Entrapment Efficiency (EE) and Release Mechanism of Rhodamine B Encapsulated in a Mixture of Chia Seed Mucilage and Sodium Alginate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chia Seed Mucilage–Alginate Solutions
2.3. Rheological and Physical Characterization of Solutions
- Surface tension (γ; dyn/cm): The surface tension of solutions was determined by the Du Noüy ring method using a tensiometer (K6, Krüss, Hamburg, Germany) coupled with a platinum–iridium ring (1.9 cm). Before each measurement, the ring and container were cleaned with distilled water and kept directly into the fire to eliminate residues. The analysis was carried out at room temperature (25 °C) and per quintuple [28].
- Density (ρ; g/cm3): The density of the solutions was measured using a 10 mL pycnometer. For this, the pycnometer was weighed empty and with distilled water. Then, each determination was made in triplicate at 25 °C.
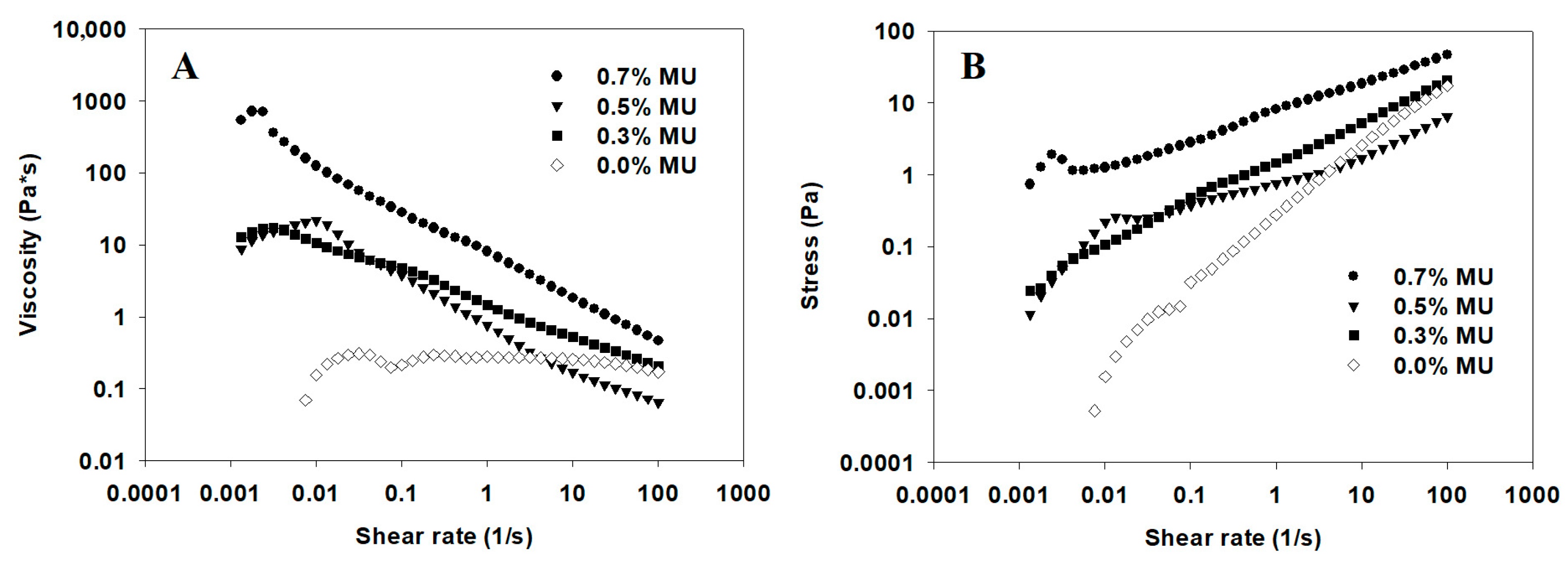
- Rheology: Rheological measurements of the mucilage–alginate solutions were performed using a Compact Modular Rheometer (Hybrid DH3 Discovery, TA Instruments, New Castle, DE, USA) equipped with a parallel plates system (40 mm diameter). Briefly, a continuous shear rate of 1 to 100 s−1 at 25 °C with a plate gap of 5921. A length of 31 µm was applied to determine rheological values. Moreover, the data were fitted to the Newtonian, Bingham, Casson, Power Law, and the Herschel–Bulkley (σ = σ0 + Kγn) models, the latter showing the best fitting to correlate the variations of the rheological behavior under constant shear, where σ is the shear stress (Pa), σ0 is yield stress, γ is the shear rate (s−1), K is the consistency coefficient (Pa·s), and nr is the flow behavior index (nr, dimensionless; shear thinning nr < 1 or shear thickening n > 1) [29]. All the determinations were performed in triplicate to guarantee reproducibility. Finally, the best model was selected based on the maximum determination coefficient (R2) and the minimum root means square error (RMSE).
