www.redalyc.org/pdf/6729/672971151001.pdf (accessed on 18 February 2023). [CrossRef]
- FAO. El Estado Mundial de la Pesca y la Acuicultura 2020. La Sostenibilidad en Acción; The Food and Agriculture Organization of the United Nations (FAO): Roma, Italy, 2020. [Google Scholar] [CrossRef]
- Gauthier, D.T. Bacterial zoonoses of fishes: A review and appraisal of evidence for linkages between fish and human infections. Vet. J. 2015, 203, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.; Dvorska, L.; Lorencova, A.; Beran, V.; Pavlik, I. Fish: A potential source of bacterial pathogens for human beings. Vet. Med. Czech. 2004, 49, 343. [Google Scholar] [CrossRef]
- Mhango, M.; Mpuchane, S.F.; Mpuchane, B.A. Incidence of indicator organisms, opportunistic and pathogenic bacteria in fish. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 4202–4218. Available online: https://www.ajol.info/index.php/ajfand/article/view/62898/50798 (accessed on 18 February 2023). [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Buras, N.; Duek, L.; Niv, S.; Hepher, B.; Sandbank, E. Microbiological aspects of fish grown in treated wastewater. Water Res. 1987, 21, 1–10. [Google Scholar] [CrossRef]
- Fuentes, M.; Valladares, J.; Grass, G.; Pico, Y. Microbiota de interés para la salud pública de Oreochromis spp. (tilapia roja) cultivada en jaulas flotantes en agua dulce. Rev. Cub. Investig. Pesq. 2011, 28, 74–80. Available online: https://aquadocs.org/bitstream/handle/1834/4662/Mayel%C3%ADn.pdf?sequence=1&isAllowed=y (accessed on 6 January 2023).
- Romero-Jarero, J.M.; Negrete-Redondo, M.D.P. Presencia de bacterias Gram positivas en músculo de pescado con importancia comercial en la zona del Caribe mexicano. Rev. Mex. Biodivers. 2011, 82, 599–606. Available online: https://www.scielo.org.mx/pdf/rmbiodiv/v82n2/v82n2a19.pdf (accessed on 10 January 2023). [CrossRef]
- Guerrero, I.; Rosmini Marcelo, A.; Armenta, R. Tecnología de Productos de Origen Acuático; Editorial Limusa: Mexico City, México, 2009; p. 532. [Google Scholar]
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in fish gastrointestinal microbiota research. Rev Aquac. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Flores, B.; González, N.; Bravo, A.; Mora-Sánchez, B.; Torres, D.; Jirón, W.; Balcázar, J.L. Identificación de bacterias patógenas en peces capturados en el Pacífico frente a Nicaragua. Cienc. Mar. 2021, 47, 175–184. [Google Scholar] [CrossRef]
- Guentzel, M.N. Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 26. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8035/ (accessed on 2 March 2023).
- Romero Cabello, R. Microbiología y Parasitología Humana: Bases Etiológicas de las Enfermedades Infecciosas y Parasitarias, 3rd ed.; Médica Panamericana: Mexico City, Mexico, 2007; p. 999. [Google Scholar]
- Daza-Hernández, A.L.; Arroyo-Escalante, S.; Bravo-Escobar, G.A. Identificación de Citrobacter koseri comonuevo patógeno en pacientes con rinitis crónica. An. Orl. Mex. 2014, 59, 1–10. Available online: https://www.medigraphic.com/pdfs/anaotomex/aom-2014/aom141a.pdf (accessed on 18 January 2023).
- Anderson, P.M.; Del, R.; Calderón, P.V. Microbiología alimentaria. In Metodología Analítica para Alimentos y Bebidas, 2nd ed.; Editorial Diaz de Santos S.A.: Madrid, Spain, 2000. [Google Scholar]
- De Pádua, S.B.; Peixoto, M.D.; Sebastião, F.A.; Pilarski, F.; Martins, M.; Ishikawa, M.M. Isolation, characterization and pathology of Citrobacter freundii infection in native Brazilian catfish Pseudoplatystoma. Braz. J. Vet. Pathol. 2014, 7, 151–157. [Google Scholar]
- Kolínská, R.; Španělová, P.; Dřevínek, M.; Hrabák, J.; Žemličková, H. Species identification of strains belonging to genus Citrobacter using the biochemical method and MALDI-TOF mass spectrometry. Folia Microbiol. 2015, 60, 53–59. [Google Scholar] [CrossRef]
- Caffer, M.I.; Lucero, C.; Pichel, M. Capítulo II.c.1.4. Salmonella, Edwardsiella, Citrobacter. En Manual de Microbiología Clínica de la Asociación Argentina de Microbiología. Volumen I Bacterias de Importancia Clínica. Editores Horacio a. Lopardo. Silvia C. Predari, Carlos Vay. 2016. Available online: https://www.aam.org.ar/descarga-archivos/Parte21Enterobacterias.pdf (accessed on 28 March 2023).
