Mathematical Modeling and Digital Simulation of Teeth Dynamics for the Approximation of Orthodontic Treatment Duration
Abstract
1. Introduction
2. Materials and Methods
- A.
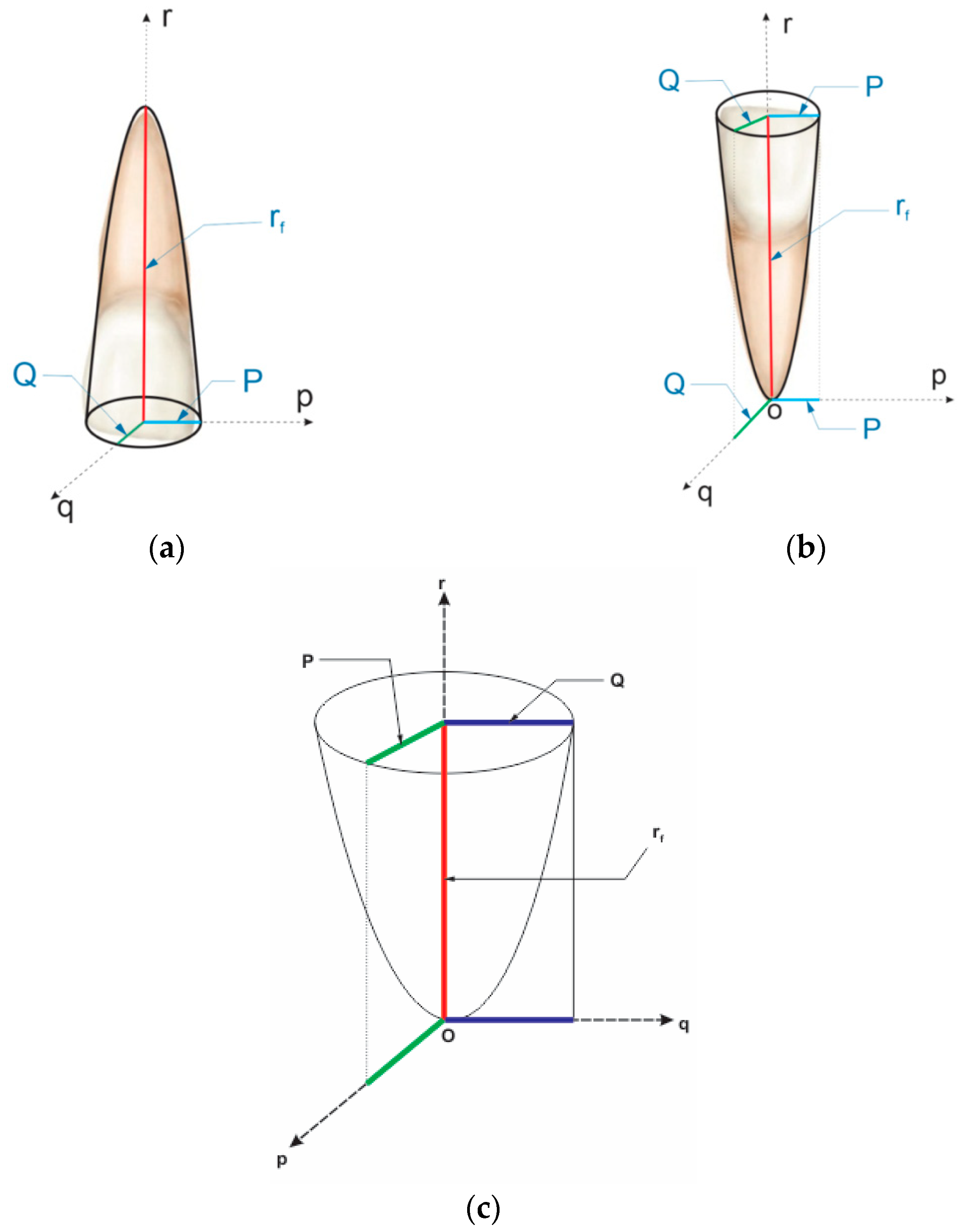
- Geometrical Core Concepts
- B.
- Analogical Model of Visible Deformation () in Elastic-Plastic Regime. Scaling the Ky Coefficient
- C.
- General Example for Scaling Ky Coefficient Based on Main Initial Data
- (1)
- Tooth’s parameters:
- (2)
- Duration of active force:
- (3)
- Orthodontic activation cycle period (as effect of one activation):
- (4)
- The elastomeric chain:
- (5)
- Time constants:
- (6)
- (7)
- The fictional axis of the tooth:
- (8)
- (9)
- (10)
- (11)
- (12)
- Scaled value for
- (1)
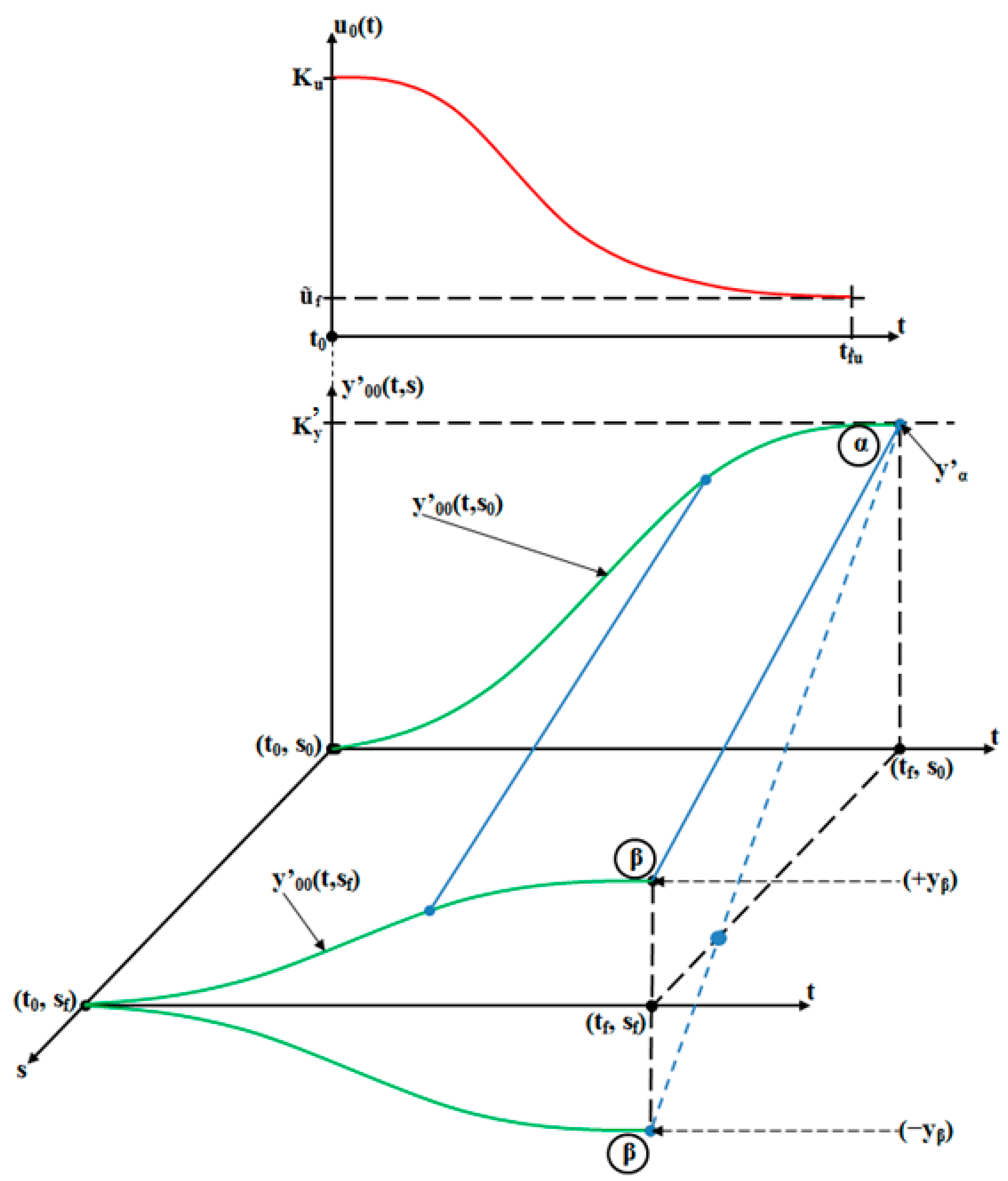
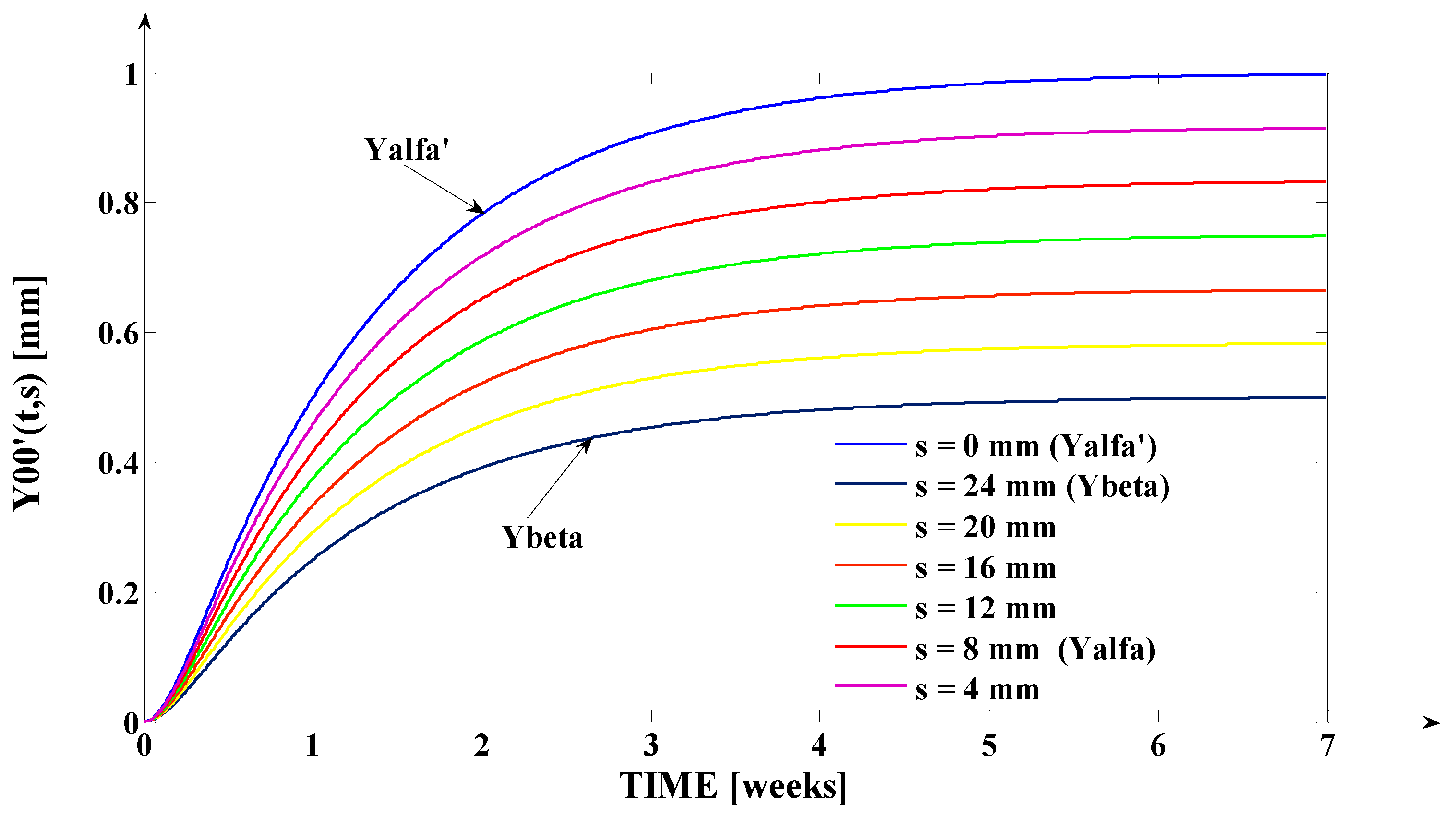
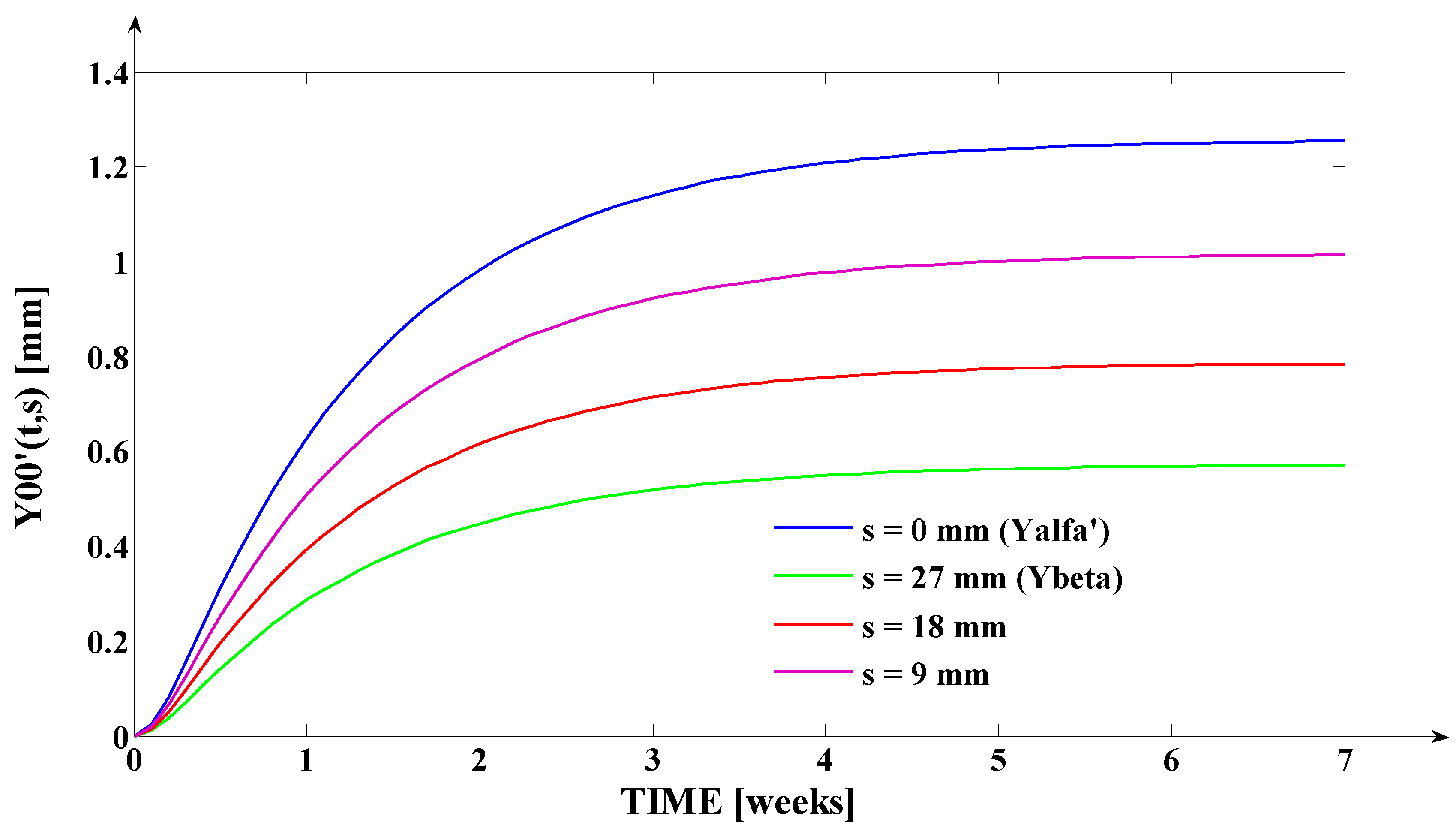
- The orthodontic activation cycle duration is = 7 weeks (the period in which the signals from Figure 6 accurately reach the new steady state regime implied by the application of the input signal), but from Figure 6 and Table 2, it results that only after four weeks, the output signal (for all values of (s) independent variable) reaches with more than 96% from the total increase due to the current activation;