2.4. Rhodamine B Encapsulation in Chia Mucilage and Alginate
2.5. Capsules Image Analysis
2.6. In Vitro Release Kinetics of Rhodamine B
2.7. Kinetic Models of Rhodamine B Release
2.8. Evaluation of Rhodamine B Entrapment Efficiency into the Capsules Using Confocal Laser Scanning Microscopy (CLSM)
2.9. Capsules Surface Morphology by Scanning Electron Microscopy
2.10. Statistical Analysis
3. Results
3.1. Rheological and Physical Characterization of Solutions
3.2. Capsules Image Analysis
3.3. In Vitro Release Kinetics of Rhodamine B
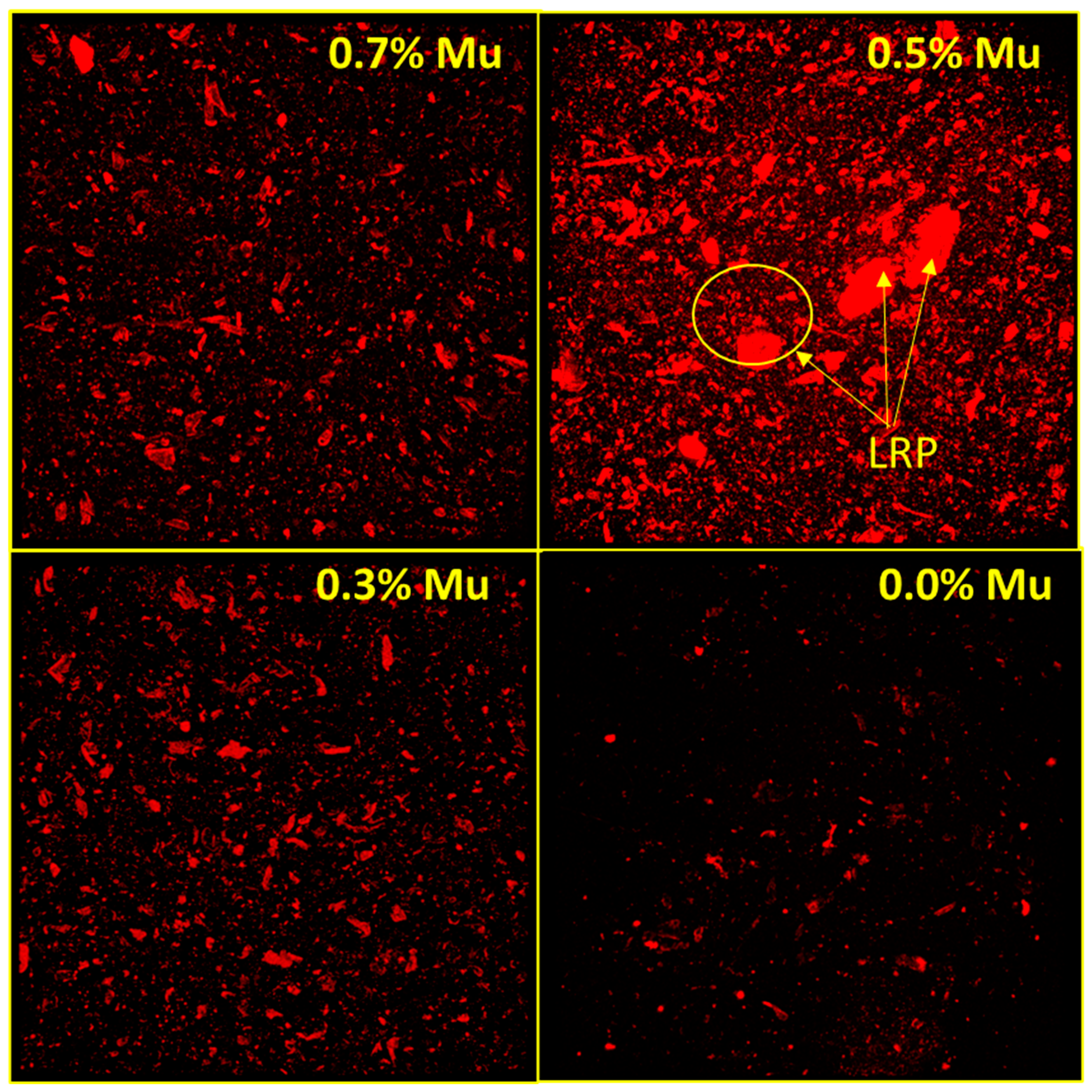
3.4. Evaluation of Rhodamine B Entrapment Efficiency into the Capsules Using Confocal Laser Scanning Microscopy (CLSM)
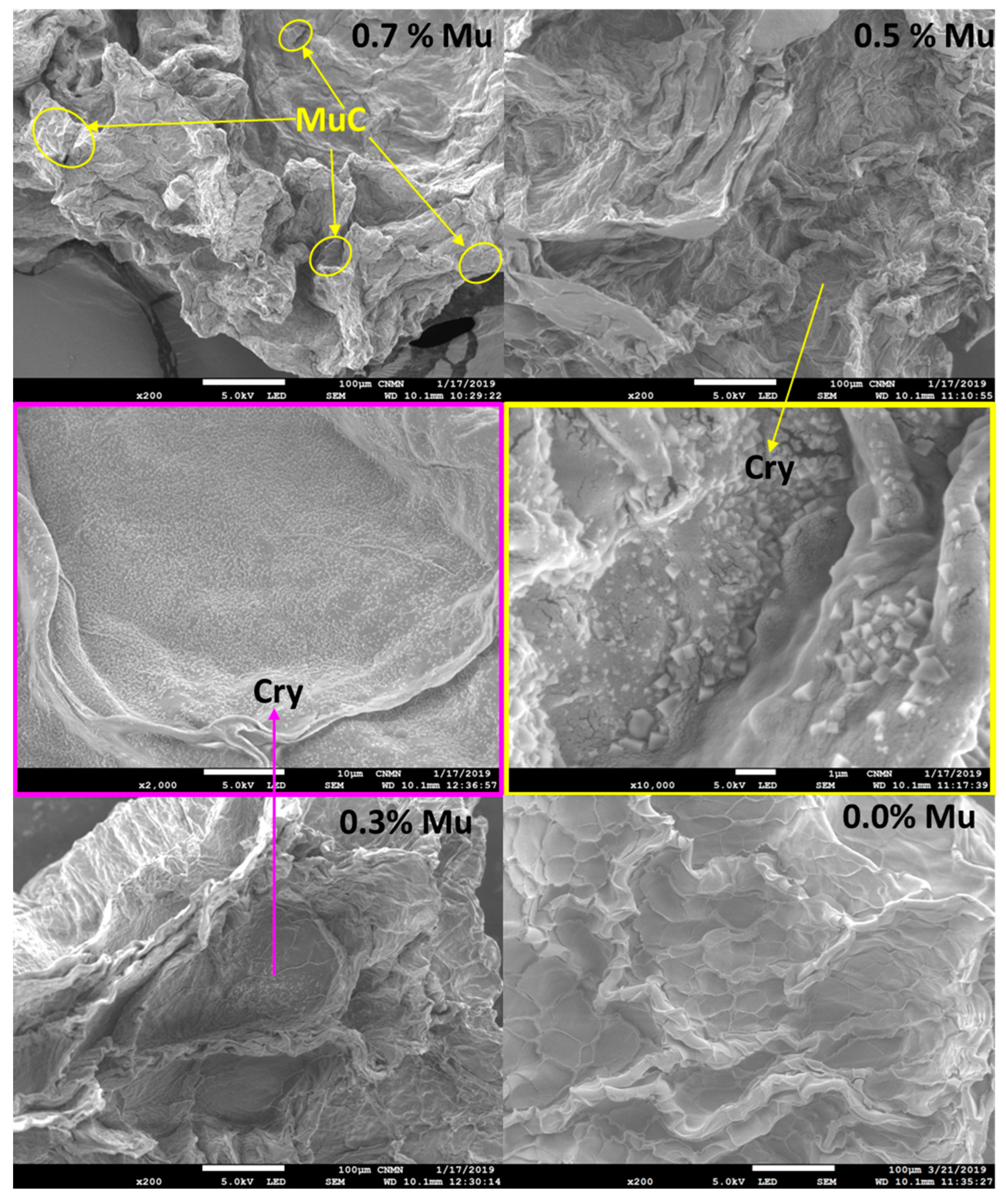
3.5. Surface Morphology of Capsules by Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, S.; Wani, I.A.; Masoodi, F.A. Extraction optimization of mucilage from Basil (Ocimum basilicum L.) seeds using response surface methodology. J. Adv. Res. 2017, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Haughn, G.W.; Western, T.L. Arabidopsis Seed Coat Mucilage is a Specialized Cell Wall that Can be Used as a Model for Genetic Analysis of Plant Cell Wall Structure and Function. Front. Plant Sci. 2012, 3, 22645594. [Google Scholar] [CrossRef] [PubMed]
- Arsovski, A.A.; Haughn, G.W.; Western, T.L. Seed coat mucilage cells of Arabidopsis thaliana as a model for plant cell wall research. Plant Signal. Behav. 2010, 5, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Western, T. The sticky tale of seed coat mucilages: Production, genetics, and role in seed germination and dispersal. Seed Sci. Res. 2012, 22, 1–25. [Google Scholar] [CrossRef]
- Clifford, S.C.; Arndt, S.K.; Popp, M.; Jones, H.G. Mucilages and polysaccharides in Ziziphus species (Rhamnaceae): Localization, composition and physiological roles during drought-stress. J. Exp. Bot. 2002, 53, 131–138. [Google Scholar] [CrossRef]
- Zimmermann, U.; Zhu, J.J.; Meinzer, F.C.; Goldstein, G.; Schneider, H.; Zimmermann, G.; Benkert, R.; Thürmer, F.; Melcher, P.; Webb, D.; et al. High Molecular Weight Organic Compounds in the Xylem Sap of Mangroves: Implications for Long-Distance Water Transport. Bot. Acta 1994, 107, 218–229. [Google Scholar] [CrossRef]
- de la Paz Salgado-Cruz, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.; Farrera-Rebollo, R.R.; Méndez-Méndez, J.V.; Díaz-Ramírez, M. Chia (Salvia hispanica L.) seed mucilage release characterization. A microstructural and image analysis study. Ind. Crops Prod. 2013, 51, 453–462. [Google Scholar] [CrossRef]
- Ferrari-Felisberto, M.H.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Pedrosa Silva Clerici, M.T.; Kil Chang, Y.; Joy Steel, C. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT-Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar] [CrossRef]
