1. Introduction
In silico modeling is a valuable tool for investigating the mechanical behavior of biological systems and exploring treatment options for various diseases [
1]. Due to advancements in computational power and imaging technology, in silico modeling has found widespread application in the field of orthopedics [
2], specifically in knee joint biomechanics, including the modeling of knee joint diseases such as osteoarthritis (OA) [
3]. Patient-specific finite element models, simulating clinical changes in the cartilage, can predict how tissue deterioration affects bone mechanics and patient outcomes [
4]. Recent studies have shown that in cases of OA and other degenerative soft tissue diseases, the stiffness of cartilage decreases [
5,
6]. Deterioration of cartilage, in turn, disrupts the natural functionality of the entire joint, in particular, having a negative impact on bone tissue, which wears out more intensively due to the redistribution of loads [
7]. In silico methods can help to analyze in more detail the consequences of soft tissue hardening and identify tendencies in their effect on the state of bone tissue, which cannot be tested by experimental methods. Numerical simulation techniques can help to save time and reduce the costs of investigations.
Despite their undeniable advantages, in silico methods present several challenges. Firstly, the development of geometrical models requires accurate processing of the joint’s anatomical geometry, which demands appropriate software packages capable of reading medical images. Secondly, the problem formulation is another difficulty, as biological tissues generally behave as nonlinear elastic or hyperelastic bodies, requiring the use of more intricate mathematical techniques, and the material data should be well known. All of the mentioned points should be verified for sufficient investigation to be performed.
Recent studies, for the most part, suggest using the finite element method for numerical modeling of the knee. A literature review has shown that the problems and research objectives that are addressed can be very diverse: authors [
8] pronounced a numerical knee model for verifying the mechanical processes in knee joint in case of arthroplasty. The geometry of the model was obtained by processing DICOM data. The three-dimensional model contains femur, tibia, ligaments, and implants, although the bone components didn’t reflect the trabecular structure; in addition, the ligaments were modeled as perfectly elastic bodies, so nonlinear behavior was not verified. The model proposed by [
9] allowed the verification of the loads occurring in cases of flexion and was characterized by complex external geometry: it contained fibula and reflected the anatomical curvature of bone surfaces, though the trabecular structure of porous bone and the hyperelasticity of cartilages and ligaments were not verified too. Other authors [
10] provided a shock wave of research on the knee joint affected with osteoarthritis. The porous material properties were taken into account, but at the same time, the representation of spongious bone was simplified, so the proposed model cannot accurately predict the interaction between cortical and trabecular bone. Some authors [
11,
12] provide numerical knee joint models with verification of nonlinear properties of soft tissues, although they assume the bone components to act as rigid bodies, so the elastic properties of femur and tibia remain uninvolved.
The present study aims to investigate the stress changes of the bones which occur in cases of deterioration of cartilage. The numerical model of the knee joint containing components of femur, tibia, meniscus, and cartilage was developed. The trabecular structure of both femur and tibia was verified. In order to assess the hyperelastic properties of soft tissues, the Mooney–Rivlin material model was applied, and the problem of nonlinear theory of elasticity was raised. The importance of the work for the field of biomedical engineering is that the proposed model can be parameterized, and this, in turn, can contribute to the further study of biomechanical processes occurring in the knee joint.
