Label-Free Detection and Classification of Glaucoma Based on Drop-Coating Deposition Raman Spectroscopy
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Tear Samples Collection
2.2. Drop-Coating Deposition Raman SPECTROSCOPIC Measurements
2.3. Data Processing and Analysis
3. Results
3.1. The Acquisition and Analysis of Raman Spectra
3.2. PCA-LDA-Based SVM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Jiang, N.; Bikkannavar, P.; Cordeiro, M.F.; Yetisen, A.K. Ophthalmic sensing technologies for ocular disease diagnostics. Analyst 2021, 146, 6416–6444. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.M.; Ng, E.; Jahmunah, V.; Koh, J.E.W.; Oh, S.L.; Han, W.S.; Yip, L.W.L.; Acharya, U.R. Automated detection of glaucoma using elongated quinary patterns technique with optical coherence tomography angiogram images. Biomed. Signal Process. Control 2021, 69, 102895. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.T.; Sakata, L.M.; Medeiros, F.A. Managing glaucoma in developing countries. Arq. Bras. Oftalmol. 2011, 74, 83–84. [Google Scholar] [CrossRef]
- Sathyamangalam, R.V.; Paul, P.G.; George, R.; Baskaran, M.; Hemamalini, A.; Madan, R.V.; Augustian, J.; Prema, R.; Lingam, V. Determinants of glaucoma awareness and knowledge in urban Chennai. Indian J. Ophthalmol. 2009, 57, 355. [Google Scholar]
- Coleman, A.L. Glaucoma. Lancet 1999, 354, 1803–1810. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Quigley, H.A.; Nickells, R.W.; Kerrigan, L.A.; Pease, M.E.; Thibault, D.J.; Zack, D.J. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Investig. Ophthalmol. Vis. Sci. 1995, 36, 774–786. [Google Scholar]
- Morgan, J.E.; Uchida, H.; Caprioli, J. Retinal ganglion cell death in experimental glaucoma. Br. J. Ophthalmol. 2000, 84, 303–310. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Vargas, J.L.C.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Doucette, L.P.; Rasnitsyn, A.; Seifi, M.; Walter, M.A. The interactions of genes, age, and environment in glaucoma pathogenesis. Surv. Ophthalmol. 2015, 60, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Grotegut, P.; Reinehr, S.; Joachim, S.C. Role of heat shock proteins in glaucoma. Int. J. Mol. Sci. 2019, 20, 5160. [Google Scholar] [CrossRef] [PubMed]
- Zukerman, R.; Harris, A.; Oddone, F.; Siesky, B.; Verticchio Vercellin, A.; Ciulla, T.A. Glaucoma Heritability: Molecular Mechanisms of Disease. Genes 2021, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhou, X.Y.; Jylha, A.; Aapola, U.; Liu, D.N.; Koh, S.K.; Tian, D.; Quah, J.; Uusitalo, H.; Beuerman, R.W. Quantitation of 47 human tear proteins using high resolution multiple reaction monitoring (HR-MRM) based-mass spectrometry. J. Proteom. 2015, 115, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Posa, A.; Bräuer, L.; Schicht, M.; Garreis, F.; Beileke, S.; Paulsen, F. Schirmer strip vs. capillary tube method: Non-invasive methods of obtaining proteins from tear fluid. Ann. Anat. Anat. Anz. 2013, 195, 137–142. [Google Scholar] [CrossRef]