- Us-Medina, U.; Ruiz-Ruiz, J.C.; Quintana-Owen, P.; Segura-Campos, M.R. Salvia hispanica mucilage-alginate properties and performance as an encapsulation matrix for chia seed oil. J. Food Process. Preserv. 2017, 41, e13270. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Ahikari, B. Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr. Polym. 2016, 136, 128–136. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Ahmed, M.A.; Gerke, H. Spectroscopic characterization of mucilage (chia seed) and polygalacturonic acid. J. Plant Nutr. Soil Sci. 2019, 182, 888–895. [Google Scholar] [CrossRef]
- Avila-de la Rosa, G.; Alvarez-Ramirez, J.; Vernon-Carter, E.J.; Carrillo-Navas, H.; Pérez-Alonso, C. Viscoelasticity of chia (Salvia hispanica L.) seed mucilage dispersion in the vicinity of an oil-water interface. Food Hydrocoll. 2015, 49, 200–207. [Google Scholar] [CrossRef]
- Brax, M.; Schaumann, G.E.; Diehl, D. Gel formation mechanism and gel properties controlled by Ca2+ in chia seed mucilage and model substances. J. Soil Sci. Plant Nutr. 2019, 182, 92–103. [Google Scholar] [CrossRef]
- de Campo, C.; Pereira-Dos Santos, P.; Hass-Costa, T.M.; Paese, K.; Stanisҫuaski-Guterres, S.; de Oliveira-Rios, A.; Hickmann-Flôres, S. Nanoencapsulation of chia seed oil with chia mucilage (Salvia hispanica L.) as wall material: Characterization and stability evaluation. Food Chem. 2017, 234, 1–9. [Google Scholar] [CrossRef]
- Fernandes-Santos, S.; Salas-Mellado, M.M. Addition of chia seed mucilage for reduction of fat content in bread and cakes. Food Chem. 2017, 227, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.; Hass-Costa, T.M.; Gomaa, A.; Subirade, M.; de Oliveira-Rios, A.; Hickmann-Flôres, S. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, A.; Langyan, N.; Ahuja, M. Evaluation of Mimosa pudica seed mucilage as sustained-release excipient. AAPS PharmSciTech. 2009, 10, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Fahami, A.; Fathi, M. Fabrication and characterization of novel nanofibers from cress seed mucilage for food applications. J. Appl. Polym. 2018, 135, 45811. [Google Scholar] [CrossRef]
- Abd-El Hafeez, S.I.; Eleraky, N.E.; Hafez, E.; Abouelmagd, S.A. Design and optimization of metformin hydrophobic ion pairs for efficient encapsulation in polymeric drug carriers. Sci. Rep. 2022, 12, 5737. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, D.; Liu, X.; Wang, Y.; Tong, J.; Zhang, M.; Ma, X. Amphiphilic sodium alginate-vinyl acetate microparticles for drug delivery. J. Oceanol. Limnol. 2019, 37, 855–862. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Reza-Bagheri, A.; Guo, X.; Li, J.; Ma, J.; Chen, L. Hydrophilic molecularly imprinted nanospheres for the extraction of rhodamine B followed by HPLC analysis: A green approach and hazardous waste elimination. Talanta 2020, 215, 120933. [Google Scholar] [CrossRef] [PubMed]