- (2)
- The evolution of the force delivered by the elastomeric chain () [grams force] is an exponentially decreasing one, from the initial value [grf] to the residual plastic strength [grf];
- (3)
- The incisal edge of the tooth [mm] follows an exponentially increasing movement evolution, which superposes, in steady state regime, to the [mm] asymptote;
- (4)
- All the evolutions of the output signal presented in Figure 6 follow increasing exponential evolutions, their dynamics being given by (T1) and (T2) time constants;
- (5)
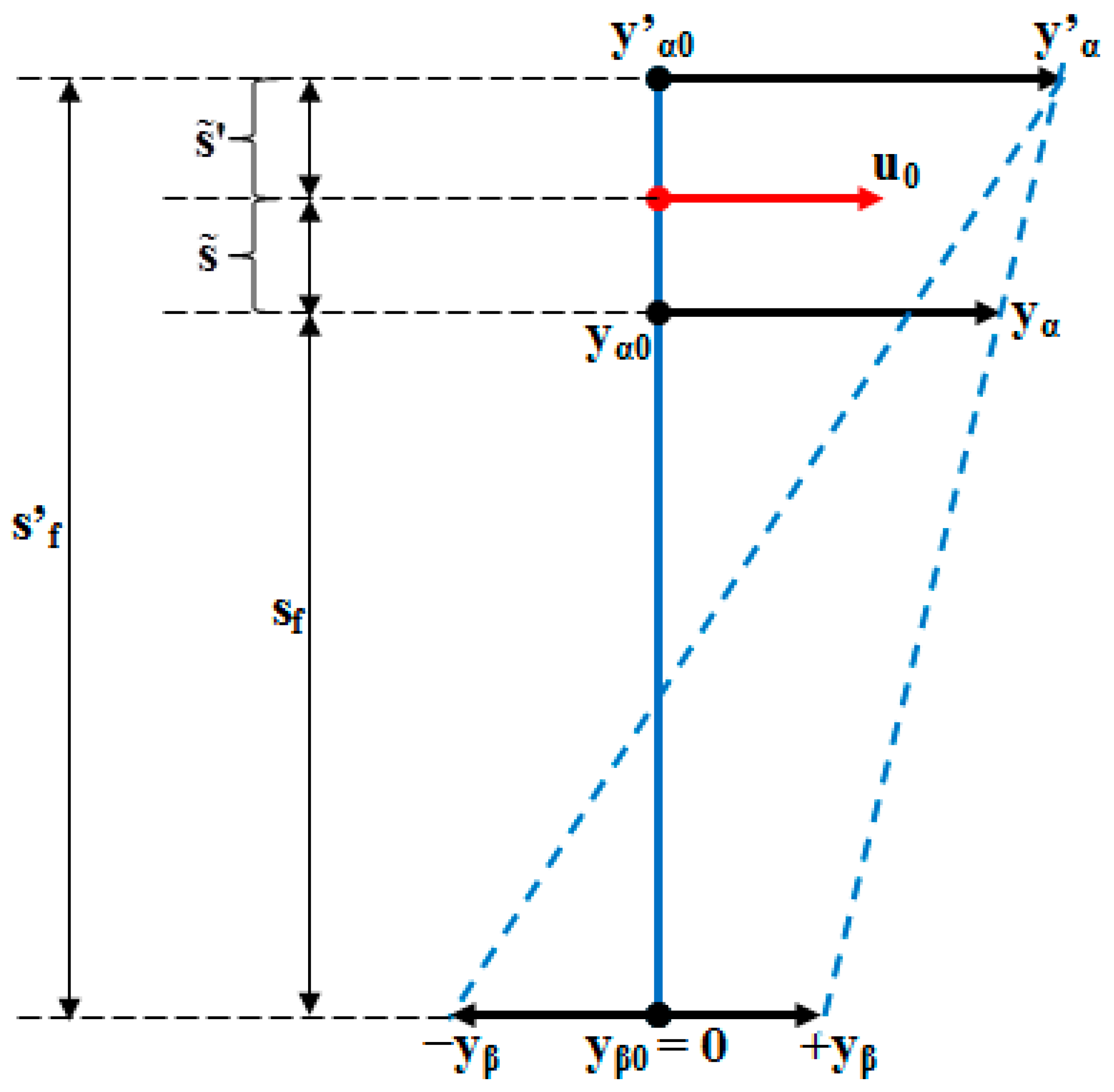
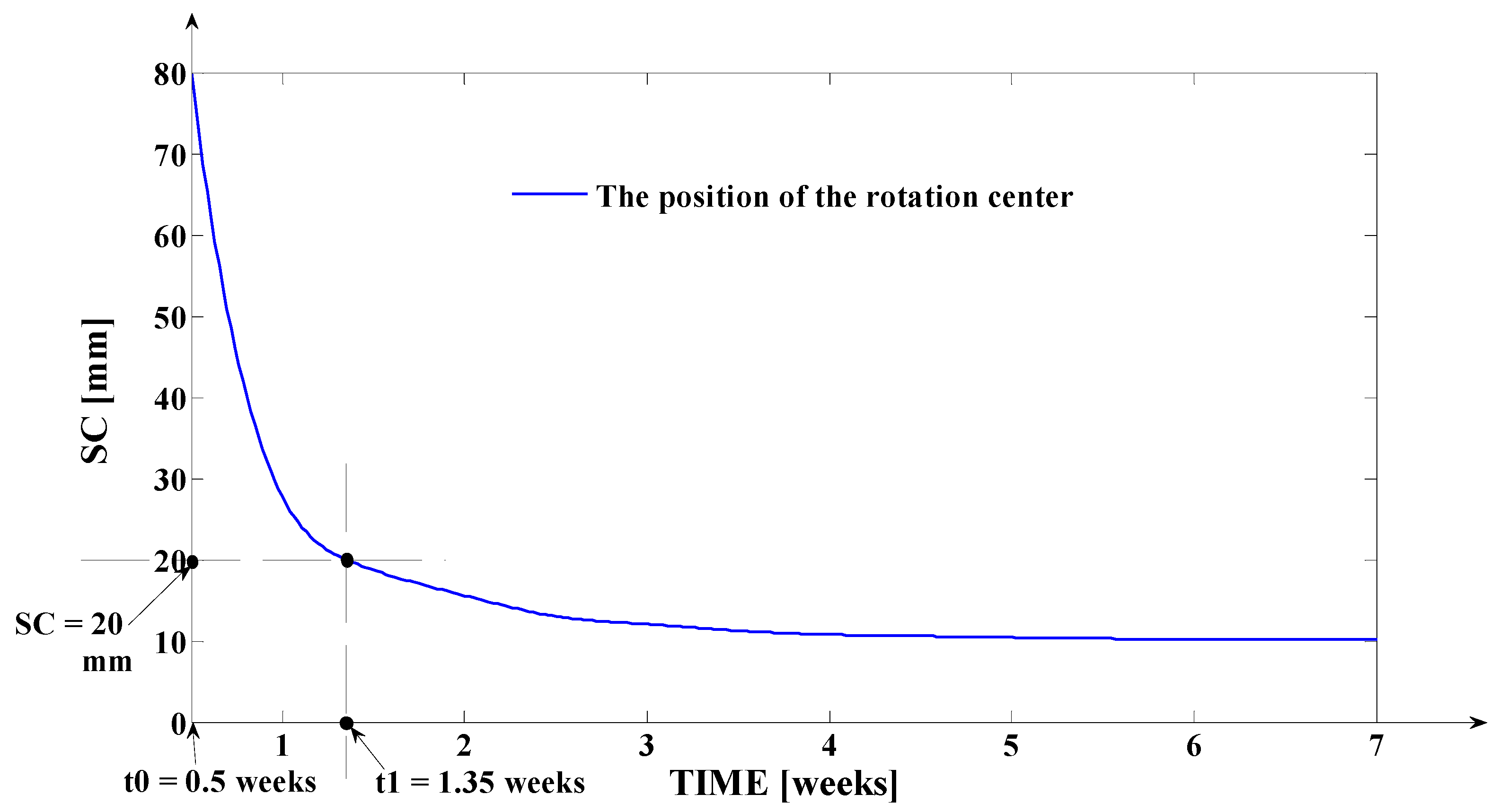
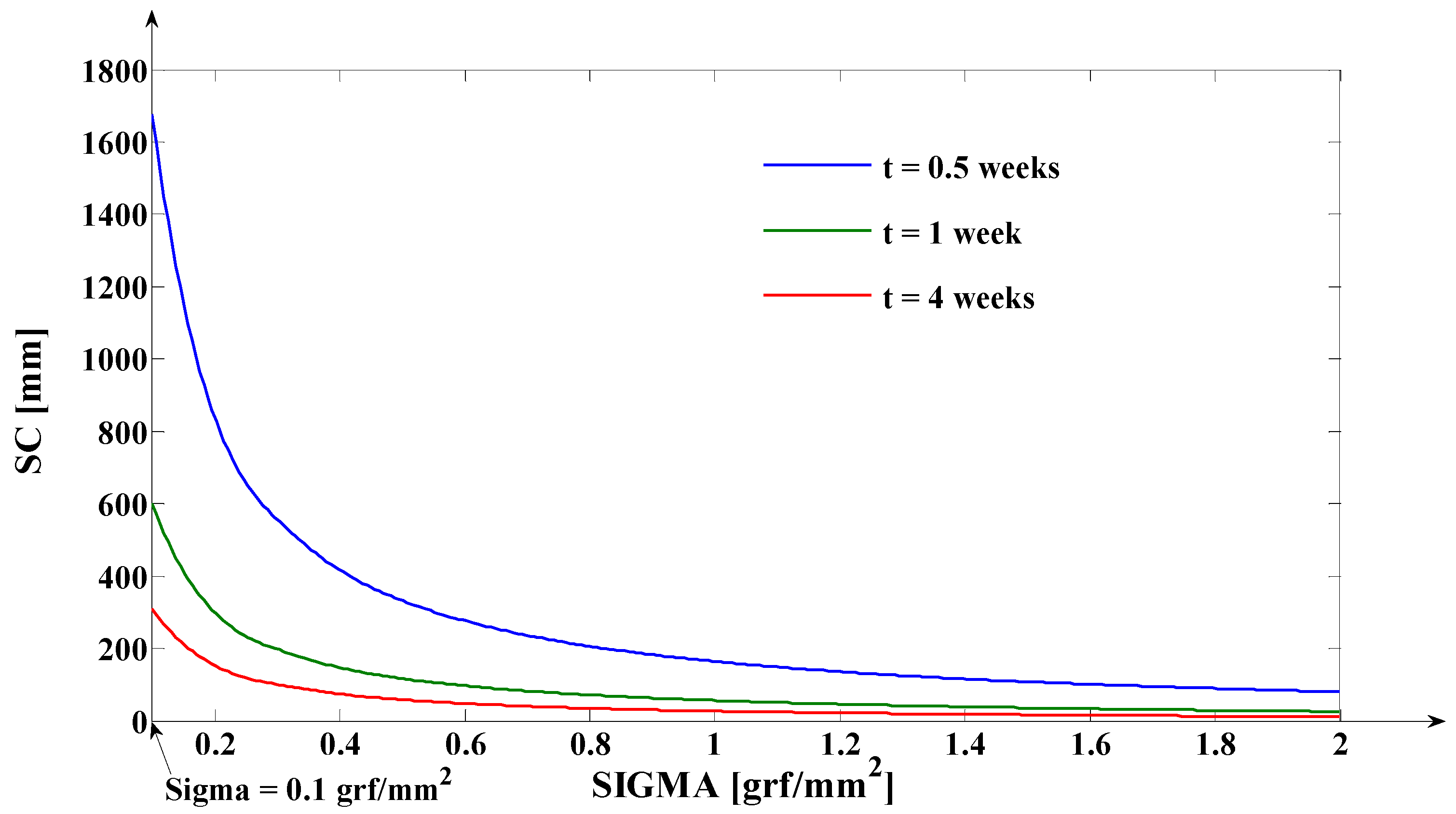
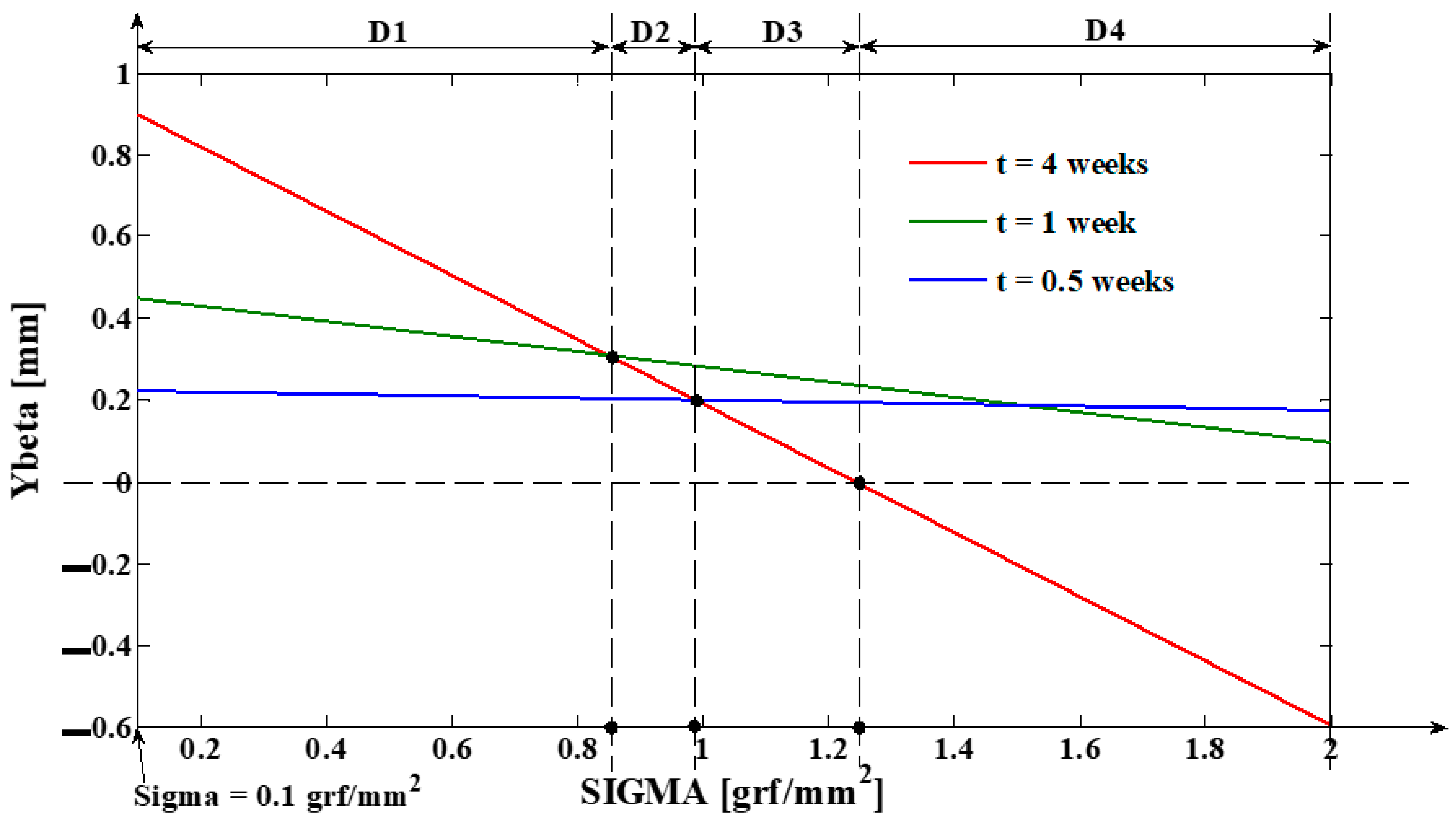
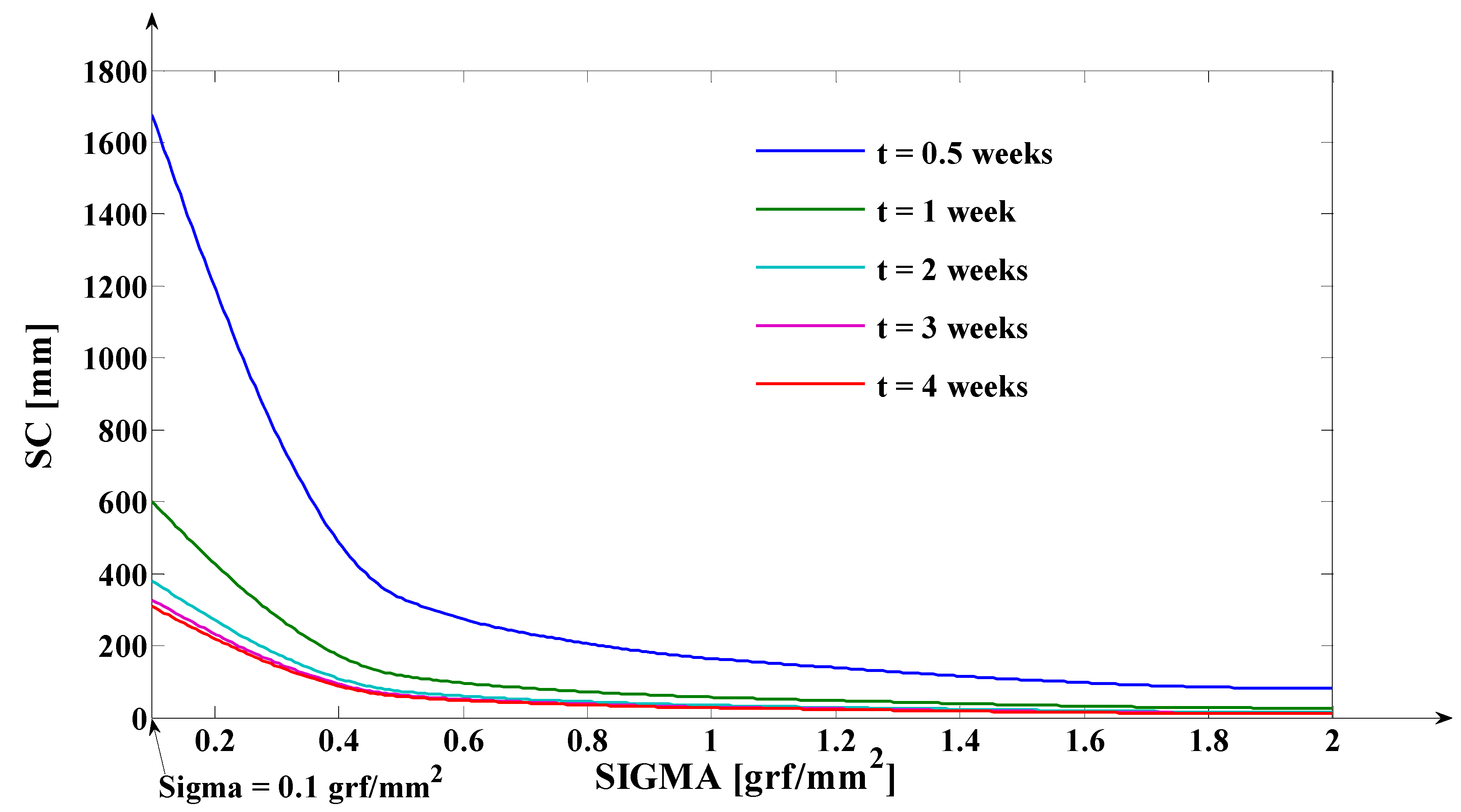
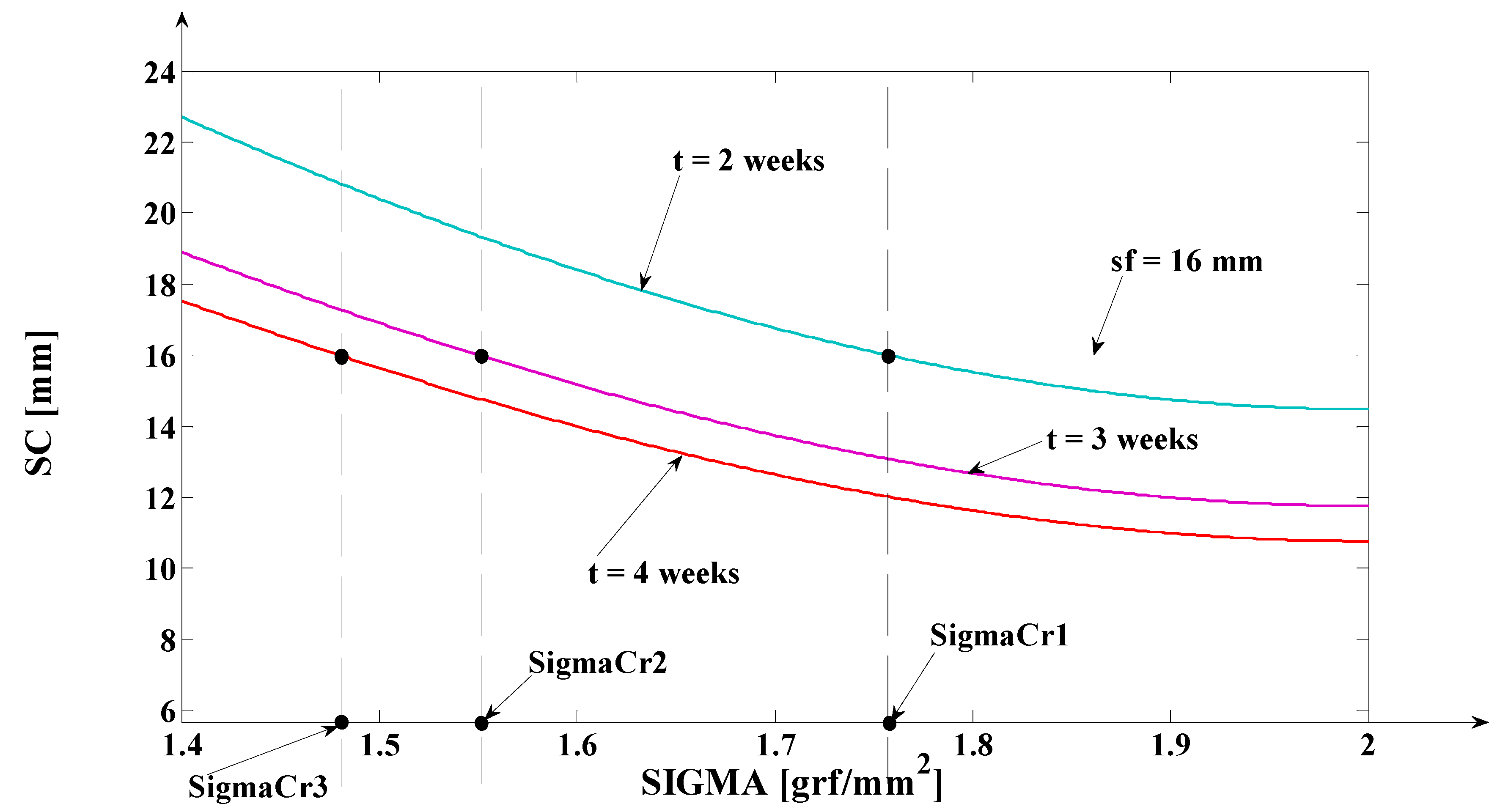
- As it results from Figure 6, the lower the value of (s) independent variable is, the higher the values of the corresponding signals are (the maximum steady-state value of the output signal is [mm] obtained for s = s0 = 0 mm and the minimum steady-state value of the output signal is y obtained for );
- (6)