3. Numerical Results and Discussion
3.1. Von Mises Stress in Case of 1 mm Displacement
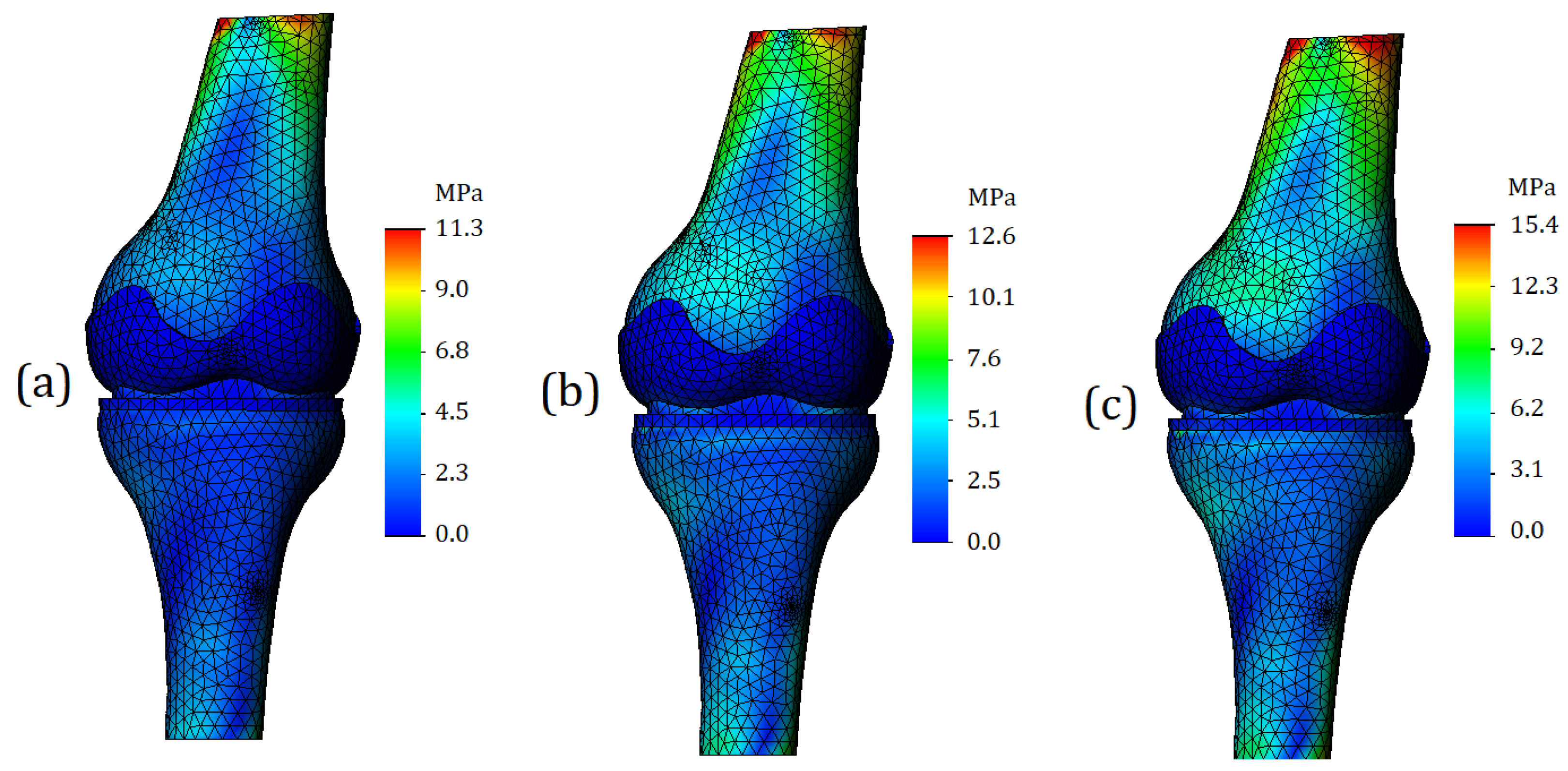
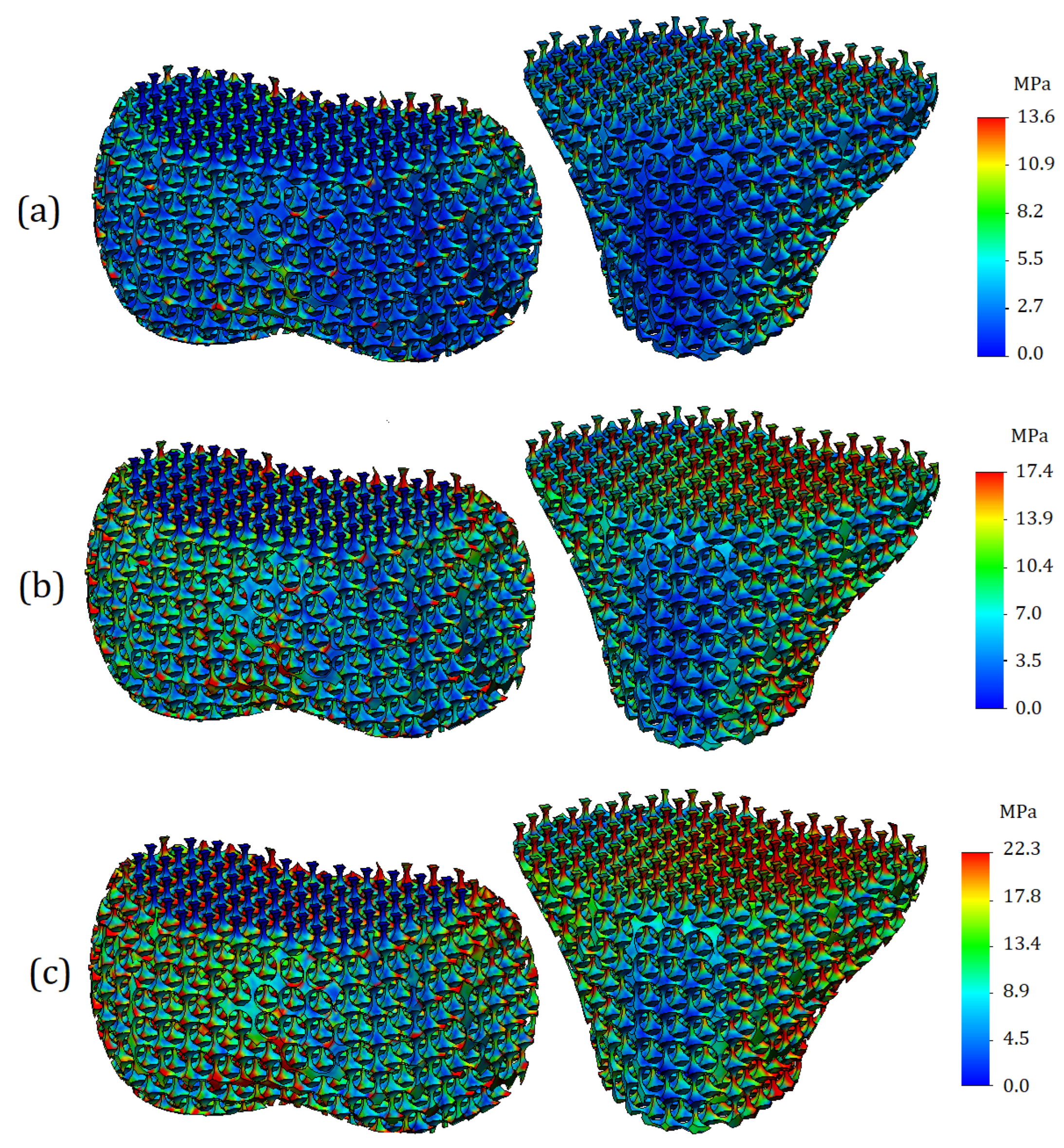
Von Mises stresses were calculated for the entire 3D model. A general view of the von Mises stress plot is shown in
Figure 6. As can be seen from the digital scale, the maximum stress values are deployed on the bone components, while the values for soft tissues (cartilage and meniscus) are significantly lower. In the case of processing a model with mild cartilage stiffness (
Figure 6a), the maximum stress values reached 11.3 MPa. For the model with moderate cartilage stiffness (
Figure 6b), the maximum stress values were 12.6 MPa and were 15.4 MPa for the model with the severe cartilage stiffness (
Figure 6c).
Regarding von Mises stress for soft tissues, as the cartilage stiffness increases, the stress values for femur cartilage were obtained at 0.4 MPa (
Figure 6a), 1.1 MPa (
Figure 6b), and 1.9 MPa (
Figure 6c). The stress values for tibia cartilage were slightly higher: 0.5 MPa (
Figure 6a), 1.8 MPa (
Figure 6b), and 2.7 MPa. This effect can be explained by the fact that tibia cartilage typically has greater stiffness, which was taken into account. The meniscus stress values varied as follows due to changes in cartilage stiffness: 1.1 MPa (
Figure 6a), 3.2 MPa (
Figure 6b), and 3.9 MPa (
Figure 6c). It should be noted that the stress values for the meniscus were higher than for other soft tissues. This can be explained by the fact that the meniscus was assigned properties characteristic of third-degree osteoarthritis [
21].
