Abstract
Food science is constantly undergoing innovation, which is why the trend toward developing nanomaterials and their use in food matrices is increasing, mainly due to the physicochemical properties nanomaterials exhibit at the nanometric scale. Therefore, it is convenient to contextualize how these nanomaterials are obtained, classified, and characterized, including interactions that occur at the biomolecule–nanostructure interface, attributed to their physical, chemical, and mechanical properties. This review discusses how nanotechnology is linked naturally to food, including macro-, micro-, and nanostructures, and how the physicochemical properties of nanomaterials influence the food industry by substantially improving the antimicrobial effects, the bioavailability of compounds, and the development of packaging. Finally, the scope of nanotechnology is broad and includes the study of new materials and existing nanostructures in foods, as well as their effects on health. Thus, the physicochemical properties at the micro- and nano-level are essential for the development of and knowledge apportion in scientific nanofood research.
1. Introduction
In recent years, the integration of nanotechnology into food science has leaned toward the use of nanoscale structures to take advantage of these nanostructures’ physical, chemical, optical, and mechanical properties in food systems. The incorporation of nanomaterials is intended to solve technological challenges in the food industry; among them is the extension of the shelf life of food, the use of additives to improve sensory and techno-functional properties, the use of antimicrobial ingredients, and the development of active packaging [1,2]. The combination of these two disciplines has resulted in the implementation of nanostructures such as nanoparticles, nanofibers, nanosheets, and nanotubes, among others, synthesized from organic (proteins, peptides, biopolymers, and lipids) and inorganic (metals, metal oxides, and synthetic polymers) compounds. Their optical, electronic, and surface properties are responsible for the desired effects in various applications. For example, the effect of decreasing antimicrobial activity is given by the chemical reactivity on the surface of the nanomaterial and the adhesion mechanisms of the microorganism [3,4]; as a consequence of this feature, submicroscopic organisms are deactivated, for example, Salmonella enterica, Escherichia coli, and Listeria innocua, due to the potential surface charge of engineered water nanostructures (EWNS) [5]. However, the antimicrobial effect is all-encompassing regarding the physicochemical interactions between bacteria and nanostructures. The surface charge (which generates electrostatic repulsions that confer thermodynamic stability on the system) and the droplet size in a nanoemulsion will influence the bioavailability and release of the compound [6]; furthermore, mechanical and migration properties are being studied as determinants of the use of nanostructures (nanoclays and nanoparticles) in active packaging [1]. Nanomaterial characteristics depend on the material’s application and can have a positive or negative effect; however, two points of control to warrant nanomaterials’ safe use in the food industry are their biological effect and potential toxicity [7]. Thinking on this, the method to obtain a nanomaterial can offer some advantages concerning toxicity. There are chemical, physical, and green synthesis methods. Nowadays, green synthesis is convenient in food and medicinal applications due to its minimal use of harmful and hazardous synthetic chemicals [8]. Despite these advantages, an illustrative comparison of the function of different methods’ components and synthesis conditions, which affect the size, morphology, and crystallinity allows for the understanding of these formation mechanisms. At the same time, characterization techniques provide information that describes the mechanical, optical, electrical, and thermal nanomaterial properties that make classification possible [9,10]. Therefore, understanding in-depth the phenomena that occur at the interface of a food matrix and a nanostructure, as well as knowing some parameters involved in determining the antimicrobial effect, the barrier properties of packaging, and delivery systems to improve the compound’s bioavailability, is of the utmost importance. With this in mind, regarding the potential of nanostructured materials, this review aims to frame the daily interrelationship between nanotechnology and food science by reviewing the classification, synthesis methods, and characterization of nanomaterials; highlighting the physicochemical properties involved in the interactions between a nanomaterial and a biomolecule of interest; and obtaining nanomaterials that, according to their properties, can be used in applications that meet the needs of the food industry (Figure 1).
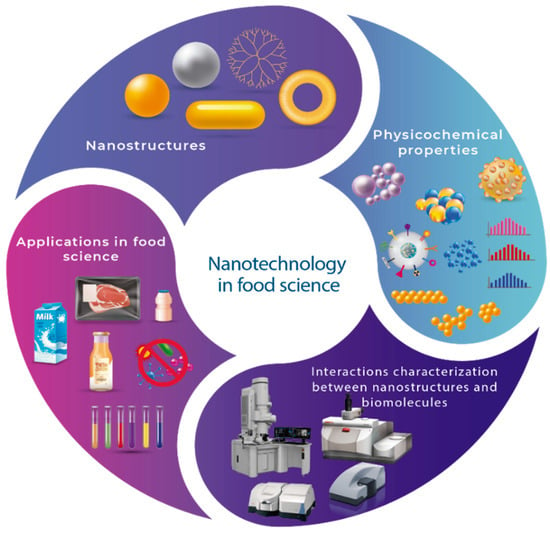
Figure 1.
Summary of the nanostructures, characterization, and nanotechnology application in food science.
2. Classification of Nanostructures
The development and use of nanomaterials is a growing area. One of the nanomaterial classification systems is based on the type of material and is divided into: (1) carbon-based nanomaterials, (2) inorganic-based nanomaterials, (3) organic-based nanomaterials excluding carbon, and (4) nanomaterials based on composites [11,12]. The second classification obeys dimensionality (Figure 2). In this case, the classification includes four groups: (1) Zero-dimensional (0D) nanomaterials have magnitudes less than 100 nm in all directions (nanoparticles, nanopores, quantum dots, proteins, and micelles). (2) First-dimensional (1D) nanomaterials are less than 100 nm only in two directions (nanorods, nanotubes, nanofibers, nanowires, collagen nanofibers, and cellulose nanofibers). (3) Second-dimensional nanomaterials (2D) correspond only to nanomaterials that are smaller than 100 nm in the thickness direction (thin films, nanocoatings, nanoplatelets, and cell membranes). (4) Third dimension nanomaterials (3D) have all dimensions outside the nanometer scale but are composed of individual nanometer-scale blocks smaller than 100 nm known as nanocomposites and nanostructured materials [13,14]. Recently, some works have proposed classifying the nanomaterials according to their toxicity and considering some attributes (material, physicochemical, toxicological, and quantum-mechanical properties) through in silico methods [15,16]. Results based on the Bayesian information criterion (BIC) analysis show that three hazard categories were defined: (1) o to low (NoL), (2) medium (M), and (3) high (H) hazard. This classification proposal resulted from a study that introduced a predictive computational framework based on the molecular and phenotypic effects of a large panel of engineered nanomaterial across multiple in vitro and in vivo models [17]. This novelty classification considers measurable physicochemical characteristics and their correlation through mathematical approximations. Research into this topic is still in progress, so the biological variables should add to have an extensive analysis.
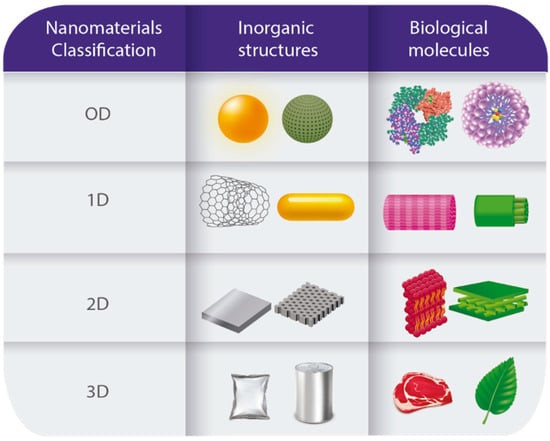
Figure 2.