- Even if the applied force () accurately decreases to the residual value () in 1.2 weeks, its effect lasts approximately seven weeks; practically, relative to the settling time of the responses presented in Figure 6, the input signal () can be assimilated with an impulse type signal; the graphical representation from Figure 3, being a qualitative one, does not respect the scale between the input and the output signal (for a better highlighting of the signals variation form).
3. Results
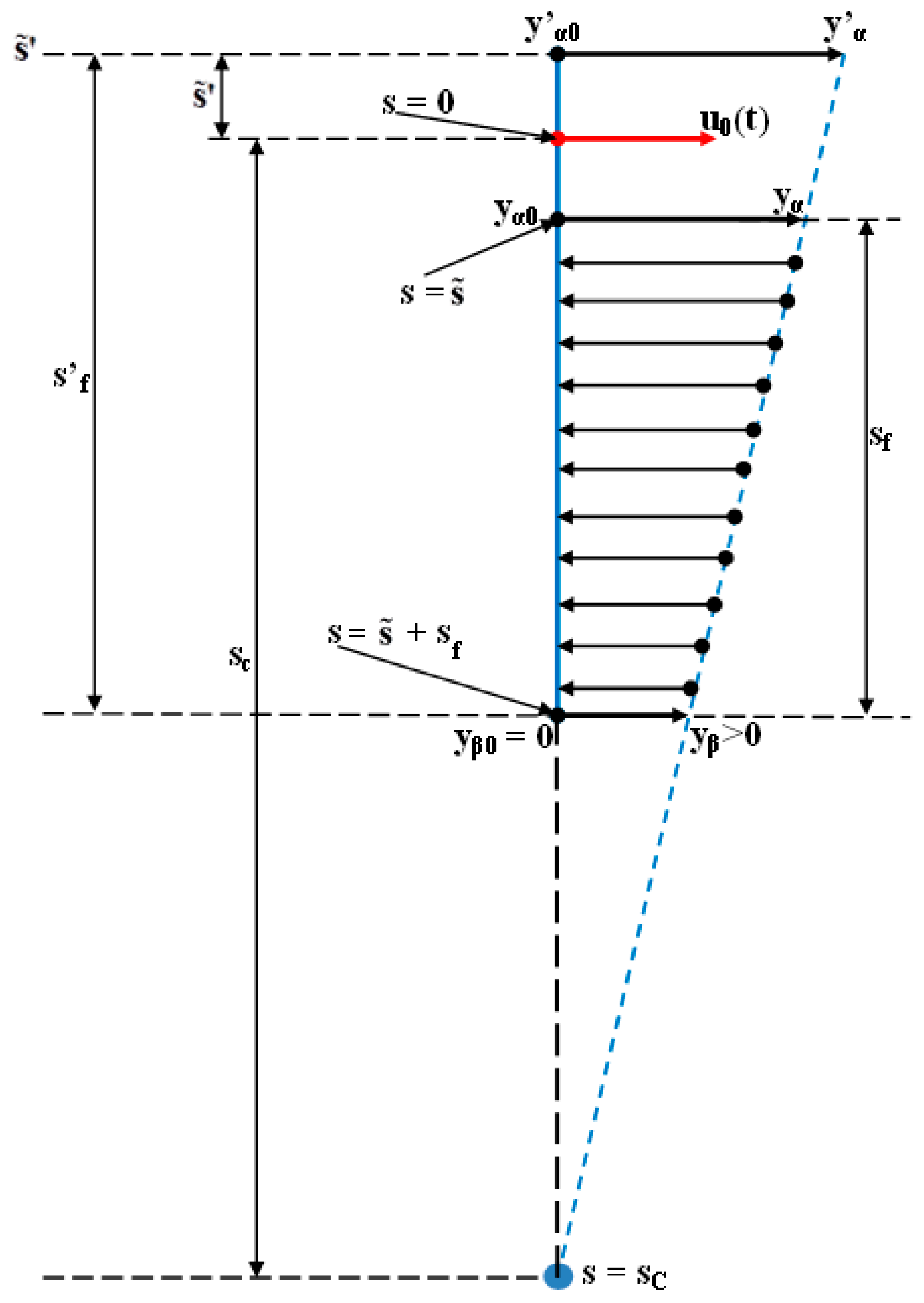
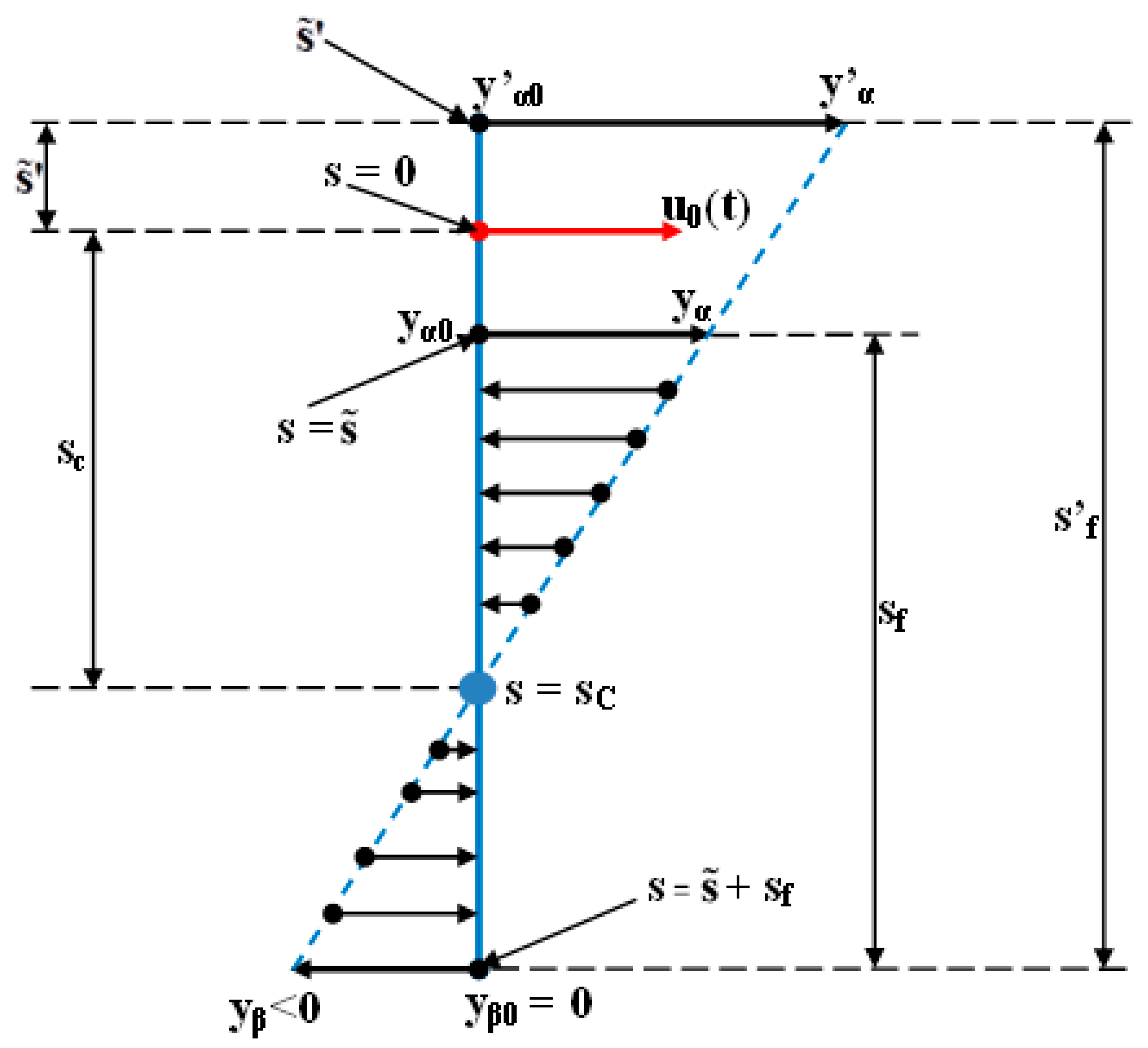
3.1. Digital Approximation of the Rotation Center (sc) and of the Tooth Apex (Tooth Depth) Movement (yβ)
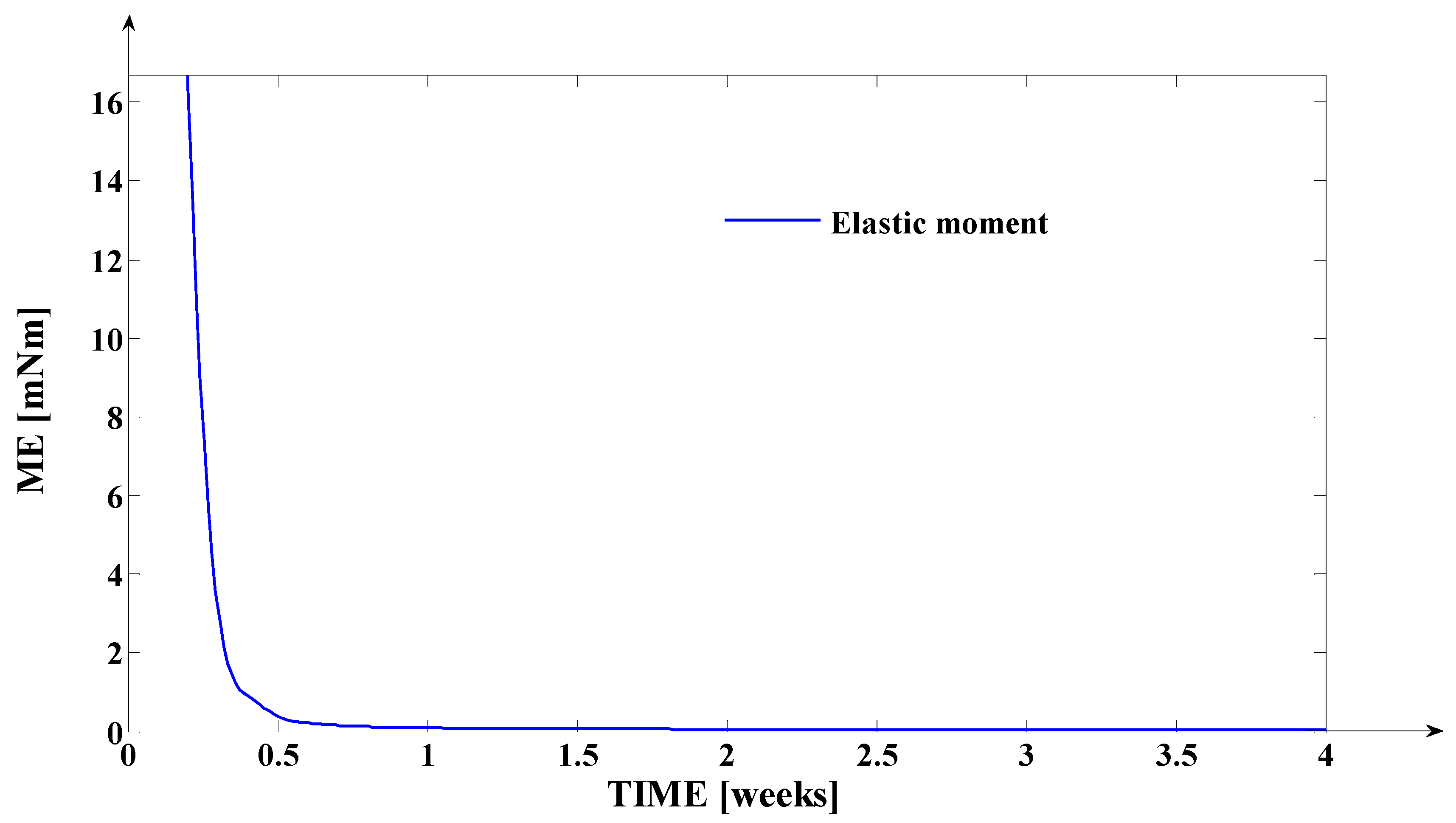
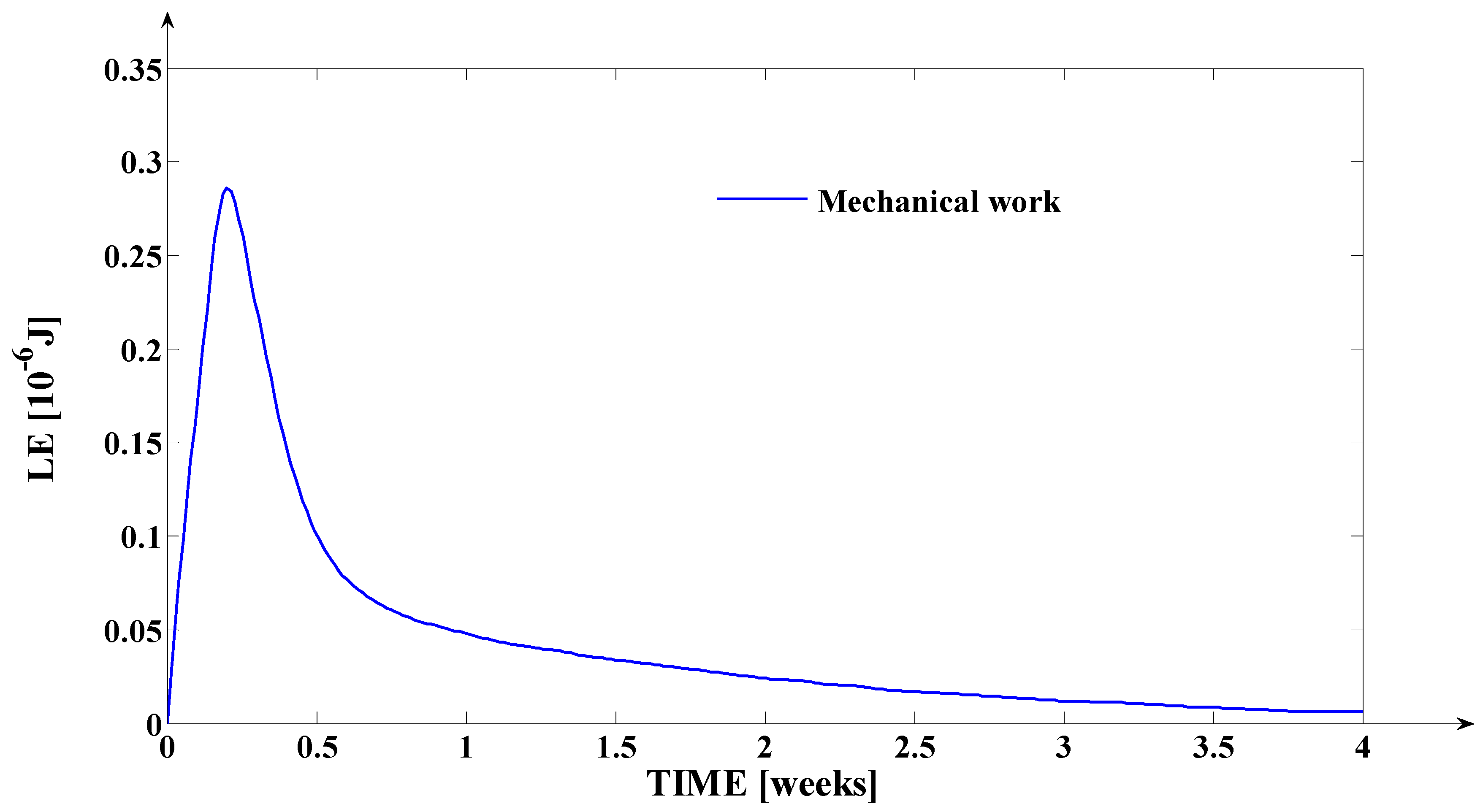
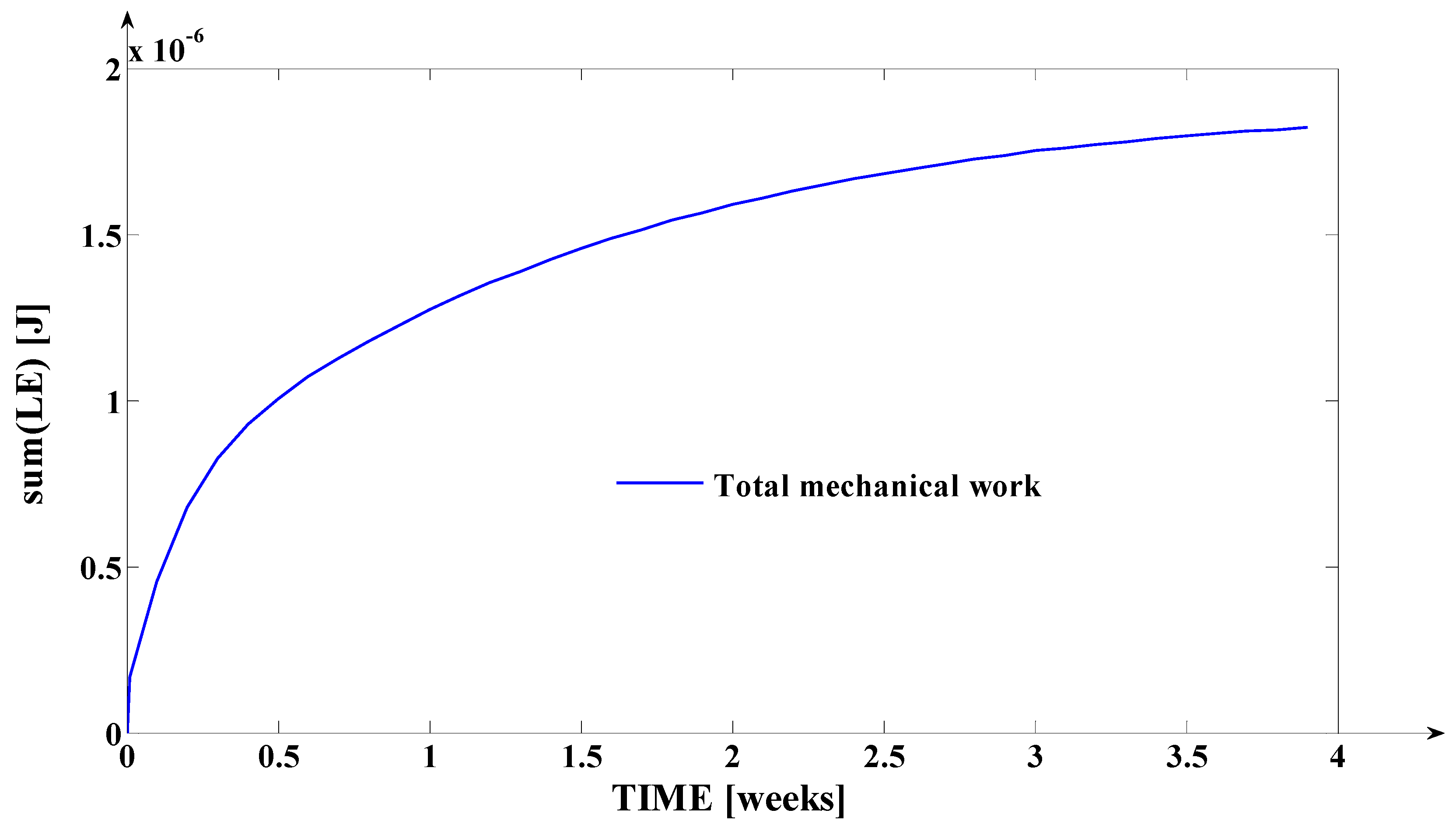
3.2. Simulation of the Evolutions of the Elastic Moment () and of the Mechanical Work in Elastic Regime (), during Orthodontic Treatment
- (1)
- The elastic moment given by the equation: expressed in [grf∙mm] or in [];
- (2)
- The mechanical work in elastic regime given by the equation: , where , and () represent the variation of () on the considered time interval; with (), the teeth’s movement for s = is logged (the movement of the point from the tooth’s fictional axis which belongs to the tooth’s transversal section upon which the force (through the bracket) is applied; the mechanical work is expressed in [grf∙mm] or in [].