- Lin, C.-C.; Kuo, M.-T.; Chang, H.-C. Raman spectroscopy–a novel tool for noninvasive analysis of ocular surface fluid. J. Med. Biol. Eng. 2010, 30, 343–354. [Google Scholar] [CrossRef]
- Leal, L.; Nogueira, M.; Canevari, R.; Carvalho, L. Vibration spectroscopy and body biofluids: Literature review for clinical applications. Photodiagnosis Photodyn. Ther. 2018, 24, 237–244. [Google Scholar] [CrossRef]
- Ballow, M.; Donshik, P.C.; Rapacz, P.; Samartino, L. Tear lactoferrin levels in patients with external inflammatory ocular disease. Investig. Ophthalmol. Vis. Sci. 1987, 28, 543–545. [Google Scholar]
- Willcox, M.D.; Lan, J. Secretory immunoglobulin A in tears: Functions and changes during contact lens wear. Clin. Exp. Optom. 1999, 82, 1–3. [Google Scholar] [CrossRef]
- Grus, F.H.; Podust, V.N.; Bruns, K.; Lackner, K.; Fu, S.; Dalmasso, E.A.; Wirthlin, A.; Pfeiffer, N. SELDI-TOF-MS ProteinChip array profiling of tears from patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 863–876. [Google Scholar] [CrossRef]
- Huang, M.-Z.; Hsu, H.-J.; Lee, J.-Y.; Jeng, J.; Shiea, J. Direct protein detection from biological media through electrospray-assisted laser desorption ionization/mass spectrometry. J. Proteome Res. 2006, 5, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Ghaffariyeh, A.; Honarpisheh, N.; Heidari, M.H.; Puyan, S.; Abasov, F. Brain-derived neurotrophic factor as a biomarker in primary open-angle glaucoma. Optom. Vis. Sci. 2011, 88, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Bucci, S.; Agnifili, L.; Fasanella, V.; D’Aguanno, S.; Mastropasqua, A.; Ciancaglini, M.; Mastropasqua, L.; Di Ilio, C.; Sacchetta, P. Differential protein expression in tears of patients with primary open angle and pseudoexfoliative glaucoma. Mol. Biosyst. 2012, 8, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, O.W.; Bell, K.; Hohenstein-Blaul, N.v.T.U.; Wilding, C.; Beck, S.; Pfeiffer, N.; Grus, F.H. Autoimmune biomarkers in glaucoma patients. Curr. Opin. Pharmacol. 2013, 13, 90–97. [Google Scholar] [CrossRef]
- Erckens, R.; Jongsma, F.; Wicksted, J.; Hendrikse, F.; March, W.; Motamedi, M. Raman spectroscopy in ophthalmology: From experimental tool to applications in vivo. Lasers Med. Sci. 2001, 16, 236–252. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chen, W. Raman spectroscopic analysis of cataract lens: A compendious review. Appl. Spectrosc. Rev. 2018, 53, 689–702. [Google Scholar] [CrossRef]
- Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, C.; Grozdanic, S.D.; Harper, M.M.; Kecova, H.; Lazic, T.; Hamouche, N. Exploring Raman spectroscopy for the evaluation of glaucomatous retinal changes. J. Biomed. Opt. 2011, 16, 107006. [Google Scholar] [CrossRef]
- Wang, Q.; Grozdanic, S.D.; Harper, M.M.; Hamouche, N.; Kecova, H.; Lazic, T.; Hernandez-Merino, E.; Yu, C. Detection and characterization of glaucoma-like canine retinal tissues using Raman spectroscopy. J. Biomed. Opt. 2013, 18, 067008. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Sun, B.; Zhang, W.; Liu, S.; Liu, J.; Lv, H.; Zhang, G.; Kang, X. Label-free serum detection based on Raman spectroscopy for the diagnosis and classification of glioma. J. Raman Spectrosc. 2020, 51, 1977–1985. [Google Scholar] [CrossRef]
- Fălămaș, A.; Rotaru, H.; Hedeșiu, M. Surface-enhanced Raman spectroscopy (SERS) investigations of saliva for oral cancer diagnosis. Lasers Med. Sci. 2020, 35, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Del Mistro, G.; Cervo, S.; Mansutti, E.; Spizzo, R.; Colombatti, A.; Belmonte, P.; Zucconelli, R.; Steffan, A.; Sergo, V.; Bonifacio, A. Surface-enhanced Raman spectroscopy of urine for prostate cancer detection: A preliminary study. Anal. Bioanal. Chem. 2015, 407, 3271–3275. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.; Cho, P. The relationship between total tear protein concentrations determined by different methods and standards. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, Y.; Mrozek, M.F.; Ortiz, C.; Davisson, V.J.; Ben-Amotz, D. Raman detection of proteomic analytes. Anal. Chem. 2003, 75, 5703–5709. [Google Scholar] [CrossRef]
- Ortiz, C.; Zhang, D.; Xie, Y.; Ribbe, A.E.; Ben-Amotz, D. Validation of the drop coating deposition Raman method for protein analysis. Anal. Biochem. 2006, 353, 157–166. [Google Scholar] [CrossRef]
- Choi, S.; Moon, S.W.; Shin, J.-H.; Park, H.-K.; Jin, K.-H. Label-free biochemical analytic method for the early detection of adenoviral conjunctivitis using human tear biofluids. Anal. Chem. 2014, 86, 11093–11099. [Google Scholar] [CrossRef]
- Filik, J.; Stone, N. Analysis of human tear fluid by Raman spectroscopy. Anal. Chim. Acta 2008, 616, 177–184. [Google Scholar] [CrossRef]
- Filik, J.; Stone, N. Investigation into the protein composition of human tear fluid using centrifugal filters and drop coating deposition Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 218–224. [Google Scholar] [CrossRef]
- Pichardo-Molina, J.; Frausto-Reyes, C.; Barbosa-García, O.; Huerta-Franco, R.; González-Trujillo, J.; Ramírez-Alvarado, C.; Gutiérrez-Juárez, G.; Medina-Gutiérrez, C. Raman spectroscopy and multivariate analysis of serum samples from breast cancer patients. Lasers Med. Sci. 2007, 22, 229–236. [Google Scholar] [CrossRef]