Nanomaterials classification. Comparison between inorganic structures with biological molecules in a nanoscale.
3. Synthesis of Nanomaterials
The synthetic processes for obtaining nanomaterials are classified into ‘top-down’ and ‘bottom-up’ methods (Figure 3). The former consists of obtaining nanostructures from the subdivision of material by physical methods to nanometer-sized particles, while the latter corresponds to the formation of nanostructures from the aggregation of atoms followed by growth in some dimension to obtain nanostructures. Its advantages include uniform size distribution and homogeneity, in contrast to the drawbacks, which include the high energy and costs and low yields. The advantages of green synthesis over physical and chemical methods remain in a nontoxic process thus it is environmentally friendly, pollution-free, low-cost, and sustainable [18]. The green synthesis classification includes biological synthesis (biosynthesis) and biomimetic synthesis. The former uses organisms (fungi, bacteria, algae, and viruses) or extracts thereof as reducing agents. The latter method refers to the use of biological molecules or principles of bioreduction for the formation of nanostructures [19,20,21,22]. In these processes, with regard to the shape and size of nanostructures, factors such as temperature, pH, extract concentration, metal precursor concentration, and incubation time are important in the reduction process of metal ions [21].
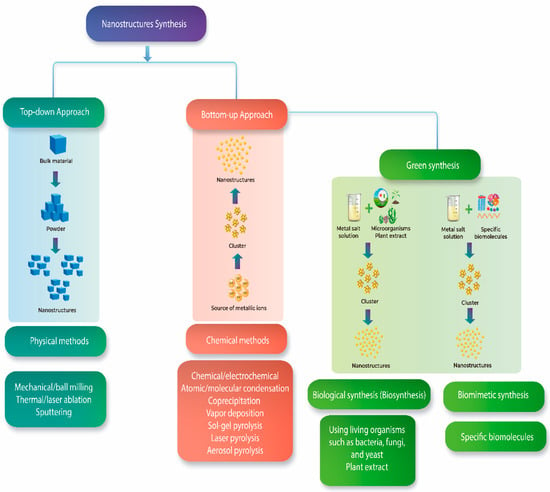
Figure 3.
Differences between nanomaterials synthesis and new eco-friendly methods.
The synthesis of nanomaterials has generally focused on the use of materials such as metals (silver, copper, gold, platinum, aluminum, magnesium, titanium, and mixtures of them); metal oxides (titanium dioxide, zinc oxide, copper oxide, magnesium oxide, calcium oxide, and aluminum oxide); biopolymers and ceramic materials such as alumina; or combinations of all these materials [14,19,20,21,22,23].
On the one hand, nanomaterials synthesized from metals stand out for their optical and electrical properties, which are attributed to their surface charge density; such nanomaterials are of interest in the development of biosensors and antimicrobial agents, among other things.
On the other hand, the synthesis of metal-oxide-based nanomaterials increases their efficiency and reactivity and modifies the properties and characteristics of the metal in the presence of oxygen [24]. The electrical (changes in conductance or electrical resistance) and chemical (adsorption and desorption of gaseous compounds given by the surface of their structure) properties of nanomaterials are of interest in systems for the monitoring and detection of volatile organic compounds (VOCs) in fresh produce, as part of food packaging, and for their antimicrobial effect [25]. The use of biopolymers has generated significant interest, which is why they exist in great variety, among which are natural biopolymers (starch, cellulose, chitosan, alginates, gluten, collagen, etc.), chemically synthesized ones (glycolic acid, poly-Ɛ-caprolactone, polybutylene succinate, polyvinyl alcohol, and polylactic acid), microbial polyesters (polyhydroxyalkanoates, polyhydroxybutyrate, etc.), and non-biodegradable polymers (nylon, polyamide, polyurethane, etc.). Nanocomposites obtained with these polymers in particular have great potential in the development of food packaging due to their thermal, optical, mechanical, barrier, and, in some cases, biodegradation properties [26]. Concerning ceramic materials, it is known that they are practically inert materials; the most used are alumina or calcium phosphate, which serve as scaffolds mainly for their porosity and resistance. For this reason, they have been used together with polymers to generate structures that allow the immobilization of bacteria, for purification processes, and in the formation of ceramic bodies as gelling agents, which are helpful in the food industry in products such as ice cream, jelly, and jam, to mention a few [27,28].
4. Characterization of Nanomaterials
The study of nanomaterials includes the characterization of their structure as well as their physicochemical, mechanical, optical, and electronic properties. One of the most robust techniques for studying nanostructures is X-ray diffraction (XRD), whose objective is to determine the lattice parameters and atomic arrangement, as well as obtain the orientation, size, and crystallinity, of the nanostructures, which in turn determines the structural arrangement. By using techniques such as transmission electron microscopy (TEM), scanning electron microscopy (SEM), and scanning transmission electron microscopy (STEM, which is a merger of TEM and SEM), a sample picture is generated using an electron beam, where the path of the electron is controlled by the use of electrostatic or electromagnetic lenses. When an input electron beam impacts the surfaces of a sample, atoms interact, resulting in the generation of secondary electrons, back-scattered electrons, diffracted electrons, and particular X-rays that vary greatly depending on the surface morphology, topography, and chemical composition. For the elemental surface analysis, energy dispersive X-ray spectroscopy (EDS) is used in conjunction with SEM and is based on the Bragg diffraction principle. This allows us to identify the chemical composition because it can detect almost all elements [29,30].
Atomic force microscopy (AFM) generates a three-dimensional nanomaterial surface image, which allows us to collect quantitative and qualitative data on morphology, size, surface roughness, and texture. To calculate the dimensions of surface features, it measures the attraction and repulsion forces through the van der Waals interactions between a probe and a sample surface. In addition, AFM can be used to estimate mechanical properties, such as stiffness or adhesion strength, and electrical properties, such as conductivity or surface potential. A modified version of AFM is magnetic force microscopy (MFM), which detects the magnetic fields for imaging down to 100 nm in resolution and can be employed to characterize and manipulate 1D and 2D nanomaterials. The operational principle of MFM is based on the quantification of long-range magnetostatic force, which is situated over the sample surface at a constant height between the magnetic sample and magnetically coated probe [31]. Furthermore, nanomaterial three-dimensional visualization is possible with confocal scanning laser microscopy (CSLM), as well as by observation of the interaction or distribution of the wall material by using fluorochromes that bind to specific functional groups of the target molecule [32]. Dynamic mechanical analysis (DMA) allows us to characterize the interfacial interactions between the polymeric matrix and the filler particles. Commonly, it is used in films that add solid particles to enhance their properties. The material is subjected to oscillating force given by temperature and frequency. In terms of the functions of stress (σ) and strain (γ) forces applied, these determine the transition temperature (Tg), storage modulus (E’), loss modulus (E”), and tan value (δ), which then translate into the flexibility film and isotropic properties of the final coating [33]. The change in the properties of the nanomaterials as a function of temperature requires a differential thermal analysis (DTA). Currently, thermal stability is evaluated by thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), thermal analysis (DTA), and dynamic mechanical analysis (DMA). The first one measures the sample weight in the function of the temperature in a specific controlled atmosphere. The second determines the material transitions. It means the melting, crystallization, and glass transition of a nanomaterial. The third method measures the materials’ melting points and decomposition in order to quantitatively assess and determine the chemical composition of nanomaterials by studying a substance’s thermal performance when it is heated. Finally, the last method is more sensitive than DTA and DSC. DMA measures damping coefficients and dynamic modulus qualities, which are significant when crystalline structures have transitioned to the amorphous phase. One of the most indispensable features is the Tg because it describes the temperature range at which the mechanical properties of the nanomaterials change from hard and brittle to more flexible, malleable, or rubbery. While the melting temperature (Tm) and energy fluctuations provide information about the amorphous content of nanomaterials, these values are gathered in a melting endotherm utilized to determine the purity of the materials [34,35,36].