4. Conclusions, Discussion, and Future Research
- (1)
- The first computer program was implemented in MATLAB and represents the software application used for the () scaling. This application implements the algorithm of generating () by solving the equality presented in Equation (5). The algorithm’s solution results by processing the evolution (from the tip of the tooth—incisal edge, part which is visible to the orthodontist, in the oral cavity).
- (2)
- The second computer program was implemented in MATLAB/SIMULINK and represents the software application used for simulating the orthodontic process as a distributed parameter one [29]. This application computes the orthodontic process’s response for a certain variation form of the input signal u0(t) (used during the orthodontic treatment) and for different values of the (s) independent variable. Certainly, for the depth , the deformation’s evolution becomes progressively even more different from as the () independent variable is getting closer to the top of the tooth’s root (apex, whose position corresponds to ()). The simulations presented in the paper (for the example in Table 2) are made considering a predetermined value of (). The () value (both in the case when it has the “+” or the “−” sign) can be only estimated (with high accuracy) but cannot be strictly known by the orthodontist.
- (3)
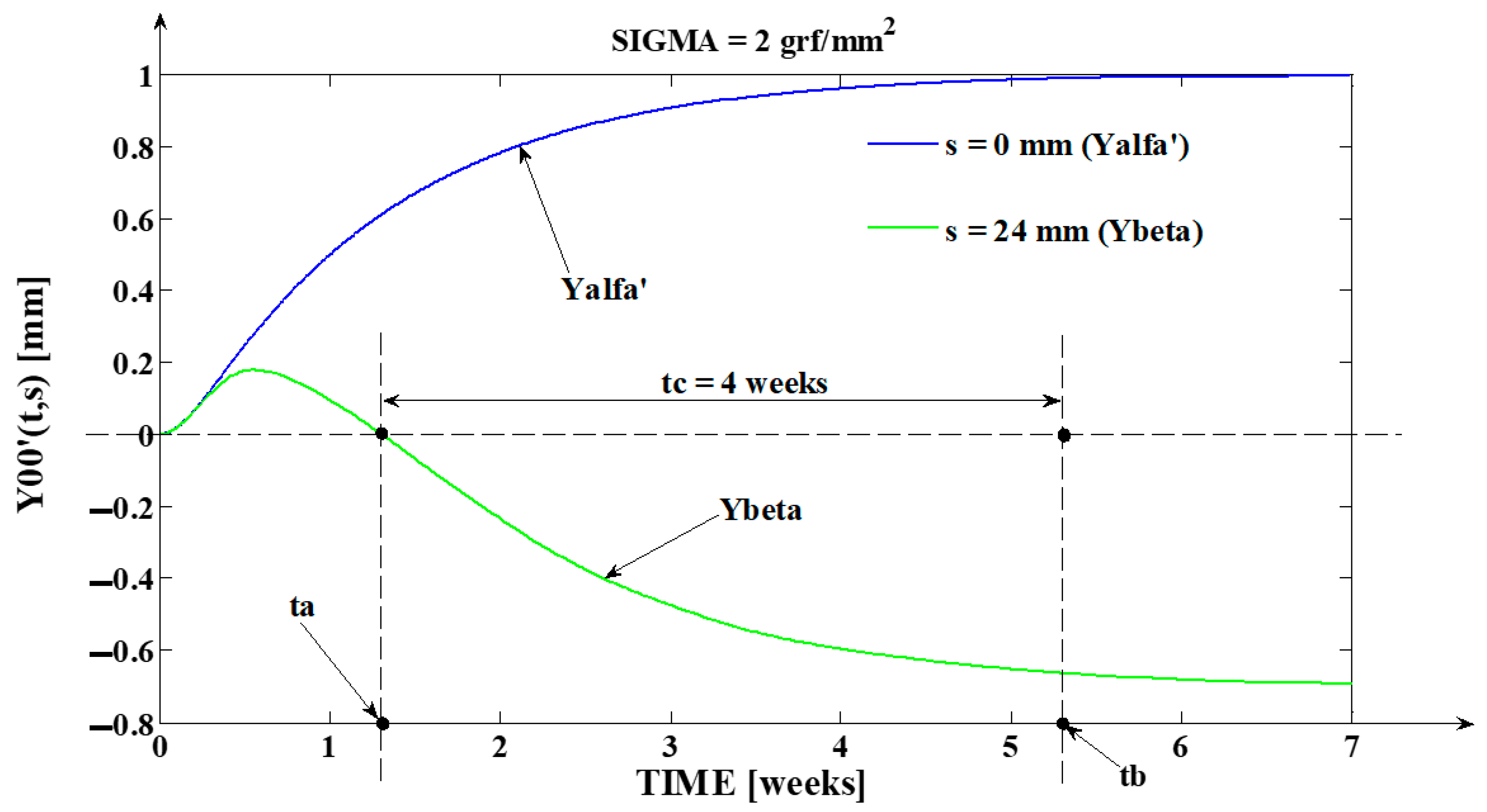
- The third program was implemented in MATLAB and represents the software application used for determining the tooth’s rotation center sc = sc(t,s) and the deformation of the tooth’s root tip. Practically, this application implements the algorithm presented in paragraph three, more exactly the algorithm of solving the transcendental Equation (14) (this procedure represents an original and precise method for determining the tooth’s rotation center and, as a direct application, a method of determining the movement of the tooth’s apex). An important advantage of the program is the fact that it can generate the computation of the rotation center position in relation to time (t), but also for different values of the tissue resistance (mathematically highlighted through the value of the coefficient). Based on the simulations’ results obtained through the third program running, the evolutions of the two mentioned signals in relation to the corresponding independent variables can be determined (of (sc) in relation to both (t) and (s), respectively of in relation to (t)). In the paper, both the interpretations of the balance methods between the modulus’s elasticity from Equation (10) with the plastic moment from Equation (11), and of the transcendent equation, in relation to variables (t) and (s), from Equation (14), are highlighted. For the initial conditions used in this program, with the presented results in Table 3, it can be observed that after ta = 1.35 weeks, the evolution of decreases at negative values, which signifies the rotation regime’s occurrence (this regime has to be avoided in orthodontic treatments; based on this remark, the third program elaborated in the research activity can assist the orthodontist in choosing the correct treatment—for example, in choosing the correct initial value of the applied force). Other interesting future studies can be made based on the plastic resistance coefficient of the environment (tissue) presented in (15), in relation to the structure of the overall analog model shown in (5) and, consequently, in relation with the , and functions.