- Macías-Cortés, E.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Moreno-Jiménezm, M.R.; Medina-Torres, L.; González-Laredo, R.F. Microencapsulation of phenolic compounds: Technologies and novel polymers. Rev. Mex. Ing. Quim. 2019, 19, 491–521. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodíguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Anjani, K.; Kailasapathy, K.; Phillips, M. Microencapsulation of enzymes for potential application in acceleration of cheese ripening. Int. Dairy J. 2007, 17, 79–86. [Google Scholar] [CrossRef]
- Dashevsky, A. Protein loss by the microencapsulation of an enzyme (lactase) in alginate beads. Int. J. Pharm. 1998, 161, 1–5. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.F.; Lehr, C.M. Visualization and quantification of polymer distribution in microcapsules by confocal laser scanning microscopy (CLSM). Int. J. Pharm. 2000, 196, 223–226. [Google Scholar] [CrossRef]
- Erfani, A.; Khosharay, S.; Aichele, C.P. Surface tension and interfacial compositions of binary glycerol/alcohol mixtures. J. Chem. Thermodyn. 2019, 135, 241–251. [Google Scholar] [CrossRef]
- Ocampo-Salinas, I.O.; Jiménez-Aparicio, A.; Perea-Flores, M.J.; Tapia-Ochoategui, A.; Salgado-Cruz, M.P.; Jiménez-Martínez, C.; Téllez-Medina, D.I.; Dávila-Ortiz, G. High-pressure homogenization and maltodextrins mixtures to microencapsulate vanilla (Vanilla planifolia) extract through freeze-drying. Rev. Mex. Ing. Quim. 2017, 16, 131–146. [Google Scholar] [CrossRef]
- Ghumman, S.A.; Mahmood, A.; Noreen, S.; Rana, M.; Hameed, H.; Ijaz, B.; Sara, H.; Aslam, A.; ur Rehman, M.F. Formulation and evaluation of quince seeds mucilage—Sodium alginate microspheres for sustained delivery of cefixime and its toxicological studies. Arab. J. Chem. 2022, 15, 103811. [Google Scholar] [CrossRef]
- Permanadewi, I.; Kumoro, A.C.; Wardhani, D.H.; Aryanti, N. Modelling of controlled drug release in gastrointestinal tract simulation. J. Phys. Conf. Ser. 2019, 1295, 012063. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Brütsch, L.; Stringer, F.J.; Kuster, S.; Windhab, E.J.; Fischer, P. Chia seed mucilage—A vegan thickener: Isolation, tailoring viscoelasticity and rehydration. Food Funct. 2019, 10, 4854–4860. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, A.; Cueva, C.; Laguna, L.; Moreno-Arribas, M.V.; Muñoz, L.A. Understanding the impact of chia seed mucilage on human gut microbiota by using the dynamic gastrointestinal model simgi®. J. Funct. Foods 2018, 50, 104–111. [Google Scholar] [CrossRef]
- Naveed, M.; Brown, L.K.; Raffan, A.C.; George, T.S.; Bengough, A.G.; Roose, T.; Sinclair, I.; Koebernick, N.; Cooper, L.; Hackett, C.A.; et al. Plant exudates may stabilize or weaken soil depending on species, origin and time: Effect of plant exudates on rhizosphere formation. Eur. J. Soil Sci. 2017, 68, 806–816. [Google Scholar] [CrossRef]