Electron spin resonance (ESR) spectroscopy is also called electron paramagnetic resonance (EPR). It is based on the absorption of microwave radiation by a paramagnetic sample (materials with unpaired electrons) placed in an external magnetic field. ESR spectra can reveal information on the structure and identity of trapped radicals. Consequently, splitting patterns in ESR spectra were initially designed to investigate biological molecules containing extremely reactive and short-lived superoxide (O2) and hydroxyl (HO) radicals, along with the production of proteins, lipids, and polysaccharides. Therefore, ESR measurement can be a helpful approach to comprehending cellular chemical interactions and establishing a link between the generation of free radicals, such as reactive oxygen and nitrogen species (ROS/RNS), and functional products in food systems. For example, this method could be used to estimate free radical scavenging capability and determine Cu2+ chelating capacity and features of irradiated foods [37]. Nuclear magnetic resonance (NMR) and solid-state nuclear magnetic resonance (SSNMR) provide information about the structure of materials, purity, ligand density, and surface chemistry. They have the potential to provide proof of cluster and nanoparticle arrangement by the effect on spin orientation induced by an electromagnetic field [36].
Phenomena such as adsorption, the electric double-layer effect, and resistance to flocculation are a consequence of attractive or repulsive forces between particles (electrostatic, hydrophobic, etc.) in dispersion mechanisms. Dynamic light scattering (DLS) provides the hydrodynamic size, while the zeta potential determines the electrokinetic potential and the surface charge. This is significant in the adsorption of biomolecules through electrostatic interactions, which relates chemically to the functional groups on the surface [29,36,38,39].
Optical absorption spectra provide information on electron density, size, and structure. Thus, UV-Vis spectroscopy is a characterization tool, since their absorption depends on the material and structure. Diffuse reflectance spectroscopy (DRS) is a technique that uses broadband light to produce reflection in the visible and near-infrared wavelength ranges. The ability to absorb the wavelengths of light is directly proportional to the electron excitation. As well, characterization by infrared spectroscopy techniques, such as Fourier-transform infrared spectroscopy (FTIR), allows for the identification of functional groups through vibrational modes. Infrared radiation is transmitted through a sample after absorbing a determined wavelength, causing vibrations and rotations in bound atoms and molecules. The gathered infrared spectrum is a molecular fingerprint of the material [36], whereas Ramman spectroscopy detects the inelastic scattering caused by a high-intensity laser light beam impacting a material with wavelengths in the visible or near-infrared ranges. The Raman spectrum provides a fingerprint of a molecule in the 500–2000 cm−1 range [29].
To characterize magnetic materials, we can use the method of vibrating sample magnetometry (VSM). VSM can measure the magnetic properties of magnetically soft (low coercivity) and hard (high coercivity) materials in many forms: solids, powders, single crystals, thin films, or liquids. In an electromagnet-based VSM, a magnetic material is vibrated within a uniform magnetic field H generated by an electromagnet, inducing an electric current in suitably placed sensing coils. The magnetic behavior is regulated in part by the particle size, which may be superparamagnetic. This state is beneficial because it reduces the magnetic attraction and helps to stabilize colloidal suspensions required for biomedical applications. Concerning in vivo studies, nanomaterial circulation time, targeting, and final biodistribution or elimination depend greatly on the particle’s size, shape, and superficial properties due to the layer spins on the surface influencing the magnetic behavior. For this quality, biosensing platforms adapt magnetic field sensors to excite the nanoparticles and detect their magnetization [40].
It should be noted that the colloidal stability of nanomaterials is linked to their surface charge, which is due to electrostatic interactions. To understand and characterize these interactions, use has been made of techniques such as isothermal titration calorimetry (ITC), which, by means of thermodynamic parameters, allows for monitoring of the formation of bonds due to the difference in the heat increment in real time [39]. This method allows us to obtain the values of thermodynamic variables such as the binding stoichiometry (n); the binding affinity, which is expressed as an association constant (Ka); and the interaction mechanism, which is explained in terms of binding enthalpy (ΔH), binding entropy (ΔS), and Gibbs free energy (ΔG). Finally, this method allows for the identification of the type of interaction from the covalent bond formation to non-covalent interactions such as hydrogen bridges or electrostatic interactions [41]. All characterization techniques provide information regarding the interactions and properties of nanomaterials; they also evaluate the stability and reproducibility of synthesis methods, which allows the processes and formulations of these nanomaterials to be improved.
The electrochemical nature of nanomaterials describes a surface phenomenon given by dynamic surfaces and interfaces. In addition, the relation of the surface–volume ratio is associated with the effect on the reactivity of a small cluster when a few atoms on a cluster size change the magnitude order. This change modifies the reactivity and selectivity of nanomaterials and can be regulated by checking the electrochemical performance. The electrochemical characterization techniques are divided into the static method (potentiometric) and dynamic techniques (voltammetry, electro-gravimetric, and cyclic voltametric). The first technique involves a galvanic cell that incorporates a reference electrode, while the dynamic techniques require a net current and a net cell reaction [42].
From the point of view of biological processes, hydrophobicity is considered a property related to protein adsorption, interaction with biological membranes, and the passage of molecules into the interior of a cell. Performing a characterization is fundamental and reflects stability in systems such as emulsions, affinity with other molecules such as proteins, and toxicity of the nanomaterial. To this end, various techniques, such as biphasic partitioning, contact angle measurement, hydrophobic interaction chromatography, and absorption tests, are used [38,43,44]. Due to the complexity that exists between organic and inorganic materials, nanomaterials require specific conditioning for their characterization. Many of these techniques are complementary, and their correlation allows for the determination of optimal conditions for the standardization of a nanomaterial synthesis method, as well as the evaluation of these in biological systems.