- Duman, M.; Saticioglu, I.B.; Buyukekiz, A.G.; Balta, F.; Altun, S. Molecular characterization and antimicrobial resistance profile of atypical Citrobacter gillenii and Citrobacter sp. isolated from diseased rainbow trout (Oncorhynchus mykiss). J. Glob. Antimicrob. Resist. 2017, 10, 136–142. [Google Scholar] [CrossRef]
- Hidayatullah, A.R.; Effendi, M.H.; Plumeriastuti, H.; Wibisono, F.M.; Hartadi, E.B.; Sofiana, E.D. A Review of the opportunistic pathogen Citrobacter freundii in piglets post weaning: Public Health Importance. Sys. Rev. Pharm. 2020, 11, 767–773. Available online: https://www.sysrevpharm.org/articles/a-review-of-the-opportunistic-pathogen-citrobacter-freundii-in-piglets-post-weaning--public-health-importance.pdf (accessed on 20 January 2023).
- Sánchez Códez, M.I.; Alonso Ojembarrena, A.; Arca Suárez, J. Gramnegativos infrecuentes como agentes etiológicos de infecciones nosocomiales en una Unidad de Cuidados Intensivos Neonatales. Rev. Esp. Quimioter. 2018, 31, 288. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6166260/pdf/revespquimioter-31-288.pdf (accessed on 12 January 2023).
- Collado García, O.; Barreto Rodríguez, H.; Rodríguez Torrens, H.; Barreto Argilagos, G.; Abreu Guirado, O. Especies bacterianas asociadas a infecciones del tracto urinario. Rev. Arch. Médico Camagüey 2017, 21, 479–486. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1025-02552017000400006&lng=es&tlng=es (accessed on 25 January 2023).
- Meseguer Ruiz, V.; Carmona Martín, M.M.; Polo Romero, F.J.; Fernández Rodríguez, A.; Barba Romero, M.A.; Sáez Mendez, L. Bacteriemia por Citrobacter freundii: Presentación de dos casos. An. Med. Interna 2002, 19, 54. Available online: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-71992002000200011&lng=es&tlng=es (accessed on 26 January 2023). [CrossRef]
- Altun, S.; Duman, M.; Buyukekiz, A.G.; Ozyigit, M.O.; Karatas, S.; Turgay, E. Isolation of Citrobacter braakii from rainbow trout (Oncorhynchus mykiss). Isr. J. Aquacult. Bamidgeh 2013, 65, 915–922. [Google Scholar]
- Jeremić, S.; Jakić-Dimić, D.; Veljović, L.J. Citrobacter freundii as a cause of disease in fish. Acta Vet. 2003, 53, 399–410. [Google Scholar] [CrossRef]
- Puello-Caballero, L.P.; Montoya-Campuzano, O.I.; Castañeda-Monsalve, V.A.; Moreno-Murillo, L.M. Caracterización de la microbiota presente en el intestino de Piaractus brachypomus (Cachamablanca). Rev. Salud Anim. 2018, 40, 1–12. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0253-570X2018000200002&lng=es&nrm=iso> (accessed on 25 February 2023).
- Lü, A.; Hu, X.; Zheng, L.; Zhu, A.; Cao, C.; Jiang, J. Isolation and characterization of Citrobacter spp. from the intestine of grass carp Ctenopharyngodon idellus. Aquaculture 2011, 313, 156–160. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A.; Munn, C.B. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish; Springer: Chichester, UK, 2007; Volume 26, p. 552. [Google Scholar] [CrossRef]
- Bandeira, G., Jr.; Dos Santos, A.C.; de Freitas Souza, C.; Baldissera, M.D.; dos Santos Moreira, K.L.; da Veiga, M.L.; Baldisserotto, B. Citrobacter freundii infection in silver catfish (Rhamdia quelen): Hematological and histological alterations. Microb. Pathog. 2018, 125, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Velasco, S.; Ponce-Alquicira, E.; Farrés-González, S.A.; Guerrero-Legarreta, I. Histamine production by two Enterobacteriaceae strains isolated from tuna (Thunnus thynnus) and jack mackerel (Trachurus murphyii). Int J. Food Prop. 2004, 7, 91–103. [Google Scholar] [CrossRef]
- Ruiz-Capillas, P.C.; Jiménez-Colmenero, F. Aminas biógenas: Importancia toxicológica. Electron. J Biomed. 2010, 3, 58–60. Available online: https://www.biomed.uninet.edu/2010/n3/ruiz-capillas.html (accessed on 26 February 2023).