As can be seen from
Figure 6, for all analyzed cases, there is a general tendency. It lies in the fact that the most loaded nodes are located on the tubular sections of the bone. The range of stress values here varies from 9 to 11 MPa; however, we can argue that the appeared concentrators are associated more with the boundary conditions than with the mechanical properties of the model components, since the locations of the stress concentrators are close to the location of the applied load. For this reason, for a more detailed analysis of the stress state of the model, it is necessary to study other plots, as well as section-views of the finite element model. They are presented below.
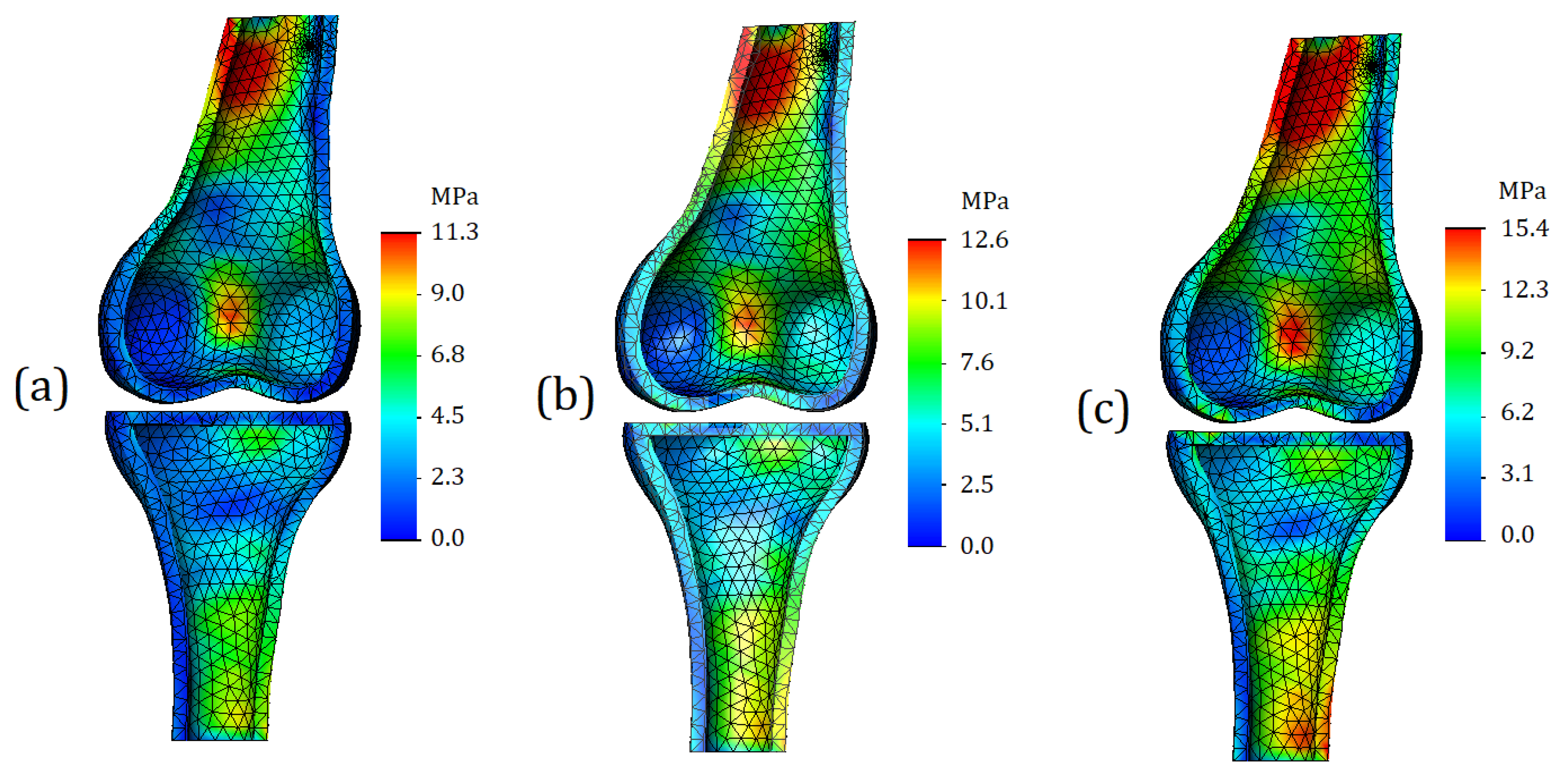
The finite element model section-view for the bone components is shown in
Figure 7. We can observe an increase in stress values on the posterior side of both bones; however, it should be noted that this distribution is related to the geometry of the bones and the loading pattern. Firstly, the tibia and femur are not strictly collinear to each other; for this reason, the displacement that is applied to the upper surface of femur causes an external moment, due to which the model is subjected not only in compression, but also undergoes bending. As a result, we get stress concentrators at the fixed base of the model, as well as in the tubular section of femur. It must be admitted that changes in the stress state in these areas are not the direct object of our study, but, at the same time, changes in stress values in these locations allow us to conclude that the properties of the meniscus affect the load distribution not only in the case of compression but also in the case of bending.