5. Interactions of Macromolecules with Nanomaterials
Understanding the interactions between a nanostructure and a biomolecule (proteins, lipids, and carbohydrates, among others) is relevant to the functionalization of nanomaterials. At this interface, interactions occur that depend mainly on the physicochemical characteristics of the surface of the nanostructure, in which one or several chemically reactive groups can coexist, generating chemical conjugation or adsorption mechanisms, and the latter is generally of a non-covalent type, such as hydrogen bridges, van der Waals forces, and ionic interactions [45]. In the case of milk proteins, they bind to molecules or ions with different affinities or degrees of specificity. For example, caseins αs1, αs2, and β bind calcium through their serine and phosphate residues; this mechanism is common in nature; for example, calcium phosphate nanoparticles are involved in the regulation of lactose synthesis [46]. With respect to these proteins, pH affects the formation of complexes between iron ions and caseins because hydrogen ions compete with metal ions for binding to proteins [47]. At low pH, protonation of the amino group of amino acid ions reduces the ability of proteins to bind iron, whereas at higher pH, these amino acids will be negatively charged and tend to form complexes with cations. The formation of these complexes also depends on the concentration of iron, the number of binding sites, and pH, all of which have an impact on the properties of the final product. In this case, the release of transported substances mainly depends on the pH and swelling behavior of the proteins in nano-vehicles in food technology [48]. One such widely studied protein is bovine serum albumin (BSA), since it naturally transports hydrophobic molecules such as vitamins, hormones, and other plasma constituents and also has three specific domains that favor binding with ions, lipids, and nucleotides [46,47,48,49]. In the case of gold nanoparticles bound to proteins, these interactions are electrostatic, hydrophobic, or via specific binding affinity [50]. Mainly, covalent conjugation strategies have been exploited by employing a thiolate group on the nanoparticle surface or by forming bonds with the amino or carboxyl group of the protein. Nanocomplexes between proteins and polysaccharides have attracted attention because of their electrostatic interactions between two or more polyelectrolytes. This is due to the affinity of proteins to bind to bioactive compounds via hydrogen bonds and hydrophobic interactions, which increases encapsulation efficiency, while polysaccharides are a protective barrier to prevent the degradation of bioactive compounds under gastric conditions. The interactions in these nanostructured systems are determined mainly by the nature of the nanostructure, as well as by biomolecular and system conditions such as pH, among others. The identification of the adsorption or chemical conjugation interactions that occur in each nanostructured system must be considered, since they will influence the internalization pathways into cells and the stability or techno-functional characteristics of a product.
6. Physical, Chemical, and Mechanical Properties of Nanomaterials
The physicochemical properties of a nanostructure are mainly determined by its size and surface area and the type of material it is made of. Among the physical properties considered are morphology, size distribution, aspect ratio, and solubility; these properties are essential to ensure homogeneity and stability in a nanometric system. In contrast, chemical properties refer to the structure, functionality, reactive sites, hydrophobicity, and surface characteristics, which are important because they allow possible interactions to be determined when functionalizing a nanostructure with another molecule of interest. Finally, mechanical properties are related to influence resistance, flexural strength, and fracture properties, which depend on the surface of the structure, porosity, and synthesis methods, among other factors. These mechanical properties vary depending on the material [8] and are essential to maintaining structural integrity.
Among the surface characteristics are hydrophobicity, which indicates the ability of the material to repel water, and hydrophilicity, which shows the affinity for interactions with water; this quality depends on the contact angle and is classified as superhydrophobic (less than 5°), hydrophilic (less than 90°), hydrophobic (90–150°), and superhydrophobic (150–180°). The latter is due to the lotus effect caused by hysteresis of the contact angle. Hydrophilic or hydrophobic surface properties are related to surface energy; therefore, when the nanomaterial has a higher surface energy, the surface is hydrophilic, resulting in a smaller contact angle, and conversely, a hydrophobic material has a lower surface energy and consequently a higher contact angle. These mechanical properties vary depending on the material [51,52]. Regarding optical and electronic properties, these are due to the existence of a more significant number of atoms on the surface of the nanostructure; this is explained by quantum confinement in which the energy levels are discretized, and the waves show their corpuscular nature [9]. For example, the confinement of energy levels can make a semiconductor material such as silicon behave as an insulator [53].
A fundamental part of the stability of nanostructured systems is due to the interactions that exist between a nanomaterial and a food matrix, which is made up of macromolecules such as carbohydrates, proteins, lipids, and phenolic compounds, among others. At the same time, these interactions are associated with the physicochemical properties of nanostructures, such as shape, porosity, surface charge, hydrophobicity, agglomeration, solubility, and optical and electronic properties; which are essential when considering the absorption and delivery systems of these macromolecules with the objective of improving bioavailability in an organism, as well as when understanding the mechanisms by which nanomaterials exhibit antimicrobial attributes or favor the improvement of food products (stability, color, taste, etc.) [54,55]. Parameters such as shape, size, roughness, and porosity are important, as the surface area depends on these; in other words, reducing the particle size of bioactive compounds can improve the properties of availability, solubility, and delivery by crossing biological barriers such as the cell membrane; for example, in the case of polymers, their size influences the degradation process [29,56]. Now, regarding the toxicity of the nanomaterials, depending on the nature of the nanomaterials, they can be toxic or nontoxic; however, this can be regulated if the oxidation state is switched. In addition, the effect on ionic transport and catalytic properties is related to the fact that most nanometric substances exist as aqueous or nonaqueous solutions. To understand the relationship between physicochemical properties, some works have studied the effect of the zeta potential and aspect ratio on cytotoxicity, highlighting the type of material; for example, oxide–silica and metal nanoparticles with high positive zeta potential value resulted in an increased cytotoxicity effect. The literature describes that metal oxide toxicity increased the toxicity levels depending on non-oxidant routes to cellular injury [15,57].
7. Nanotechnology in Food Science
In nature, most foods contain nanostructures with specific functions as part of their structural organization, which includes a hierarchical perspective of macro-, micro-, and nanostructures. One example of this describes the importance of calcium oxalate crystals in cacti at the nanostructural level; they allow calcium to be bioavailable in the youngest cladodes, while the function of cellulose nanofibers is to provide structure to the cell wall in the microstructure of cactus cladodes (Figure 4) [58].
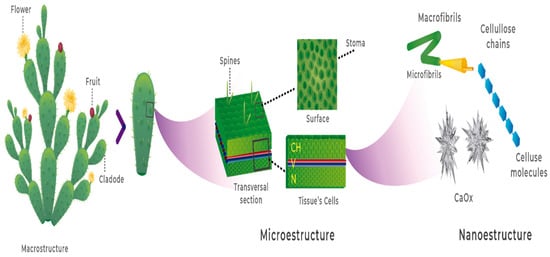
Figure 4.
Hierarchical structural organization of Nopal, showing cellulose nanofiber as a part of the nanostructure (adapted from [58]).
This is due to the ability of cellulose nanofibers to form a network that results in a flexible, semi-crystalline structure with high mechanical strength [59]. Seen in this way, nanotechnology maintains a close link with food science, both in the structural organization of food and in the use of nanostructures (dendrimers, nanoparticles, nanocomposites, nanofibers, and liposomes) obtained from metals, metal oxides, and natural and synthetic biopolymers—all to generate knowledge that benefits the food industry. For example, devices are being developed to detect analytes or microorganisms relevant to bioavailability, i.e., the specific delivery of components such as vitamins, and to participate in food fortification, the improvement of sensory properties, and the extension of shelf life through the development of smart packaging with antimicrobial activity, among other applications [60].