- Galleguillos, A.M. 21. Aminas Biogénicas—Nuevos Indicadores Químicos Utilizados Como Criterios de Calidad en Harina de Pescado. In Control de Calidad de Insumos y Dietas Acuícolas. I Curso Regional de Capacitación (Santiago de Chile, 20/9-8/10/1993) Organizado por el Proyecto AQUILA II y Ejecutado por Fundación Chile; Programa Cooperativo Gubernamental. gcp/rla/102/it. Proyecto Aquila II. Editor Emilio Castro Campos; Documento de Campo No 16; FAO-ITALIA: Rome, Italy, 1994; Available online: https://www.fao.org/3/ab482s/AB482S00.htm#TOC (accessed on 20 February 2023)documento de campo No 16. 1994.
- Izquierdo, P.; Sandrea, L.; Allara, M.; González, P.; García, A.; Valecillos, Y. Evaluación bacteriológica y contenido de histamina en pescado desmenuzado precocido en Venezuela. Rev. Científica 2004, 14, 467–473. Available online: https://www.redalyc.org/pdf/959/95914513.pdf (accessed on 20 February 2023).
- Niven, C.F., Jr.; Jeffrey, M.B.; Corlett, D.A., Jr. Differential plating medium for quantitative detection of histamine-producing bacteria. Appl. Environ. Microbiol. 1981, 41, 321–322. [Google Scholar] [CrossRef]
- Graü, C.; Sánchez, D.; Zerpa, A.; Ballenilla, O.; Berti, O. Estudio de la microflora asociada a la formación de histamina en sardina (Sardinella aurita). Rev. Científica Fac. Cienc. Vet. 2003, 13, 199–205. Available online: https://produccioncientificaluz.org/index.php/cientifica/article/download/14979/14956/#:~:text=El%20objetivo%20de%20este%20trabajo,%2D0%2C5%C2%B0C (accessed on 15 February 2023).
- Torres, G.; Izquierdo, P.; Aliara, M.; García, A. Efecto de la temperatura y tiempo de almacenamiento sobre el crecimiento de bacterias productoras de histamina en dos especies de pescado: Lisa (Mugil curema) y róbalo (Centropomus undecimalis). Rev. Científica Fac. Cienc. Vet. 2003, 13, 263–269. Available online: https://produccioncientificaluz.org/index.php/cientifica/article/view/14986 (accessed on 20 February 2023).
- Restrepo, M.; Torres, G.; Medina, Z.; García, A.; Piñero, M.; Allara, M. Efecto del pH sobre la Producción de Histamina por Enterobacterias Presentes en Músculo de Cachama Negra (Colossoma Macropomum). Rev. Científica 2015, 25, 11–18. Available online: https://www.redalyc.org/pdf/959/95934122002.pdf (accessed on 10 January 2023).
- Moreira, J.D.O.V. Segurança Microbiológica e Bactérias Produtoras de Histamina em Cavala (Scomberomorus Cavalla Cuvier, 1829) e Dourado (Salminus Brasiliensis Cuvier, 1816) Comercializados em MACEIÓ-AL. Master’s Thesis, Universidade Federal de Alagoas-UFAL, Macejo, Brazil, 2018. Available online: https://www.repositorio.ufal.br/jspui/bitstream/riufal/3821/1/Seguran%C3%A7a%20microbiol%C3%B3gica%20e%20bact%C3%A9rias%20produtoras%20de%20histamina%20em%20cavala%20%28Scomberomorus%20cavalla%20Cuvier%2C%201829%29%20e%20dourado%20%28Salminus%20brasiliensis%20Cuvier%2C%201816%29%20comercializados%20em%20MACEI%C3%93-AL.pdf (accessed on 31 March 2023).