- Naveed, M.; Ahmed, M.A.; Benard, P.; Brown, L.K.; Goerge, T.S.; Bengough, A.G.; Roose, T.; Koebernick, N.; Hallett, P.D. Surface tension, rheology and hydrophobicity of rhizodeposits and seed mucilage influence soil water retention and hysteresis. Plant Soil 2019, 437, 65–81. [Google Scholar] [CrossRef]
- Simas-Tosin, F.F.; Barraza, R.R.; Petkowicz, C.L.O.; Silveira, J.L.M.; Sassaki, G.L.; Santos, E.M.R.; Gorin, P.A.J.; Iacomimi, M. Rheological and structural characteristics of peach tree gum exudate. Food Hydrocoll. 2010, 24, 486–493. [Google Scholar] [CrossRef]
- Paolicelli, P.; Varani, G.; Pacelli, S.; Ogliani, E.; Nardoni, M.; Petralito, S.; Adrover, A.; Casadei, M.A. Design and characterization of a biocompatible physical hydrogel based on scleroglucan for topical drug delivery. Carbohydr. Polym. 2017, 174, 960–969. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, R.; Cao, N.; Sun, X.; Sui, Z.; Guo, Q. A systematical rheological study of polysaccharide from Sophora alopecuroides L. seeds. Carbohydr. Polym. 2017, 180, 63–71. [Google Scholar] [CrossRef]
- Soto-Caballero, M.C.; Valdez-Fragoso, A.; Salinas-López, A.N.; Verardo, V.; Mujica-Paz, H. Rheological parameters of xanthan gum/pectin solutions as a function of temperature and composition. Rev. Mex. Ing. Quim. 2016, 15, 859–868. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Zhang, H. Rheological study of gum arabic solutions: Interpretation based on molecular self-association. Food Hydrocoll. 2009, 23, 2394–2402. [Google Scholar] [CrossRef]
- Deore, U.V.; Mahajan, H.S. Isolation and characterization of natural polysaccharide from Cassia Obtustifolia seed mucilage as film forming material for drug delivery. Int. J. Biol. Macromol. 2018, 115, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Camargo, S.; Acuña-Avila, P.E.; Rodríguez-Huezo, M.E.; Román-Guerrero, A.; Varela-Guerrero, V.; Pérez-Alonso, C. Effect of chia mucilage addition on oxidation and release kinetics of lemon essential oil microencapsulated using mesquite gum —Chia mucilage mixtures. Food Res. Int. 2019, 116, 1010–1019. [Google Scholar] [CrossRef]
- Maqsood, H.; Uroos, M.; Muazzam, R.; Naz, S.; Muhammad, N. Extraction of basil seed mucilage using ionic liquid and preparation of AuNps/mucilage nanocomposite for catalytic degradation of dye. Int. J. Biol. Macromol. 2020, 164, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.S. Natural Polysaccharides and their interactions with dye molecules: Applications in effluent treatment. Environ. Sci. Technol. 2004, 38, 4905–4909. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Iacovino, S.; Cinelli, G.; Messia, M.C.; Marconi, E.; Lopez, F. Effect of additives on chia mucilage suspensions: A rheological approach. Food Hydrocoll. 2020, 109, 106118. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Khaksar, A.M. Characterization and likelihood application of extracted mucilage from Hollyhocks plant as a natural polymer in enhanced oil recovery process by alkali-surfactant-polymer (ASP) slug injection into sandstone oil reservoirs. J. Mol. Liq. 2020, 320, 114445. [Google Scholar] [CrossRef]
- Huang, X.; Kakuda, Y.; Cui, W. Hydrocolloids in emulsions: Particle size distribution and interfacial activity. Food Hydrocoll. 2001, 15, 533–542. [Google Scholar] [CrossRef]
- Brummer, Y.; Cui, W.; Wang, Q. Extraction, purification and physicochemical characterization of fenugreek gum. Food Hydrocoll. 2003, 17, 229–236. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A.; Mohebbi, M.; Malaekeh-Nikouei, B. New studies on basil (Ocimum bacilicum L.) seed gum: Part I—Fractionation, physicochemical and surface activity characterization. Food Hydrocoll. 2016, 52, 350–358. [Google Scholar] [CrossRef]
- Lee, B.B.; Chan, E.S.; Ravindra, P.; Khan, T.A. Surface tension of viscous biopolymer solutions measured using the du Nouy ring method and the drop weight methods. Polym. Bull. 2012, 69, 471–489. [Google Scholar] [CrossRef]
- Sitthithaworn, W.; Khongkaw, M.; Wiranidchapong, C.; Koobkokkruad, T. Mucilage powder from Litsea glutinosa leaves stimulates the growth of cultured human hair follicles. Songklanakarin J. Sci. Technol. 2018, 40, 1076–1080. [Google Scholar] [CrossRef]
- Huh, C.; Mason, S.G. A rigorous theory of ring tensiometry. Colloid Polym. Sci. 1975, 253, 566–580. [Google Scholar] [CrossRef]
- Deng, W.; Jeng, D.S.; Toorop, P.E.; Squire, G.R.; Iannetta, P.P.M. A mathematical model of mucilage expansion in myxospermous seeds of Capsella bursa-pastoris (Shepherd’s purse). Ann. Bot. 2012, 109, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Davarcı, F.; Turan, D.; Ozcelik, B.; Poncelet, D. The influence of solution viscosities and surface tension on calcium-alginate microbead formation using dripping technique. Food Hydrocoll. 2017, 62, 119–127. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; Salgado-Cruz, M.D.L.P.; Morales-Sánchez, E.; Alamilla-Beltrán, L.; Farrera-Rebollo, R.R.; Valdespin-León, M.; Calderón-Domínguez, G. Effect of electrohydrodynamic atomization conditions on morphometric characteristics and mechanical resistance of chia mucilage-alginate particles. CYTA J. Food 2020, 18, 461–471. [Google Scholar] [CrossRef]
- Santhanes, D.; Teng, L.Y.; Sheng, F.S.; Coombes, A.G.A. Exploiting the versatility of oral capsule formulations based on high M-alginate for targeted delivery of poorly water soluble drugs to the upper and lower GI tract. J. Drug Deliv. Sci. Technol. 2018, 46, 384–391. [Google Scholar] [CrossRef]