7.1. Antimicrobial Activity
Nanomaterials synthesized from metals and metal oxides, such as silver, zinc oxide, copper, selenium, magnesium, and gold [23], have demonstrated an antimicrobial effect attributed to the cellular internalization of nanoparticles, direct interaction between the microorganism with the surface of the nanostructure, and the effect of metal ions at the nanoscale. Generally, this antimicrobial activity depends on the composition, concentration of the metal oxide, and physicochemical properties of the nanostructure, such as size, shape, surface charge, and ligands that facilitate cell internalization. On the other hand, the effect of metal ions is due to their specific binding affinity for functional groups of biomolecules present in the cell wall structures of microorganisms. In the case of bacteria, amino (–H), carboxyl (–COOH), hydroxyl (–OH), and thiol (–SH) groups in particular participate in these interactions, which lead to changes in membrane permeability and initiate the processes of lipoperoxidation and protein denaturation [61,62]. Among these, silver ions possess the highest toxicity to microorganisms, followed by other metal ions according to the following sequence: Ag > Hg > Cu > Cd > Cr > Pb > Co > Au > Zn > Fe > Mn > Mo > Sn [63]. The toxicity of silver ions is due to their ability to bind to the thiol group of a protein, altering its three-dimensional structure; they can also interfere with the transport and release of potassium (K+) in cells, thus affecting the membrane potential [64]. Similarly, the interaction mechanism between iron nanoparticles and the bacterial cell wall disrupts the permeability of the plasma membrane, involving the release of reactive oxygen species that can damage DNA and proteins, eventually causing cell death [65]. The antimicrobial activity of nanostructures is determined by the effect of coating agents and by the use of antibiotics or extracts with antimicrobial activity, which, in conjunction with the shape, size, and concentration, can increase the desired effect on a microorganism. Concerning polymeric nanocomposites, the preference for the use of biopolymers is increasing due to the availability in nature of these matrices, such as cellulose, chitosan, agarose, alginic acid, pectin, and starch, among others. In addition to their availability, they are also biodegradable and low-cost. Due to their structure, these matrices have oxygen-rich functional groups and act as good reducing and stabilizing agents and coatings. Therefore, biopolymers are considered emerging nanomaterials that can increase antimicrobial activity in combination with inorganic materials. One of the most popular biopolymers is chitosan, which is a heteropolysaccharide noted for its biocompatibility.
7.2. Packaging with Nanostructures
A vital area within the food industry is packaging because packaging is both the container and the barrier that protects the product during storage, handling, distribution, and consumption. Materials used in packaging, such as plastics, metals, glass, paper, polymers, or combinations of them (laminated paper), are chosen according to their physical properties and chemical composition. Nanomaterial scientists have turned their attention to food packaging, intending to improve the mechanical and optical properties as well as the chemical reactivity of packaging material. At the same time, improvements in packaging have positive effects along the product distribution chain [66]; therefore, knowing the characteristics of packaging is fundamental to protecting food against the effects of light, temperature, and humidity, among other factors that can affect product quality. Packaging is exposed to mechanical damage (shock, compression, etc.), hence the need to evaluate its effect on food. Mechanical properties can be identified on the basis of the structural changes in the material when an external force is applied, evaluated by measuring tensile strength or the modulus of elasticity (Young’s modulus). This explains the relationship between stress and deformation due to the chemical structure (atomic arrangement); thus, the higher the value of Young’s modulus, the greater the rigidity and the lower the deformation of the material. Mechanical resistant evaluation includes elongation at break (EAB) and tensile strength (TS) tests. The main function of a gasket is to protect against microbial contamination, permeability, and exchange of gases such as oxygen, which are the criteria for the choice of material. Water vapor permeability provides a measure of the ease with which water vapor can penetrate a material, and it indicates moisture barrier properties [67]. This phenomenon is explained by molecular diffusion or by flow through pores, and its measurement indicates the amount of water vapor transmitted per unit area and unit time; it is usually expressed as the water vapor transmission rate (WVTR) [66]. Another type of protection provided by packaging is against light; therefore, optical properties evaluate the effect of light in either inhibiting or favoring spoilage reactions such as lipid oxidation, generation of off-flavors, and degradation of nutritional compounds. In part, the changes that occur in the product are associated with the transparency of the packaging (opaque, translucent, or transparent); in this sense, the optical property is weighed by transmittance, which is determined using spectrophotometry. This property is explained by the Beer–Lambert law (T = (I⁄I_0) = e(-kz), where T is the transmittance (given in percentage), I0 and I are the intensity of transmitted and incident light, respectively, k is the absorbance, and z is the thickness of the material [66,67,68]. Another characteristic to consider in packaging is chemical reactivity. This characteristic describes the chemical migration of substances from the packaging into the food, which is not necessarily a negative effect, since bioactive packaging tends to release components into the product that extend the shelf life through the use of antimicrobial agents [1,66]. In this sense, nanomaterials favor the barrier properties of packaging (mechanical, optical, and permeability) attributed to the dimensions of these nanostructures. Several works have described the use of nanoparticles, nanotubes, nanoclays, nanoparticles, nanofibers, organic materials such as polysaccharides (cellulose, starch, chitosan, gluten, polylactic acid, and polyvinyl alcohol, among others), and inorganic materials such as metal oxides (zinc oxide, copper oxide, titanium dioxide, and silicon dioxide, among others) in packaging [69]. Innovation in packaging has increased the use of nanomaterials, which can include nanocomposite films, nanoliposomes, and/or nanoparticles in protein-based films, which improve their mechanical, thermal, and barrier properties [70]. Mainly, these properties are given for the active compounds and the physicochemical nanoscale properties, and this allows advancement in the use and design of nanocomposite systems.
7.2.1. Smart Nano-Packaging
Innovative packaging systems go beyond preserving and protecting food from spoilage. They seek to improve the food’s attributes and the packaging functionality, as well as adding new features. In this regard, smart packaging tries to merge all these concepts. These things considered, it is classified into two groups: active packaging and intelligent packaging. This area is in full development and can provide answers regarding the prevention of foodborne infections as well as sustainability concerns. Smart packaging is described as packaging that incorporates both active and intelligent systems that work in tandem. The food validity period can be bound using some factors from food product data, such as saturated and unsaturated fatty acid content, enzyme activity, water activity, pH, or protein content [71].
7.2.2. Active Nano-Packaging
Active food packaging is a cutting-edge packaging technique that contributes significantly to food quality and safety. Active packaging is made by adding active agents into packaging materials, and it operates by absorbing food-derived chemicals or releasing active agents into the environment. The most promising uses of active food packaging technology appear to be antioxidant and antibacterial packaging (Table 1) [72,73].

Table 1.
Properties of active nano-packaging.
7.2.3. Intelligent Nano-Packaging
Intelligent packaging, unlike active packaging solutions, does not directly affect food shelf life. It is defined as “materials and articles that monitor the condition of packaged food or the environment surrounding the food” [77]. Where there is interaction with biomolecules, indicators are regarded as intelligent or interactive. In some cases, the alteration can be visualized by a color change due to pH modification (Table 2). To monitor changes in the gas profile inside the package, some films or labels are used as a gas indicator [73].

Table 2.
Properties of intelligent nano-packaging.
7.3. Bioavailability of Bioactive Compounds
Bioactive compounds have been used more frequently in supplements and food products due to their diverse beneficial effects on health; however, the challenge in the industry lies in the solubility, stability, and susceptibility to degradation of these compounds. In this sense, nanotechnology has found an area of opportunity in the development of methods to solve this problem and improve their bioavailability. Nanoencapsulation is a technology that allows the encapsulation of bioactive compounds on a nanometer scale [32,81] using nanoemulsions, nanosuspensions, nanoparticles, liposomes, and micelles as nanocarriers [82]. The stability of these delivery systems is related to their internal and external morphology, size, and area–volume ratio on the surface of the nanostructure, and the above characteristics are determinants of sedimentation stability, aggregation stability, solubility, and bioaccessibility.