- Surya, T.; Sivaraman, B.; Alamelu, V.; Priyatharshini, A.; Arisekar, U.; Sundhar, S. Rapid methods for histamine detection in fishery products. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2035–2046. [Google Scholar] [CrossRef]
- Cordeiro, K.S.; Galeno, L.S.; Mendonça, C.J.S.; Carvalho, I.A.; Costa, F.N. Occurrence of pathogenic and spoilage bacteria in salmon sashimi: Histamine and antimicrobial susceptibility evaluation. Braz. J. Food Technol. 2020, 23, e2019085. [Google Scholar] [CrossRef]
- Gram, L. Microbiological spoilage of fish and seafood products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA; Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK, 2009; pp. 87–119. [Google Scholar] [CrossRef]
- Guergué-Díaz de Cerio, O.; Barrutia-Borque, A.; Gardeazabal-García, J. Escombroidosis: Abordaje práctico. Actas Dermosifiliogr. 2016, 107, 567–571. [Google Scholar] [CrossRef]
- Álvarez, D.; Jiménez-Díaz, M.; Arias-Echandi, L. Estudio de la estabilidad microbiológica de la barracuda a través del tiempo de almacenaje en Costa Rica. Arch. Latinoam. Nutr. 2011, 61, 183–188. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0004-06222011000200010&lng=es&tlng=es (accessed on 22 February 2023).
- Elika. Histamina. Elika Fundación Vasca para la Seguridad Agroalimentaria. 2021. Available online: https://seguridadalimentaria.elika.eus/fichas-de-peligros/histamina/#limites (accessed on 25 February 2023).
- FDA—Food and Drug Administration. Bad Bug Book, Foodborne Pathogenic Microorganisms and Natural Toxins, 2nd ed.; Benner, R.A., Jr., Ed.; Scombrotoxin; 2012; pp. 208–209. Available online: https://www.fda.gov/media/83271/download (accessed on 3 February 2023).
- Bou, G.; Fernández-Olmos, A.; García, C.; Sáez-Nieto, J.A.; Valdezate, S. Métodos de identificación bacteriana en el laboratorio de microbiología. Enferm. Infecc. Microbiol. Clin. 2011, 29, 601–608. [Google Scholar] [CrossRef]
- Díaz, C.A.E.; Silva, G.J.A. Patrón de Bandas Genéticas en Pseudomonas Aeruginosa y Citrobacter Diversus Aislados en Productos Agrícolas y Aguas de Riego. Bachelor’s Thesis, Universidad Nacional de Chimborazo, Riobamba, Chimborazo, 2021. Available online: http://dspace.unach.edu.ec/bitstream/51000/8093/3/6.-TESIS%20Evelyn%20Alexandra%20D%C3%ADaz%20Cadena%20%20Y%20Jhonny%20Alfredo%20Silva.pdf (accessed on 15 February 2023).
- Madigan, M.T.; Martinko, J.M.; Parker, J.B. Biología de los Microorganismos, 10th ed.; Pearson Educación S.A.: Madrid, Spain, 2004; p. 1096. [Google Scholar]
- Gary, W.; Procop, D.L.; Church, G.S.; Hall, W.M.; Janda, E.W.; Koneman, P.C.; Schreckenberger, G.L. Woods. In Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 7th ed.; Editorial Jones & Bartlett Learning: Burlington, MA, USA, 2017. [Google Scholar]
- Pławinska-Czarnak, J.; Wódz, K.; Kizerwetter-Swida, M.; Nowak, T.; Bogdan, J.; Kwiecinski, P.; Kwiecinski, A.; Anusz, K. Citrobacter braakii Yield False-Positive Identification as Salmonella, a Note of Caution. Foods 2021, 10, 2177. [Google Scholar] [CrossRef]
- Jiménez, F.; Garro, L.; Rodríguez, E.; Zeledón, Z. Evaluación de la presencia de bacterias en alimentos y en el ambiente de una sección de oncología de un hospital nacional, San José, Costa Rica. Arch. Latinoam. Nutr. 2004, 54, 303–307. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0004-06222004000300008&lng=es&tlng=es (accessed on 8 February 2023).