- Estrada-Villegas, G.M.; Martínez-Hernández, R.C.; Morales, J.; Olayo, R. Incorporation of fluoroquinolone/beta cyclodextrin inclusion complex from polylactic acid electrospun fibers and modeling of the release behavior. Rev. Mex. Ing. Quim. 2019, 18, 737–747. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Pradhan, J.; Hasnain, M.S. Fenugreek seed mucilage-alginate mucoadhesive beads of metformin HCl: Design, optimization and evaluation. Int. J. Biol. Macromol. 2013, 54, 144–154. [Google Scholar] [CrossRef]
- Velázquez-Gutiérrez, S.K.; Figueira, A.C.; Rodríguez-Huezo, M.E.; Román-Guerrero, A.; Carrillo-Navas, H.; Pérez-Alonso, C. Sorption isotherms, thermodynamic properties and glass transition temperature of mucilage extracted from chia seeds (Salvia hispanica L.). Carbohydr. Polym. 2015, 121, 411–419. [Google Scholar] [CrossRef]
- Javanbakht, V.; Shafiei, R. Preparation and performance of alginate/basil seed mucilage biocomposite for removal of eriochrome black T dye from aqueous solution. Int. J. Biol. Macromol. 2020, 152, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Xin, F.; Sui, M.; Liu, X.; Zhao, C.; Yu, Y. Biodegradable poly(N-isopropylacrylamide-co-N-maleylgelatin) hydrogels with adjustable swelling behavior. Colloid Polym. Sci. 2019, 297, 763–769. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Bajpai, J.; Shukla, S. Water sorption through a semi-interpenetrating polymer network (IPN) with hydrophilic and hydrophobic chains. React. Funct. Polym. 2002, 50, 9–21. [Google Scholar] [CrossRef]
- Montoya-Álvarez, M.; Quinchía-Figueroa, A.M.; González-Murillo, O.; Araque-Marín, P. An evaluation of the controlled release of ammoniacal nitrogen from residual cellulose/polyvinyl alcohol hydrogels as an alternative to traditional fertilization processes. Dyna 2018, 85, 187–193. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; He, L.; Yang, B.; Zhu, S.; Yao, M. Thermal-responsive poly (N-isopropyl acrylamide)/sodium alginate hydrogels: Preparation, swelling behaviors, and mechanical properties. Colloid Polym. Sci. 2016, 294, 1959–1967. [Google Scholar] [CrossRef]
- Peppas, N.A.; Franson, N.M. The swelling interface number as a criterion for prediction of diffusional solute release mechanisms in swellable polymers. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 983–997. [Google Scholar] [CrossRef]
- Ruiz-Ruiz-Riancho, N.; Garcia, A.; Grossegger, D.; Saadoon, T.; Hudson-Griffiths, R. Properties of ca-alginate capsules to maximise asphalt self-healing properties. Constr. Build. Mater. 2021, 284, 122728. [Google Scholar] [CrossRef]


| Mucilage Concentration (MU% w) | Alginate Concentration (A% w) |
|---|---|
| 0.7 | 0.3 |
| 0.5 | 0.5 |
| 0.3 | 0.7 |
| 0.0 | 1.0 |
| Mucilage Concentration (%) | Herschel–Bulkley Model | |||||