At the same time, bioaccessibility is the first stage of oral bioavailability and considers the release of the bioactive compound (which depends on the texture of the food matrix), physicochemical characteristics of the carrier delivering the bioactive compound, and the presence of enzymes in the digestion process [83]. Therefore, stability is also accompanied by the choice of the matrix material and its physicochemical properties to evaluate the effect on the entrapment efficiency as well as to understand the mechanism of release and diffusion through different kinetic models [32,84]. Therefore, the molecular weight and chemical structure of bioactive compounds are parameters involved mainly in the bioavailability solubility, lipophilicity, and permeability and have an influence on the bioaccessibility and absorption process by cells [6,83,85]. The main interactions responsible for the structural organization of food components are hydrophobic and electrostatic interactions, hydrogen bonding, and entropy effects [86]; these molecular forces are essential in colloidal systems, which is why it the important to characterize these interactions to improve the release processes of additives or molecules of interest.
8. Challenges and Future Perspective
Every day, we interact with the nanometric scale as part of many structures in nature, and as a part of the micro- and macrostructures that are part of our environment. This includes food systems distribution; in this way, nanostructures allow for the possibility to improve the quality of food products and extend their shelf life. At the same time, the fusion of these sciences allows us to contribute in many ways, for example, to take advantage of new synthesis methods such as green synthesis, techniques to characterize nanomaterials, and natural sources to obtain them, as well as to reduce waste in a responsible environment.
The main objective is to generate safe nanomaterials for human food consumption. For this reason, the evaluation of the physicochemical and techno-functional properties gives information that is interpreted in order to discern the stability of nanomaterials in a food matrix; furthermore, knowledge about their toxicity effects represents a challenge. In this matter, it is important to understand the pharmacokinetics of nanomaterials, including the phenomenon of transport that occurs at the interface and regulates the internalization and uptake of cells. The benefits and contributions are more notable every day of products with nanostructures, such as smart packaging, products with bioactive compounds for their distribution, or products with nanomaterials developed to avoid foodborne disease caused by microorganisms; these are all part of a new nanofood system.
9. Conclusions
Nanomaterials are increasingly found in food products or at some stage of the distribution chain; therefore, the concern to determine the effects and risks to consumer health has been considered within the evaluations for these nanomaterials, being the “size effect” a crucial aspect in nanotoxicology. Shape and size characteristics, functional, and physicochemical properties are involved in the toxicity of nanomaterials; therefore identifying this relationship between physicochemical properties and their effect is another way to categorize nanomaterials allowing a preliminary discard in the toxicity of nanomaterials and thus ensuring its use in antimicrobial, packaging and bioavailability applications.
Currently, the relevance of the characterization of the physicochemical properties of nanomaterials in conjunction with bioinformatics tools allows the prediction of the toxicity of nanomaterials. Thus, it not only facilitates clustering and risk assessment of nanomaterials, but at the same time, it can support the safe design of nanomaterials.
Author Contributions
Conceptualization, A.L.G.-G. and A.L.M.-A.; formal analysis, M.d.J.P.-F.; investigation, A.L.G.-G., A.L.M.-A., D.d.R.M.-C., J.E.B.-M. and M.d.J.P.-F.; resources, A.L.M.-A., M.d.J.P.-F. and G.D.-O.; data curation, A.L.M.-A., M.d.J.P.-F. and G.D.-O.; writing—original draft preparation, A.L.G.-G. and A.L.M.-A.; writing—review and editing, A.L.G.-G., A.L.M.-A., D.d.R.M.-C. and J.E.B.-M.; visualization, A.L.G.-G., D.d.R.M.-C. and J.E.B.-M.; supervision, A.L.M.-A., M.d.J.P.-F. and G.D.-O.; project administration, G.D.-O.; funding acquisition, A.L.M.-A., M.d.J.P.-F. and G.D.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Secretaría de Investigación y Posgrado-Instituto Politécnico Nacional [20200291, 20210016, 20220524, 20230083], as well as by Scholarship Consejo Nacional deHumanidades Ciencias y Tecnologías [Scolarship 38993].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All authors give thanks to Consejo Nacional de Humanidades Ciencias y Tecnologías (CONAHCYT) and the Instituto Politécnico Nacional (IPN) for scholarships given to the students Ana Luisa Gómez-Gómez, Deyanira del Rosario Moguel-Concha, and José Eduardo Borges-Martínez.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuorwsel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of Mechanical Properties, Migration, and Potential Applications in Active Food Packaging Systems Containing Nanoclays and Nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Nanostructures: Current Uses and Future Applications in Food Science. J. Food Drug. Anal. 2017, 25, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Parkash, V.; Gaur, A.; Agnihotri, R. Nanoparticles from Fungal Resources: Importance and Applications. In Nanotechnology for Food, Agriculture, and Environment; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–18. ISBN 978-3-030-31938-0. [Google Scholar]
- Jiang, W.; Yang, K.; Vachet, R.W.; Xing, B. Interaction between Oxide Nanoparticles and Biomolecules of the Bacterial Cell Envelope as Examined by Infrared Spectroscopy. Langmuir 2010, 26, 18071–18077. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nanomicro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Emam-Djomeh, Z.; Rezvankhah, A. Chapter Three-Targeted Release of Nanoencapsulated Food Ingredients. In Release and Bioavailability of Nanoencapsulated Food Ingredients; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 5, pp. 79–120. ISBN 978-0-12-815665-0. [Google Scholar]
- Zhou, H.; McClements, D.J. Recent Advances in the Gastrointestinal Fate of Organic and Inorganic Nanoparticles in Foods. Nanomaterials 2022, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Nooshkam, M.; Zargar, M.; Garavand, F.; Ghosh, S.; Hadidi, M.; Forough, M. Green Synthesis of Nanomaterials for Smart Biopolymer Packaging: Challenges and Outlooks. J. Nanostructure Chem. 2023. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Khan, S.T.; Al-Warthan, A.; Siddiqui, M.R.H.; Tahir, M.N.; Tremel, W. Plant Extracts as Green Reductants for the Synthesis of Silver Nanoparticles: Lessons from Chemical Synthesis. Dalton Trans. 2018, 47, 11988–12010. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Green Synthesis of Nanomaterials from Sustainable Materials for Biosensors and Drug Delivery. Sens. Int. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Sannino, D. Types and Classification of Nanomaterials. In Nanotechnology: Trends and Future Applications; Tahir, M.B., Rafique, M., Sagir, M., Eds.; Springer: Singapore, 2021; pp. 15–38. ISBN 978-981-15-9437-3. [Google Scholar]