- Kwak, H.L.; Han, S.K.; Park, S.; Park, S.H.; Shim, J.Y.; Oh, M.; Kim, H.Y. Development of a Rapid and Accurate Identification Method for Citrobacter Species Isolated from Pork Products Using a Matrix-Assisted Laser-Desorption Ionization Time-of-Flight Mass Spectrometry (MALDITOF MS). J. Microbiol. Biotechnol. 2015, 25, 1537–1541. [Google Scholar] [CrossRef]
- Książczyk, M.; Kuczkowski, M.; Dudek, B.; Korzekwa, K.; Tobiasz, A.; Korzeniowska-Kowal, A.; Bugla-Płoskońska, G. Application of routine diagnostic procedure, VITEK 2 compact, MALDI-TOF MS, and PCR assays in identification procedure of bacterial strain with ambiguous phenotype. Curr. Microbiol. 2016, 72, 570–582. [Google Scholar] [CrossRef]
- Walczak, N.; Puk, K.; Guz, L. Bacterial flora associated with diseased freshwater ornamental fish. J. Vet. Res. 2017, 61, 445. [Google Scholar] [CrossRef]
- Hwang, B.S.; Wang, J.T.; Choong, Y.M. A rapid gas chromatographic method for the determination of histamine in fish and fish products. Food Chem. 2003, 82, 329–334. [Google Scholar] [CrossRef]
- NOM-242-SSA1-2009. Productos y Servicios. Productos de la Pesca Frescos, Refrigerados, Congelados Y Procesados. Especificaciones Sanitarias y Métodos de Prueba. Norma Oficial Mexicana. Gobierno de México. Available online: https://www.dof.gob.mx/normasOficiales/4295/salud2a/salud2a.htm#:~:text=Productos%20y%20servicios-,%20NOR-MA%20Oficial%20Mexicana%20NOM%2D242%2DSSA1%2D2009%2C%20Productos,que%20dice%3A%20Estados%20Unidos%20Mexicanos (accessed on 10 February 2023).
- Altieri, I.; Semeraro, A.; Scalise, F.; Calderari, I.; Stacchini, P. European official control of food: Determination of histamine in fish products by a HPLC–UV-DAD method. Food Chem. 2016, 211, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhuang, D.; Hu, X.; Zhang, S.; He, Z.; Zeng, M.; Chen, X. Rapid determination of histamine in fish by thin-layer chromatography-image analysis method using diazotized visualization reagent prepared with p-nitroaniline. Anal. Methods. 2018, 10, 3386–3392. [Google Scholar] [CrossRef]
- Manterola, J.; Bó, M.; Sanzano, P. Determinación de Histamina en Conservas de Pescado Mediante la Técnica de Cromatografía en Capa Delgada. Bachelor’s Thesis, Facultad de Ciencias Veterinarias-UNCPBA, Tandil, Argentina, 2017. Available online: https://ridaa.unicen.edu.ar:8443/server/api/core/bitstreams/b5d42125-705b-4115-b88e-8653c7219f8b/content (accessed on 20 February 2023).
- Arciniega, G. Determinación de Histamina por el Método de ELISA en Pescado Fresco Comercializado en el Mercado Municipal “El Arenal” de la Ciudad de Cuenca. In Conference Proceedings UTMACH, 2017 . Volume 1, pp. 1160–1170. Available online: https://investigacion.utmachala.edu.ec/proceedings/index.php/utmach/article/view/199 (accessed on 6 February 2023).
- Ma, H.; Shieh, K.J. ELISA technique. Nat Sci. 2006, 4, 36–37. [Google Scholar]
- Munir, M.A.; Mackeen, M.M.M.; Heng, L.Y.; Badri, K.H. Study of histamine detection using liquid chromatography and gas chromatography. ASM Sci. J. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Muscarella, M.; Magro, S.L.; Campaniello, M.; Armentano, A.; Stacchini, P. Survey of histamine levels in fresh fish and fish products collected in Puglia (Italy) by ELISA and HPLC with fluorimetric detection. Food Control. 2013, 31, 211–217. [Google Scholar] [CrossRef]
- Munir, M.A.; Badri, K.H. The importance of derivatizing reagent in chromatography applications for biogenic amine detection in food and beverages. J. Anal. Methods Chem. 2020, 2020, 5814389. [Google Scholar] [CrossRef]
- Balbuena Rivarola, E.D. Manual Básico de Sanidad Piscícola; Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO): Rome, Italy, 2011; Available online: https://www.fao.org/3/as830s/as830s.pdf (accessed on 12 January 2023).
- OMS. Cinco Claves para Una Mayor Inocuidad de los Productos de Acuicultura con Objeto de Proteger la Salud Pública; 2016; Organización Mundial de la Salud (OMS): Geneva, Switzerland; Available online: https://apps.who.int/iris/rest/bitstreams/1067738/retrieve#:~:text=Mantener%20una%20buena%20higiene%20personal%202.,salud%20de%20los%20peces%205 (accessed on 20 February 2023).