- (4)
- Finally, the fourth synthesis program implemented in MATLAB considers another dimension of the tooth. This program implements, as a novelty, the algorithms of determining the instantaneous values of the following physical quantities: the elastic moment () expressed both in [grf∙mm] and in [μNm]; the mechanical work in elastic regime () expressed both in [grf∙mm] and in [μJ]; the cumulated mechanical work in elastic regime (ΣLE(t)) expressed in [μJ]. Based on the obtained values (after the simulation), the fourth program generates, also, the evolution of these signals in relation to time (t). The interpretations of the obtained evolutions present a valuable practical relevance. Also, the obtained values of these quantities show the “physical measure” of the applied treatment.
- (5)
- Finally, the fifth synthesis program is implemented in MATLAB/SIMULINK. This program runs the orthodontic process’s mathematical model for the new dimension of the tooth and for the value of the tissue plastic resistance coefficient. The simulations’ results obtained after running this program are presented in Figure 18 (in the simulations, the predetermined value is used). In Figure 18, the deformations are highlighted for four constant depths: . Using this approach, it becomes, therefore, convenient to follow these deformations, either in relation to time or in relation to depth
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fish, G.D. Some engineering principles of possible interest to orthodontics. Dent. Cosm. 1917, 59, 881–889. [Google Scholar]
- Burstone, C.J. The biomechanics of tooth movement. In Vistas in Orthodontics; Kraus, B.S., Riedel, R.A., Eds.; Lea & Febiger: Philadelphia, PA, USA, 1962; pp. 197–213. [Google Scholar]
- Zhou, X.; Gan, Y.; Zhao, Q.; Xiong, J.; Xia, Z. Simulation of orthodontic force of archwire applied to full dentition using virtual bracket displacement method. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3189. [Google Scholar] [CrossRef] [PubMed]
- Hack, D.C. The science of mechanics and its importance to analysis and research the field of orthodontics. Am. J. Orthop. 1963, 49, 330–344. [Google Scholar] [CrossRef]
- Rodriguez Janez, E. 1001 Tips for Orthodontics and Its Secrets; Amolca: Medellín, Colombia, 2008. [Google Scholar]
- Proffit, W.R. Contemporary Orthodontics, 4th ed.; Mosby: St. Louis, MI, USA, 2007. [Google Scholar]
- Jeon, P.D.; Turley, P.K.; Ting, K. Three-dimensional finite element analysis of stress in the periodontal ligament of the maxillary first molar with simulated bone loss. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.; Pae, E.-K.; Park, Y.; Kim, K.-H.; Burstone, C.J. Effect of root and bone morphology on the stress distribution in the periodontal ligament. Am. J. Orthod. Dentofac. Orthop. 2000, 117, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ona, M.; Wakabayashi, N. Influence of Alveolar Support on Stress in Periodontal Structures. J. Dent. Res. 2006, 85, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Dannan, A. An update on periodonthicorthodontic interrelationships. J. Indian Soc. Periodontol. 2010, 14, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Moga, R.A.; Olteanu, C.D.; Botez, M.D.; Buru, S.M. Finite elements analysis of tooth—A comparative analysis of multiple failure criteria. Int. J. Res. Public Health 2023, 20, 4133. [Google Scholar] [CrossRef] [PubMed]
- Diarra, A.; Mushegyan, V.; Naveau, A. Finite Element Analysis Generates an Increasing Interest in Dental Research: A Bibliometric Study. Open Dent. J. 2016, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Kawamura, J.; Fukui, H. Finite element analysis of the effect of force directions on tooth movement in extraction space closure with miniscrew sliding mechanics. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Kong, W.D.; Cen, W.J.; Zhou, X.Z.; Zhang, W.; Li, Q.T.; Guo, H.Y.; Yu, J.W. Finite element analysis of the effect of powerarm locations on tooth movement in extraction space closure with miniscrew anchorage in customized lingual orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.F.; Mojra, A.; Shirazi, M. A numerical-experimental study on thermal evaluation of orthodontic tooth movement during initial phase of treatment. J. Therm. Biol. 2019, 80, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Coloşi, T.; Abrudean, M.; Ungureşan, M.-L.; Mureşan, V. Numerical Simulation of Dis-Tributed Parameter Processes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Li, H.-X.; Qi, C. Spatio-Temporal Modeling of Nonlinear Distributed Parameter Systems: A Time/Space Separation Based Approach, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Haykin, S. Neural Networks and Learning Machines, 3rd ed.; Pearson: Hamilton, ON, Canada, 2009. [Google Scholar]
- Maren, A.; Harston, C.; Pap, R. Handbook of Neural Computing Applications; Academic Press Inc.: New York, NY, USA, 1990. [Google Scholar]
- Norgaard, M.; Ravn, O.; Poulsen, N.K.; Hansen, L.K. Neural Networks for Modelling and Control of Dynamics Systems; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Bunta, O.; Muresan, V.; Sas, D.; Colosi, T. Mathematical formalisms used in the orthodontic dynamics. In Proceedings of the 2017 14th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 1–2 June 2017; pp. 196–199. [Google Scholar] [CrossRef]
- Nemeş, O.; Mureşan, V.; Coloşi, T. Modeling and Simulation of an Orthodontic Process. Appl. Mech. Mater. 2015, 811, 365–370. [Google Scholar] [CrossRef]
- Kardach, H.; Biedziak, B.; Olszewska, A.; Golusińska-Kardach, E.; Sokalski, J. The mechanical strength of orthodontic elastomeric memory chains and plastic chains: An in vitro study. Adv. Clin. Exp. Med. 2017, 26, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, M.; Rashed, R.; Khodarahmi, N. Evaluation of the effects of three different mouthwashes on the force decay of orthodontic chains. Dent. Res. J. 2015, 12, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Aldrees, A.M.; Al-Foraidi, S.A.; Murayshed, M.S.; Almoammar, K.A. Color stability and force decay of clear orthodontic elastomeric chains: An in vitro study. Int. Orthod. 2015, 13, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Shetty, S.; Krithika, M.J.; Cyriac, B. Effect of commonly used beverage, soft drink, and mouthwash on force delivered by elastomeric chain: A comparative in vitro study. J. Int. Oral Health 2014, 6, 7–10. [Google Scholar] [PubMed]
- Sang, T.; Wu, J. Factors on force degradation of elastomeric chains in vitro. Shanghai Kou Qiang Yi Xue 2008, 17, 638–642. [Google Scholar] [PubMed]
- Mirhashemi, A.; Saffarshahroudi, A.; Sodagar, A.; Atai, M. Force-Degradation Pattern of Six Different Orthodontic Elastomeric Chains. J. Dent. Tehran Univ. Med. Sci. 2012, 9, 204–215. [Google Scholar]
- Golnaraghi, F.; Kuo, B.C. Automatic Control Systems, 9th ed.; Wiley Publishing House: Hoboken, NJ, USA, 2009. [Google Scholar]