- Oshima, Y.; Shinzawa, H.; Takenaka, T.; Furihata, C.; Sato, H. Discrimination analysis of human lung cancer cells associated with histological type and malignancy using Raman spectroscopy. J. Biomed. Opt. 2010, 15, 017009. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xie, X.; Li, H.; Lv, G.; Lv, X.; Tang, J.; Yue, X.; Mo, J. Rapid, noninvasive screening of ocular diseases using tear raman spectroscopy and different classification algorithms. Laser Phys. 2019, 30, 015701. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2015, 50, 46–111. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, X.-W.; Liang, G.; Pan, C.-W. Metabolomics in glaucoma: A systematic review. Investig. Ophthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef] [PubMed]
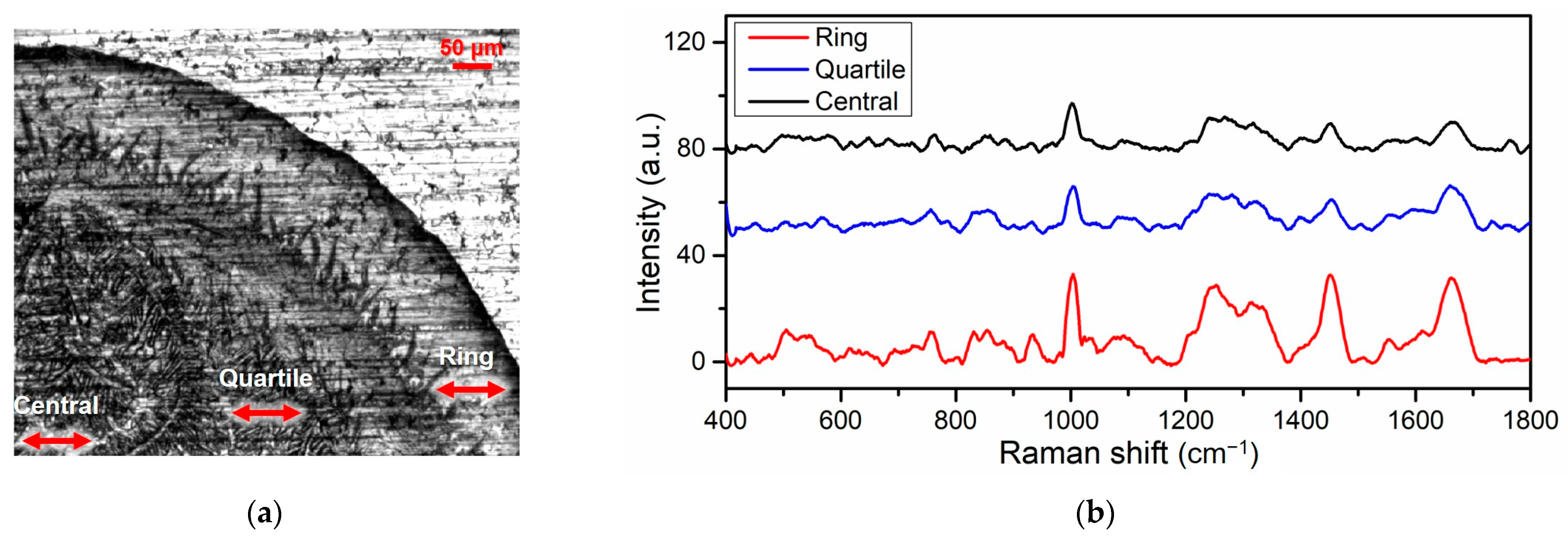


| Groups | Eye (OD/OS) | IOP (mmHg ± SD) |
|---|---|---|
| POAG | 13/14 | 20.14 ± 7.88 |
| PACG | 9/10 | 23.41 ± 15.78 |
| Normal | 14/13 | 15.03 ± 2.08 |
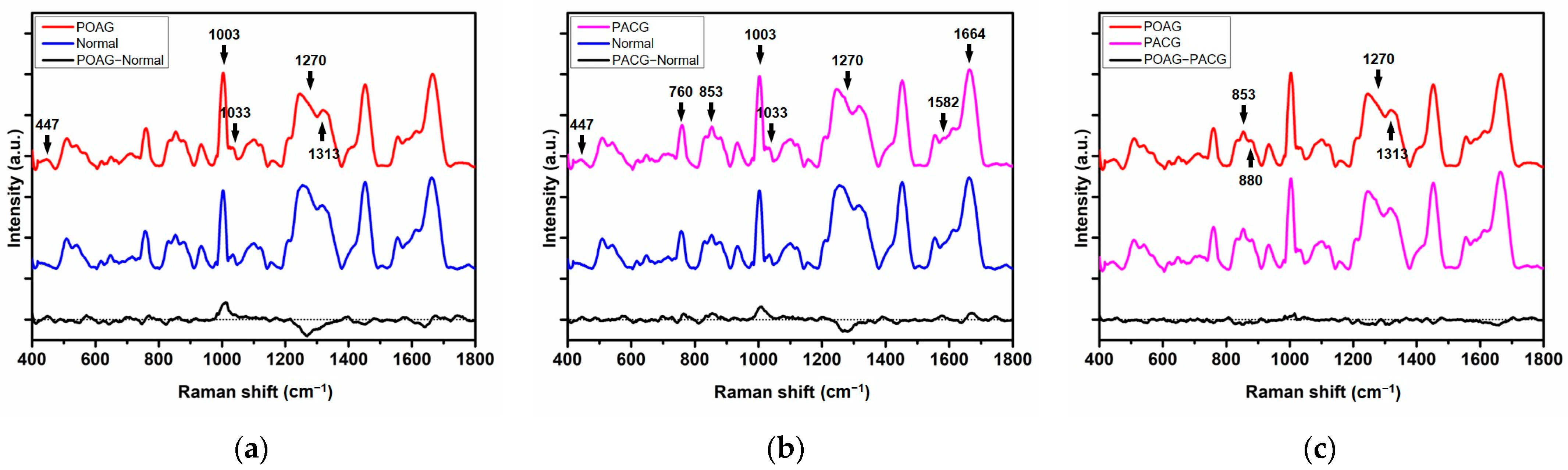
| Raman Shift (cm−1) | Tentative Assignment | Molecular Origin |
|---|---|---|
| 447 | Ring torsion | Phenylalanine |
| 760 | Ring breathing mode | Tryptophan |
| 853 | Ring breathing mode | Tyrosine |
| 879 | Hydroxyproline, tryptophan | |
| 1003 | Phenylalanine | |
| 1033 | Phenylalanine | |
| 1270 | Amide III | |
| 1313 | CH3CH2 twisting mode | Collagens and lipids |
| 1582 | Phenylalanine | |
| 1664 | Amide I |
| Actual Group | Predicted Group | Evaluation Parameters | |||||
|---|---|---|---|---|---|---|---|
| POAG | PACG | Normal | Sensitivity | Specificity | Accuracy | ||
| Experiment A | POAG | 24 | 3 | 0 | 88.9% | 97.8% | 93.2% |
| PACG | 1 | 17 | 1 | 89.5% | 94.4% | ||
| Normal | 0 | 0 | 27 | 100% | 97.8% | ||
| Experiment B | POAG | 4 | 1 | 0 | 80.0% | 100% | 90.9% |
| PACG | 0 | 7 | 1 | 87.5% | 92.9% | ||
| Normal | 0 | 0 | 9 | 100.00% | 92.3% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lin, H.; He, Q.; Zuo, C.; Lin, M.; Xu, T. Label-Free Detection and Classification of Glaucoma Based on Drop-Coating Deposition Raman Spectroscopy. Appl. Sci. 2023, 13, 6476. https://doi.org/10.3390/app13116476
Li Y, Lin H, He Q, Zuo C, Lin M, Xu T. Label-Free Detection and Classification of Glaucoma Based on Drop-Coating Deposition Raman Spectroscopy. Applied Sciences. 2023; 13(11):6476. https://doi.org/10.3390/app13116476
Chicago/Turabian StyleLi, Yao, Huishan Lin, Qiming He, Chengguo Zuo, Mingkai Lin, and Tao Xu. 2023. "Label-Free Detection and Classification of Glaucoma Based on Drop-Coating Deposition Raman Spectroscopy" Applied Sciences 13, no. 11: 6476. https://doi.org/10.3390/app13116476
APA StyleLi, Y., Lin, H., He, Q., Zuo, C., Lin, M., & Xu, T. (2023). Label-Free Detection and Classification of Glaucoma Based on Drop-Coating Deposition Raman Spectroscopy. Applied Sciences, 13(11), 6476. https://doi.org/10.3390/app13116476