For this study, the stress in the area of the articular surfaces is of much greater interest. Plots of the stress are shown in
Figure 8. It should be noted that
Figure 8 depicts exactly the articular surfaces of the bone, while cartilage and soft tissues are hidden.
As can be seen from
Figure 8a, in the case of cartilage with mild stiffness, there are no stress concentrators on the articular surfaces. The zones of increased stress are not clearly expressed, and basically the model shows a uniform distribution of stresses. In the case of moderate cartilage stiffness (
Figure 8b), the zones of increased stress are more clearly expressed; in addition, their area is noticeably larger. For the model with severe cartilage (
Figure 8c), stress concentrators are identified in four locations (two for tibia and two for femur). However, compared to the model shown in
Figure 8b, the area of the high stress remains approximately the same.
The stress plots of the trabecular tissue are shown in
Figure 9. As can be seen from the plots presented, as the cartilage stiffness increases, not only do the values of the stresses themselves change, but also their distribution changes. With mild cartilage, the zones of increased stress values are more likely to be located outside, while with an increase in stiffness, stress concentrators spread deeper and cover both vertical and horizontal trabeculae. The values of the maximum stresses on the trabeculae themselves turned out to be lower than the stresses on the cortical bone: 9.6 MPa for mild stiffness cartilage, 11.2 MPa for moderate stiffness cartilage, and 12.9 MPa for severe stiffness cartilage.