- Reghunadhan, A.; Kalarikkal, N.; Thomas, S. Mechanical Property Analysis of Nanomaterials. In Characterization of Nanomaterials: Advances and Key Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 191–212. ISBN 9780081019733. [Google Scholar]
- Dolez, P.I. Nanomaterials Definitions, Classifications, and Applications. In Nanoengineering: Global Approaches to Health and Safety Issues; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–40. ISBN 9780444627452. [Google Scholar]
- Cassano, A.; Robinson, R.L.M.; Palczewska, A.; Puzyn, T.; Gajewicz, A.; Tran, L.; Manganelli, S.; Cronin, M.T.D. Comparing the CORAL and Random Forest Approaches for Modelling the In Vitro Cytotoxicity of Silica Nanomaterials. Altern. Lab. Anim. 2016, 44, 533–556. [Google Scholar] [CrossRef]
- Ha, M.K.; Trinh, T.X.; Choi, J.S.; Maulina, D.; Byun, H.G.; Yoon, T.H. Toxicity Classification of Oxide Nanomaterials: Effects of Data Gap Filling and PChem Score-Based Screening Approaches. Sci. Rep. 2018, 8, 3141. [Google Scholar] [CrossRef]
- Fortino, V.; Kinaret, P.A.S.; Fratello, M.; Serra, A.; Saarimäki, L.A.; Gallud, A.; Gupta, G.; Vales, G.; Correia, M.; Rasool, O.; et al. Biomarkers of Nanomaterials Hazard from Multi-Layer Data. Nat. Commun. 2022, 13, 3798. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Chen, T.; Yao, Q.; Zang, S.; Xie, J. Toward Greener Synthesis of Gold Nanomaterials: From Biological to Biomimetic Synthesis. Coord. Chem. Rev. 2021, 426, 213540. [Google Scholar] [CrossRef]
- Noah, N. Green Synthesis: Characterization and Application of Silver and Gold Nanoparticles. In Green. Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–135. ISBN 9780081025796. [Google Scholar]
- Rastogi, A.; Singh, P.; Haraz, F.A.; Barhoum, A. Biological Synthesis of Nanoparticles: An Environmentally Benign Approach. In Fundamentals of Nanoparticles: Classifications, Synthesis Methods, Properties and Characterization; Elsevier: Amsterdam, The Netherlands, 2018; pp. 571–604. ISBN 9780323512558. [Google Scholar]
- Prathna, T.C.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Biomimetic Synthesis of Silver Nanoparticles by Citrus Limon (Lemon) Aqueous Extract and Theoretical Prediction of Particle Size. Colloids Surf. B Biointerfaces 2011, 82, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and Metal Nanoparticles and Their Antimicrobial Activity in Food Packaging Applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajjadi, M.; Sajadi, S.M.; Atarod, M. Types of Nanostructures. In Interface Science and Technology; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 28, pp. 29–80. [Google Scholar]
- Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J. Polymer Nanocomposites for Food Packaging Applications. In Functional and Physical Properties of Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2016; pp. 29–55. [Google Scholar]
- Condi Mainardi, J.; Rezwan, K.; Maas, M. Embedding Live Bacteria in Porous Hydrogel/Ceramic Nanocomposites for Bioprocessing Applications. Bioprocess. Biosyst. Eng. 2019, 42, 1215–1224. [Google Scholar] [CrossRef]
- Chandradass, J.; Kim, K.H.; Bae, D.s.; Prasad, K.; Balachandar, G.; Divya, S.A.; Balasubramanian, M. Starch Consolidation of Alumina: Fabrication and Mechanical Properties. J. Eur. Ceram. Soc. 2009, 29, 2219–2224. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Nanostructured Materials for Food Applications: Spectroscopy, Microscopy and Physical Properties. Bioengineering 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Merugu, R.; Gothalwal, R. Microscopic Techniques for Characterisation of Nanomaterials: A Minireview. Mater. Today Proc. 2021, 47, 4753–4757. [Google Scholar] [CrossRef]
- Impundu, J.; Hussain, S.; Minani, E.; Liu, H.; Li, Y.J.; Sun, L. Local Magnetic Characterization of 1D and 2D Carbon Nanomaterials with Magnetic Force Microscopy Techniques: A Review. Mater. Today Commun. 2023, 35, 106103. [Google Scholar] [CrossRef]
- Perea-Flores, M. de J.; Aguilar-Morán, H.F.; Calderón-Domínguez, G.; García-Hernández, A.B.; Díaz-Ramírez, M.; Romero-Campos, H.E.; Cortés-Sánchez, A.D.J.; Salgado-Cruz, M. Entrapment Efficiency (EE) and Release Mechanism of Rhodamine B Encapsulated in a Mixture of Chia Seed Mucilage and Sodium Alginate. Appl. Sci. 2023, 13, 1213. [Google Scholar] [CrossRef]
- Bashir, M.A. Use of Dynamic Mechanical Analysis (DMA) for Characterizing Interfacial Interactions in Filled Polymers. Solids 2021, 2, 108–120. [Google Scholar] [CrossRef]
- Seifi, H.; Gholami, T.; Seifi, S.; Ghoreishi, S.M.; Salavati-Niasari, M. A Review on Current Trends in Thermal Analysis and Hyphenated Techniques in the Investigation of Physical, Mechanical and Chemical Properties of Nanomaterials. J. Anal. Appl. Pyrolysis 2020, 149, 104840. [Google Scholar] [CrossRef]
- Vijayabhaskar, S.; Rajmohan, T.; Nirmal, U.; Somnath Sarma, V.S. Preparation, Mechanical Properties and Thermal Analysis of Basalt Fiber Reinforced with Polypropylene (BFRPP) Composites. In Bio-Fiber Reinforced Composite Materials: Mechanical, Thermal and Tribological Properties; Palanikumar, K., Thiagarajan, R., Latha, B., Eds.; Springer Nature: Singapore, 2022; pp. 255–279. ISBN 978-981-16-8899-7. [Google Scholar]
- Barhoum, A.; García-Betancourt, M.L.; Rahier, H.; Van Assche, G. Physicochemical Characterization of Nanomaterials: Polymorph, Composition, Wettability, and Thermal Stability. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 255–278. ISBN 9780128135167. [Google Scholar]
- Barba, F.J.; Roohinejad, S.; Ishikawa, K.; Leong, S.Y.; El-Din A Bekhit, A.; Saraiva, J.A.; Lebovka, N. Electron Spin Resonance as a Tool to Monitor the Influence of Novel Processing Technologies on Food Properties. Trends Food Sci. Technol. 2020, 100, 77–87. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of Nanoparticles with Proteins: Relation to Bio-Reactivity of the Nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef]
- Huang, R.; Lau, B.L.T. Biomolecule-Nanoparticle Interactions: Elucidation of the Thermodynamics by Isothermal Titration Calorimetry. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 945–956. [Google Scholar] [CrossRef]
- Dodrill, B.; Lindemuth, J.R. Vibrating Sample Magnetometry. In Magnetic Measurement Techniques for Materials Characterization; Franco, V., Dodrill, B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 15–37. ISBN 978-3-030-70443-8. [Google Scholar]
- Prozeller, D.; Morsbach, S.; Landfester, K. Isothermal Titration Calorimetry as a Complementary Method for Investigating Nanoparticle-Protein Interactions. Nanoscale 2019, 11, 19265–19273. [Google Scholar] [CrossRef]
- Nnamchi, P.S.; Obayi, C.S. Chapter 4-Electrochemical Characterization of Nanomaterials. In Characterization of Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 103–127. ISBN 978-0-08-101973-3. [Google Scholar]
- Valsesia, A.; Desmet, C.; Ojea-Jiménez, I.; Oddo, A.; Capomaccio, R.; Rossi, F.; Colpo, P. Direct Quantification of Nanoparticle Surface Hydrophobicity. Commun. Chem. 2018, 1, 53. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Advanced Analytical Techniques for the Measurement of Nanomaterials in Food and Agricultural Samples: A Review. Environ. Eng. Sci. 2013, 30, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Parra, M.J.A.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D. Milk Proteins as Vehicles for Bioactives. Curr. Opin. Colloid. Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Sugiarto, M.; Ye, A.; Singh, H. Characterisation of Binding of Iron to Sodium Caseinate and Whey Protein Isolate. Food Chem. 2009, 114, 1007–1013. [Google Scholar] [CrossRef]