- Love, J. Process Automation Handbook, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Oskui, I.Z.; Hashemi, A.; Jafarzadeh, H.; Kato, A. Finite element investigation of human maxillary incisor under traumatic loading: Static vs dynamic analysis. Comput. Methods Programs Biomed. 2018, 155, 121–125. [Google Scholar] [CrossRef] [PubMed]

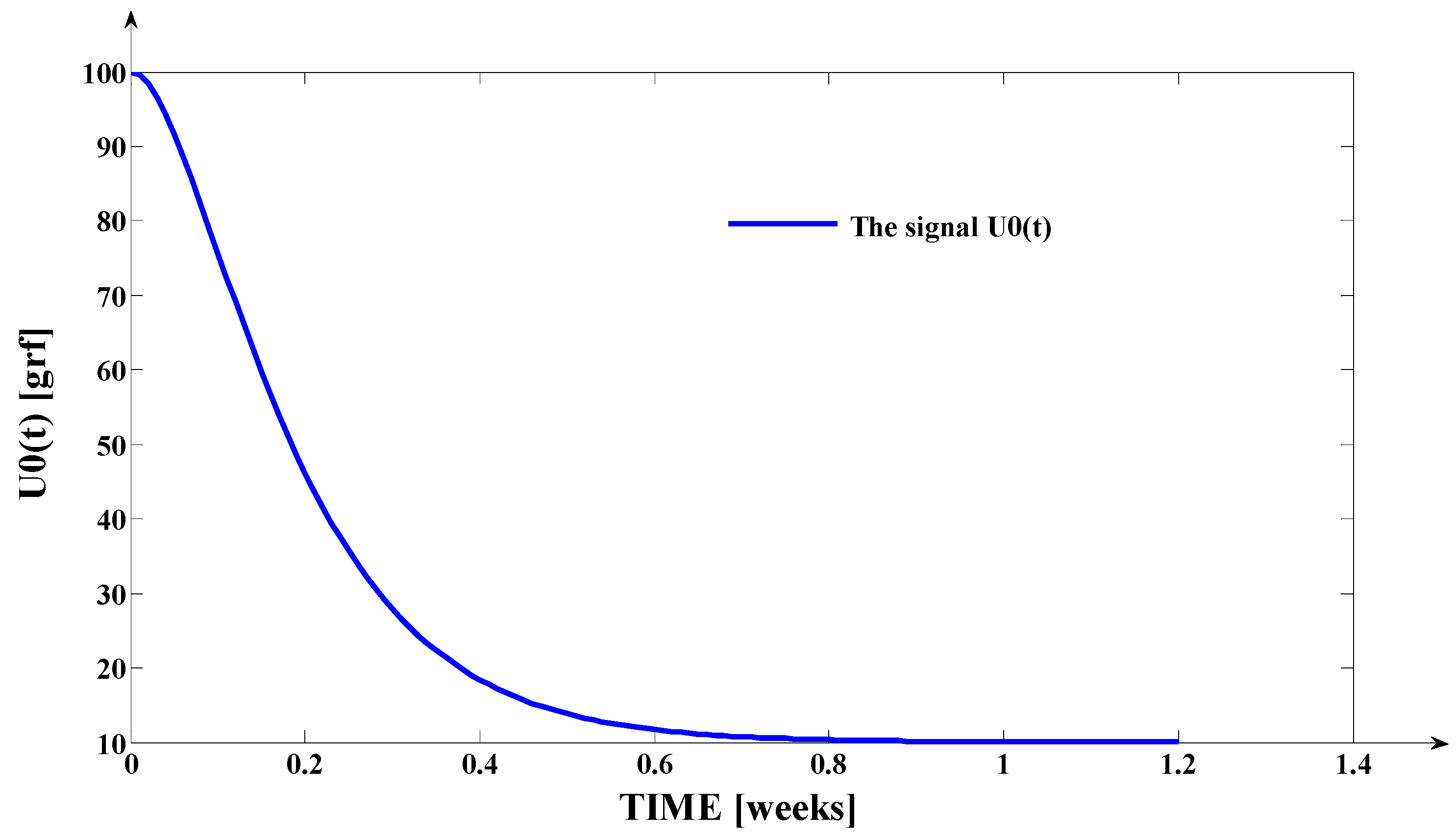
| t [Weeks] | u0 [grf] |
|---|---|
| 0 | 100 |
| 0.1 | 75.77 |
| 0.2 | 46.22 |
| 0.3 | 27.92 |
| 0.4 | 18.41 |
| 0.5 | 13.83 |
| 0.6 | 11.71 |
| 0.7 | 10.76 |
| 0.8 | 10.33 |
| 0.9 | 10.14 |
| 1 | 10.06 |
| t [Weeks] | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 0.24 | 0.22 | 0.2 | 0.18 | 0.16 | 0.14 | 0.12 |
| 1 | 0.49 | 0.45 | 0.41 | 0.37 | 0.33 | 0.29 | 0.249 |
| 1.5 | 0.669 | 0.61 | 0.55 | 0.5 | 0.44 | 0.39 | 0.33 |
| 2 | 0.78 | 0.71 | 0.65 | 0.58 | 0.52 | 0.45 | 0.39 |
| 2.5 | 0.85 | 0.78 | 0.71 | 0.64 | 0.57 | 0.499 | 0.42 |
| 3 | 0.9 | 0.83 | 0.75 | 0.679 | 0.6 | 0.52 | 0.45 |
| 3.5 | 0.93 | 0.86 | 0.78 | 0.7 | 0.62 | 0.54 | 0.469 |
| 4 | 0.96 | 0.88 | 0.8 | 0.72 | 0.64 | 0.56 | 0.48 |
| 4.5 | 0.97 | 0.89 | 0.81 | 0.73 | 0.649 | 0.56 | 0.48 |
| 5 | 0.98 | 0.9 | 0.819 | 0.73 | 0.655 | 0.57 | 0.49 |
| 5.5 | 0.989 | 0.907 | 0.824 | 0.742 | 0.659 | 0.57 | 0.494 |
| 6 | 0.993 | 0.91 | 0.828 | 0.745 | 0.662 | 0.579 | 0.496 |
| 6.5 | 0.996 | 0.913 | 0.83 | 0.747 | 0.664 | 0.581 | 0.498 |
| 7 | 0.997 | 0.914 | 0.831 | 0.748 | 0.665 | 0.582 | 0.4989 |
| 2 | ||||
|---|---|---|---|---|
| t [Weeks] | u0 [grf] | sc [mm] | ||
| 0 | 100 | 0 | - | 0 |
| 0.5 | 13.83 | 0.247 | 79.808 | 0.1763 |
| 1 | 10.06 | 0.4995 | 25.744 | 0.0965 |
| 1.5 | 10.001 | 0.669 | 17.808 | −0.0672 |
| 2 | 10 | 0.782 | 14.48 | −0.2336 |
| 2.5 | 10 | 0.844 | 13.024 | −0.3458 |
| 3 | 10 | 0.906 | 11.76 | −0.4737 |
| 3.5 | 10 | 0.939 | 11.152 | −0.5483 |
| 4 | 10 | 0.9606 | 10.768 | −0.6005 |
| 4.5 | 10 | 0.97 | 10.608 | −0.623 |
| 5 | 10 | 0.98 | 10.448 | −0.647 |
| 5.5 | 10 | 0.989 | 10.304 | −0.6704 |
| 6 | 10 | 0.993 | 10.24 | −0.6806 |
| 6.5 | 10 | 0.996 | 10.192 | −0.688 |
| 7 | 10 | 0.997 | 10.176 | −0.691 |
| t [Weeks] | u0 [grf] | sc [mm] | ME [grf × mm] | ME [mNm] | LE [grf × mm] | LE ([mJ]) | ΣLE[grf⋅mm] ([μJ]) | ||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.1 | 75.77 | 19,933 | 0.0189 | 1,510,300 | 154 | 0.0191 | 1.656 | 0.169 | 1.656 (0.169) |
| 0.2 | 46.22 | 3538 | 0.0648 | 163,530 | 16.675 | 0.0652 | 2.811 | 0.286 | 4.467 (0.455) |
| 0.3 | 27.92 | 1120.2 | 0.1215 | 31,276 | 3.189 | 0.124 | 2.179 | 0.222 | 6.646 (0.675) |
| 0.4 | 18.41 | 488.342 | 0.1779 | 8990.4 | 0.916 | 0.1865 | 1.447 | 0.147 | 8.093 (0.822) |
| 0.5 | 13.83 | 273.258 | 0.228 | 3779.2 | 0.385 | 0.2485 | 0.999 | 0.101 | 9.092 (0.923) |
| 0.6 | 11.71 | 185.283 | 0.2707 | 2169.7 | 0.221 | 0.3081 | 0.761 | 0.077 | 9.853 (1.000) |
| 0.7 | 10.76 | 143.038 | 0.3074 | 1539.1 | 0.156 | 0.3648 | 0.637 | 0.065 | 10.49 (1.065) |
| 0.8 | 10.33 | 119.34 | 0.3392 | 1232.8 | 0.125 | 0.418 | 0.561 | 0.057 | 11.051 (1.122) |
| 0.9 | 10.14 | 104.363 | 0.367 | 1058.2 | 0.107 | 0.4679 | 0.51 | 0.052 | 11.561 (1.174) |
| 1 | 10.06 | 93.959 | 0.3914 | 945.227 | 0.0964 | 0.5146 | 0.471 | 0.048 | 12.032 (1.222) |
| 1.1 | 10.02 | 86.173 | 0.4125 | 863.453 | 0.088 | 0.5583 | 0.438 | 0.044 | 12.470 (1.266) |
| 1.2 | 10.01 | 80.155 | 0.431 | 802.351 | 0.0818 | 0.5992 | 0.409 | 0.041 | 12.879 (1.307) |
| 1.3 | 10.005 | 75.276 | 0.4469 | 753.136 | 76.798 | 0.6374 | 0.382 | 0.039 | 13.261 (1.346) |
| 1.4 | 10.002 | 71.264 | 0.4608 | 712.782 | 72.683 | 0.6734 | 0.36 | 0.036 | 13.621 (1.382) |
| 1.5 | 10.001 | 67.898 | 0.4727 | 679.047 | 69.243 | 0.707 | 0.336 | 0.034 | 13.957 (1.416) |
| 1.6 | 10 | 65.042 | 0.483 | 650.42 | 66.324 | 0.7385 | 0.315 | 0.032 | 14.272 (1.448) |
| 1.7 | 10 | 62.594 | 0.4919 | 625.94 | 63.828 | 0.7679 | 0.294 | 0.03 | 14.566 (1.478) |
| 1.8 | 10 | 60.486 | 0.4997 | 604.86 | 61.678 | 0.7957 | 0.278 | 0.028 | 14.844 (1.506) |
| 1.9 | 10 | 58.65 | 0.5064 | 586.5 | 59.806 | 0.8216 | 0.259 | 0.026 | 15.103 (1.532) |
| 2 | 10 | 57.052 | 0.5122 | 570.52 | 58.176 | 0.8457 | 0.241 | 0.024 | 15.344 (1.556) |
| 2.1 | 10 | 55.641 | 0.5172 | 556.41 | 56.738 | 0.8683 | 0.226 | 0.023 | 15.570 (1.579) |
| 2.2 | 10 | 55.4 | 0.5216 | 544 | 55.472 | 0.8895 | 0.212 | 0.021 | 15.782 (1.600) |
| 2.3 | 10 | 53.295 | 0.5254 | 532.95 | 54.345 | 0.9093 | 0.198 | 0.02 | 15.980 (1.62) |
| 2.4 | 10 | 52.309 | 0.5287 | 523.09 | 53.34 | 0.9278 | 0.185 | 0.018 | 16.165 (1.638) |
| 2.5 | 10 | 51.442 | 0.5317 | 514.42 | 52.456 | 0.9451 | 0.173 | 0.017 | 16.338 (1.655) |
| 2.6 | 10 | 50.66 | 0.5342 | 506.6 | 51.658 | 0.961 | 0.159 | 0.016 | 16.497 (1.671) |
| 2.7 | 10 | 49.946 | 0.5364 | 499.46 | 50.93 | 0.9761 | 0.515 | 0.015 | 16.648 (1.686) |
| 2.8 | 10 | 49.317 | 0.5383 | 493.17 | 50.289 | 0.9899 | 0.138 | 0.014 | 16.786 (1.700) |
| 2.9 | 10 | 48.739 | 0.54 | 487.39 | 49.699 | 1.0031 | 0.132 | 0.013 | 16.918 (1.713) |
| 3 | 10 | 48.229 | 0.5415 | 482.29 | 49.179 | 1.015 | 0.129 | 0.012 | 17.047 (1.725) |
| 3.1 | 10 | 47.705 | 0.5428 | 477.05 | 48.645 | 1.0273 | 0.123 | 0.012 | 17.17 (1.737) |
| 3.2 | 10 | 47.332 | 0.5439 | 473.32 | 48.265 | 1.0367 | 0.104 | 0.009 | 17.274 (1.746) |
| 3.2 | 10 | 46.954 | 0.545 | 469.54 | 47.879 | 1.0465 | 0.098 | 0.009 | 17.372 (1.755) |
| 3.4 | 10 | 46.614 | 0.546 | 466.14 | 47.533 | 1.0555 | 0.09 | 0.009 | 17.462 (1.764) |
| 3.5 | 10 | 46.291 | 0.5467 | 462.91 | 47.203 | 1.0637 | 0.082 | 0.008 | 17.544 (1.772) |
| 3.6 | 10 | 46.002 | 0.5474 | 460.02 | 46.909 | 1.0715 | 0.078 | 0.008 | 17.622 (1.789) |
| 3.7 | 10 | 45.747 | 0.5481 | 457.47 | 46.649 | 1.0786 | 0.071 | 0.007 | 17.693 (1.787) |
| 3.8 | 10 | 45.509 | 0.5486 | 455.09 | 46.406 | 1.0851 | 0.065 | 0.006 | 17.758 (1.793) |
| 3.9 | 10 | 45.288 | 0.5491 | 452.88 | 46.18 | 1.0913 | 0.062 | 0.006 | 17.82 (1.799) |
| 4 | 10 | 45.045 | 0.5495 | 450.45 | 45.933 | 1.0981 | 0.06 | 0.006 | 17.88 (1.805) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunta, O.; Festila, D.; Muresan, V.; Coloși, T.; Stan, O.P.; Unguresan, M.L.; Baciut, M. Mathematical Modeling and Digital Simulation of Teeth Dynamics for the Approximation of Orthodontic Treatment Duration. Appl. Sci. 2023, 13, 5932. https://doi.org/10.3390/app13105932
Bunta O, Festila D, Muresan V, Coloși T, Stan OP, Unguresan ML, Baciut M. Mathematical Modeling and Digital Simulation of Teeth Dynamics for the Approximation of Orthodontic Treatment Duration. Applied Sciences. 2023; 13(10):5932. https://doi.org/10.3390/app13105932
Chicago/Turabian StyleBunta, Olimpia, Dana Festila, Vlad Muresan, Tiberiu Coloși, Ovidiu Petru Stan, Mihaela Ligia Unguresan, and Mihaela Baciut. 2023. "Mathematical Modeling and Digital Simulation of Teeth Dynamics for the Approximation of Orthodontic Treatment Duration" Applied Sciences 13, no. 10: 5932. https://doi.org/10.3390/app13105932
APA StyleBunta, O., Festila, D., Muresan, V., Coloși, T., Stan, O. P., Unguresan, M. L., & Baciut, M. (2023). Mathematical Modeling and Digital Simulation of Teeth Dynamics for the Approximation of Orthodontic Treatment Duration. Applied Sciences, 13(10), 5932. https://doi.org/10.3390/app13105932