|---|---|---|---|---|---|---|
| R2 | RMSE | nr (Dimensionless) | K(Pa·sn) | Density (g/cm3) | Surface Tension (dyn/cm) | |
| 0.7 | 0.9985 | 0.3997 | 0.386 ± 0.0 a | 7.57 ± 0.29 a | 0.75 ± 0.006 a | 80.33 ± 0.58 a |
| 0.5 | 0.9948 | 0.1081 | 0.599 ± 00 b | 0.39 ± 0.01 b | 0.82 ± 0.0 b | 62.67 ± 0.06 b |
| 0.3 | 0.9998 | 0.0896 | 0.595 ± 0.01 c | 1.328 ± 0.05 c | 0.93 ± 0.0 c | 62.67 ± 0.58 b |
| 0 | 0.9997 | 0.1099 | 0.801 ± 0.00 d | 0.44 ± 0.01 d | 0.95 ± 0.0 d | 58.17 ± 0.58 c |
|
Mucilage Concentration (%) |
Diameter (cm) |
Major Axis (cm) |
Minor Axis (cm) | Circularity |
|---|---|---|---|---|
| 0.7 | 0.24 ± 0.015 a | 0.25 ± 0.010 a | 0.20 ± 0.008 a | 0.84 ± 0.028 a |
| 0.5 | 0.21 ± 0.012 b | 0.20 ± 0.015 b | 0.19 ± 0.010 a | 0.85 ± 0.007 a |
| 0.3 | 0.20 ± 0.006 b | 0.19 ± 0.008 b | 0.17 ± 0.007 b | 0.86 ± 0.020 a |
| 0.0 | 0.15 ± 0.000 c | 0.15 ± 0.001 c | 0.14 ± 0.002 c | 0.89 ± 0.015 b |
| Mucilage Concentration (%) | L | a * |
|---|---|---|
| 0.7 | 4.99 ± 0.12 a | 11.98 ± 0.22 a |
| 0.5 | 12.11 ± 1.51 c | 22.54 ± 3.06 b |
| 0.3 | 3.94 ± 0.38 a,d | 8.49 ± 0.81 a,d |
| 0.0 | 1.23 ± 0.16 e | 3.77 ± 0.52 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perea-Flores, M.d.J.; Aguilar-Morán, H.F.; Calderón-Domínguez, G.; García-Hernández, A.B.; Díaz-Ramírez, M.; Romero-Campos, H.E.; Cortés-Sánchez, A.D.J.; Salgado-Cruz, M.d.l.P. Entrapment Efficiency (EE) and Release Mechanism of Rhodamine B Encapsulated in a Mixture of Chia Seed Mucilage and Sodium Alginate. Appl. Sci. 2023, 13, 1213. https://doi.org/10.3390/app13021213
Perea-Flores MdJ, Aguilar-Morán HF, Calderón-Domínguez G, García-Hernández AB, Díaz-Ramírez M, Romero-Campos HE, Cortés-Sánchez ADJ, Salgado-Cruz MdlP. Entrapment Efficiency (EE) and Release Mechanism of Rhodamine B Encapsulated in a Mixture of Chia Seed Mucilage and Sodium Alginate. Applied Sciences. 2023; 13(2):1213. https://doi.org/10.3390/app13021213
Chicago/Turabian StylePerea-Flores, María de Jesús, Héctor Filiberto Aguilar-Morán, Georgina Calderón-Domínguez, Alitzel Belem García-Hernández, Mayra Díaz-Ramírez, Hugo Enrique Romero-Campos, Alejandro De Jesús Cortés-Sánchez, and Ma. de la Paz Salgado-Cruz. 2023. "Entrapment Efficiency (EE) and Release Mechanism of Rhodamine B Encapsulated in a Mixture of Chia Seed Mucilage and Sodium Alginate" Applied Sciences 13, no. 2: 1213. https://doi.org/10.3390/app13021213
APA StylePerea-Flores, M. d. J., Aguilar-Morán, H. F., Calderón-Domínguez, G., García-Hernández, A. B., Díaz-Ramírez, M., Romero-Campos, H. E., Cortés-Sánchez, A. D. J., & Salgado-Cruz, M. d. l. P. (2023). Entrapment Efficiency (EE) and Release Mechanism of Rhodamine B Encapsulated in a Mixture of Chia Seed Mucilage and Sodium Alginate. Applied Sciences, 13(2), 1213. https://doi.org/10.3390/app13021213