- Kimpel, F.; Schmitt, J.J. Review: Milk Proteins as Nanocarrier Systems for Hydrophobic Nutraceuticals. J. Food Sci. 2015, 80, R2361–R2366. [Google Scholar] [CrossRef]
- Li, J.; Yao, P. Self-Assembly of Ibuprofen and Bovine Serum Albumin-Dextran Conjugates Leading to Effective Loading of the Drug. Langmuir 2009, 25, 6385–6391. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Ahmad Daud, N.; Talib, Z.A. Chapter 8-Functionalization of Graphene Oxide via Gamma-Ray Irradiation for Hydrophobic Materials. In Synthesis, Technology and Applications of Carbon—Nanomaterials; Rashid, S.A., Raja Othman, R.N.I., Hussein, M.Z., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–203. ISBN 978-0-12-815757-2. [Google Scholar]
- Wang, S.; Jiang, L. Definition of Superhydrophobic States. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Schodek, D.L.; Ferreira, P.; Ashby, M.F. Nanomaterials, Nanotechnologies and Design: An Introduction for Engineers and Architects; Butterworth-Heinemann: Oxford, UK, 2009; ISBN 0080941532. [Google Scholar]
- Cao, S.; Xu, S.; Wang, H.; Ling, Y.; Dong, J.; Xia, R.; Sun, X. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical Properties Determine Nanomaterial Cellular Uptake, Transport, and Fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of Nanomaterials: Exposure, Pathways, Assessment, and Recent Advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef] [PubMed]
- Perucini-Avendaño, M.; Nicolás-García, M.; Jiménez-Martínez, C.; Perea-Flores, M.d.J.; Gómez-Patiño, M.B.; Arrieta-Báez, D.; Dávila-Ortiz, G. Cladodes: Chemical and Structural Properties, Biological Activity, and Polyphenols Profile. Food Sci. Nutr. 2021, 9, 4007–4017. [Google Scholar] [CrossRef]
- Khan, A.; Wen, Y.; Huq, T.; Ni, Y. Cellulosic Nanomaterials in Food and Nutraceutical Applications: A Review. J. Agric. Food Chem. 2018, 66, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patra, F.; Shah, N.; Khedkar, C. Application of Nanotechnology in the Food Industry: Present Status and Future Prospects. In Impact of Nanoscience in the Food Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–27. ISBN 9780128114933. [Google Scholar]
- Stanić, V.; Tanasković, S.B. Antibacterial Activity of Metal Oxide Nanoparticles. In Nanotoxicity: Prevention and Antibacterial Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–274. ISBN 9780128199435. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Stevens, S.E. Multiple Parameters for the Comprehensive Evaluation of the Susceptibility of Escherichia coli to the Silver Ion. Biometals 1998, 11, 27–32. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Saqib, S.; Munis, M.F.H.; Zaman, W.; Ullah, F.; Shah, S.N.; Ayaz, A.; Farooq, M.; Bahadur, S. Synthesis, Characterization and Use of Iron Oxide Nano Particles for Antibacterial Activity. Microsc. Res. Tech. 2019, 82, 415–420. [Google Scholar] [CrossRef]
- Berk, Z. Food Process. Engineering and Technology; Academic Press: Cambridge, MA, USA, 2018; ISBN 0128120541. [Google Scholar]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.d.F.F.; Mattoso, L.H.C. Antimicrobial and Physical-Mechanical Properties of Pectin/Papaya Puree/Cinnamaldehyde Nanoemulsion Edible Composite Films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Patwa, R.; Kumar, A.; Katiyar, V. Plasticizing Effect of Coconut Oil on Morphological, Mechanical, Thermal, Rheological, Barrier, and Optical Properties of Poly(Lactic Acid): A Promising Candidate for Food Packaging. J. Appl. Polym. Sci. 2017, 134, 45390. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Esposto, B.S.; Cristina Da Silva, N.; Tapia-Blácido, D.R.; Jafari, S.M. Reinforced Nanocomposites for Food Packaging. In Handbook of Food Nanotechnology: Applications and Approaches; Elsevier: Amsterdam, The Netherlands, 2020; pp. 533–574. ISBN 9780128158661. [Google Scholar]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Protein-Based Films: Advances in the Development of Biomaterials Applicable to Food Packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Nemes, S.A.; Szabo, K.; Vodnar, D.C. Applicability of Agro-Industrial By-Products in Intelligent Food Packaging. Coatings 2020, 10, 550. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, X.; Jin, T.Z. Active Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-08-100596-5. [Google Scholar]
- Mirza Alizadeh, A.; Masoomian, M.; Shakooie, M.; Zabihzadeh Khajavi, M.; Farhoodi, M. Trends and Applications of Intelligent Packaging in Dairy Products: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, L.; Cen, C.; Liu, Y.; Li, H.; Wang, Y. The Nano Antibacterial Composite Film Carboxymethyl Chitosan/Gelatin/Nano ZnO Improves the Mechanical Strength of Food Packaging. Int. J. Biol. Macromol. 2022, 220, 462–471. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Akhila, K.; Ramakanth, D.; Gaikwad, K.K. Guar Gum/Carboxymethyl Cellulose Based Antioxidant Film Incorporated with Halloysite Nanotubes and Litchi Shell Waste Extract for Active Packaging. Int. J. Biol. Macromol. 2022, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.-C.; Ficai, A.; Ene, V.-L.; Vasile, B.-S.; Andronescu, E.; Holban, A.-M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An Overview of the Intelligent Packaging Technologies in the Food Sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Wei, L. A Biopolymer-Based PH Indicator Film for Visually Monitoring Beef and Fish Spoilage. Food Biosci. 2022, 46, 101523. [Google Scholar] [CrossRef]
- Cheng, M.; Yan, X.; Cui, Y.; Han, M.; Wang, X.; Wang, J.; Zhang, R. An Eco-Friendly Film of PH-Responsive Indicators for Smart Packaging. J. Food Eng. 2022, 321, 110943. [Google Scholar] [CrossRef]
- Karzarjeddi, M.; Ismail, M.Y.; Antti Sirviö, J.; Wang, S.; Mankinen, O.; Telkki, V.-V.; Patanen, M.; Laitinen, O.; Liimatainen, H. Adjustable Hydro-Thermochromic Green Nanofoams and Films Obtained from Shapable Hybrids of Cellulose Nanofibrils and Ionic Liquids for Smart Packaging. Chem. Eng. J. 2022, 443, 136369. [Google Scholar] [CrossRef]
- Quintanilla-Carvajal, M.X.; Camacho-Díaz, B.H.; Meraz-Torres, L.S.; Chanona-Pérez, J.J.; Alamilla-Beltrán, L.; Jimenéz-Aparicio, A.; Gutiérrez-López, G.F. Nanoencapsulation: A New Trend in Food Engineering Processing. Food Eng. Rev. 2010, 2, 39–50. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in Applications of Nanotechnology in Global Food Industry. Food Chem. 2021, 342, 128318. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of Nutraceuticals: Role of the Food Matrix, Processing Conditions, the Gastrointestinal Tract, and Nanodelivery Systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Katouzian, I.; Rajabi, H.; Ganje, M. Nanoencapsulation Technologies for the Food and Nutraceutical Industries Bioavailability and Release of Bioactive Components from Nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017; pp. 494–523. [Google Scholar]
- Davidov-Pardo, G.; Pérez-Ciordia, S.; Marín-Arroyo, M.R.; McClements, D.J. Improving Resveratrol Bioaccessibility Using Biopolymer Nanoparticles and Complexes: Impact of Protein-Carbohydrate Maillard Conjugation. J. Agric. Food Chem. 2015, 63, 3915–3923. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).