- CA. Código Internacional Recomendado de Prácticas—Principios Generales de Higiene de los Alimentos. Codex Alimentarius—Higiene de los Alimentos—Textos Básicos, 2nd ed.; WHO: Geneva, Switzerland; FAO: Rome, Italy, 1997. Available online: https://www.fao.org/3/y1579s/y1579s00.htm#Contents (accessed on 25 February 2023).
- OMS. Manual Sobre las CINCO claves Para la Inocuidad de los Alimentos; Organización Mundial de la Salud (OMS): Geneva, Switzerland, 2006; Available online: https://www.who.int/es/publications/i/item/9789241594639 (accessed on 3 March 2023).
- OMS. Código de Prácticas para el Pescado y los Productos Pesqueros, 2nd ed.; WHO: Geneva, Switzerland; FAO: Rome, Italy, 2012. Available online: https://www.fao.org/3/i2382s/i2382s.pdf (accessed on 13 January 2023).
- OIRSA. Manual de Buenas Prácticas de Manufactura para Productos Acuícolas y Pesqueros; Dirección Regional de Inocuidad de Alimentos, Organismo Internacional Regional de Sanidad Agropecuaria (OIRSA): San Salvador, El Salvador, 2017; Available online: https://www.oirsa.org/contenido/biblioteca/-Manual%20de%20buenas%20pr%C3%A1cticas%20de%20manufactura%20en%20productos%20acu%C3%ADcolas%20y%20pesqueros%20-%20OIRSA.pdf (accessed on 23 February 2023).
- Izquierdo, P.; Aliara, M.; Torres, G.; Fernández, A.; Paulinkevicius, M.; Fuenmayor, J. Bacterias productoras de histamina en tres especies de pescado. Rev. Científica Fac. Cienc. Vet. 2001, 11, 431–436. Available online: https://produccioncientificaluz.org/index.php/cientifica/article/view/14799 (accessed on 16 January 2023).
- Avdalov, N. Manual de Control de Calidad de los Productos de la Acuicultura. Perú; Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO): Rome, Italy, 2012; Available online: https://www.infopesca.org/sites/default/files/complemento/publilibreacceso/320/manual-de-control-de-calidad-de-los-productos-de-la-acuicultura.pdf (accessed on 23 March 2023).
- Lloret, J.; Ferrer, D.; Font, T.; Pedro-Botet, J.M.N.M.; Bartra, J.; Demestre, M. Salud y Pescado Beneficios y Riesgos. Histamina; Universitat de Girona: Girona, Spain; Gobierno de España: Madrid, Spain, 2015. Available online: http://salutipeix.udg.edu/es/histamina-indicador-fiable.html (accessed on 13 February 2023).
- ANVISA. Ministério da Saúde—MS Agência Nacional de Vigilância Sanitária–Resolução de Diretoria Colegiada—RDC Nº 12, de 2 de Janeiro de 2001. Governo Brasileiro: Brasilia, Brazil. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/legislacao-1/biblioteca-de-normas-vinhos-e-bebidas/resolucao-rdc-no-12-de-2-de-janeiro-de-2001.pdf/@@download/file/resolucao-rdc-no-12-de-2-de-janeiro-de-2001.pdf (accessed on 15 February 2023).
- RM. Resolución Ministerial N.° 591-2008-MINSA. “Norma Sanitaria que Establece los Criterios Microbiológicos de Calidad Sanitaria e Inocuidad para los Alimentos y Bebidas de Consumo Humano”; Gobierno de Perú: Lima, Peru, 2008. Available online: https://www.gob.pe/institucion/minsa/normas-legales/247682-591-2008-minsa (accessed on 14 February 2023).
- RTCA. Reglamento Técnico Centroamericano. Alimentos. Criterios Microbiológicos para la Inocuidad de Alimentos. RTCA 67.04.50:08 Anexo de Resolución No. 243-2009. 2009. Available online: https://www.oirsa.org/contenido/2017/El_Salvador_INOCUIDAD/26.%20RTCA%2067%2004%2050%2008%20CRITERIOS%20MICROBIOLOGICOS%20PARA%20LA%20INOCUIDAD%20DE%20ALIMENTOS.pdf (accessed on 15 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).