3.2. Von Mises Stress in Case of 2 mm Displacement
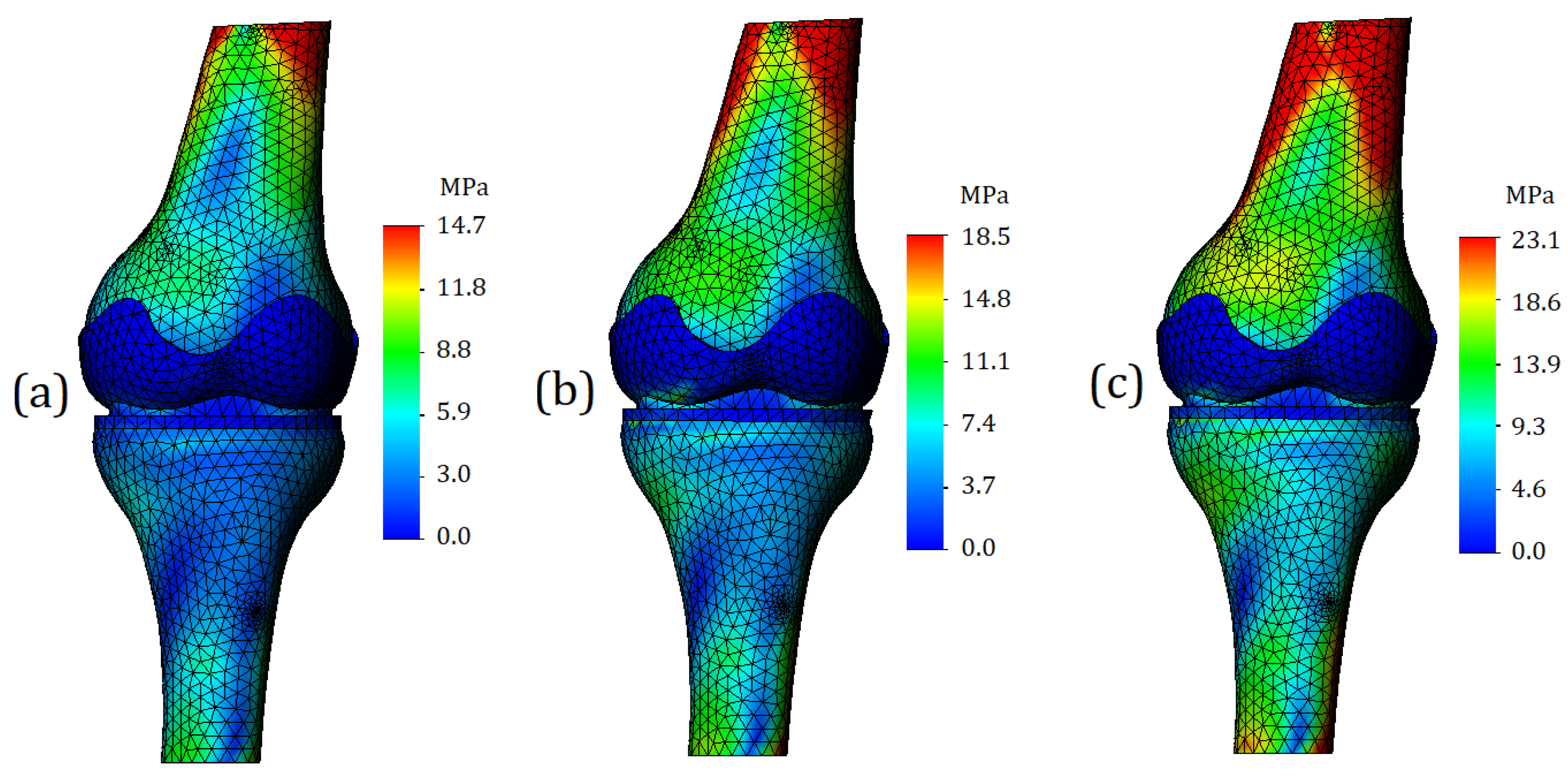
Von Mises stress plots in case of running (2 mm displacement) are shown in
Figure 10. In the general view of the finite element model, we can see that the “red” zones of stress values increased both in area and in numerical values. For the model with mild cartilage stiffness, the maximum stress was 14.7 MPa (
Figure 10a); for a model with moderate cartilage stiffness the maximum stress was 18.5 MPa (
Figure 10b); for the model with the severe cartilage stiffness the maximum stress was 3.1 MPa (
Figure 10c). The model components representing cartilage, as in the previous calculation case, turned out to be painted in blue; however, the stress value in the cartilages became higher. As the stiffness increased in the model, the stress values were 1.1 MPa for the tibia cartilage and 0.8 MPa for the femur cartilage (
Figure 10a), 6.7 MPa for the tibia cartilage and 1.4 MPa for the femur cartilage (
Figure 10b), and 10.1 MPa for the tibia cartilage and 3.6 MPa for the femur cartilage (
Figure 10c).
A significant increase in stress was also observed for the meniscus. In the case of mild cartilage stiffness, the value has risen to 4.2 MPa (
Figure 10a). In the case of moderate cartilage stiffness, it was 5.1 MPa (
Figure 10b), and in the case of severe cartilage stiffness—8.1 MPa (
Figure 10c).
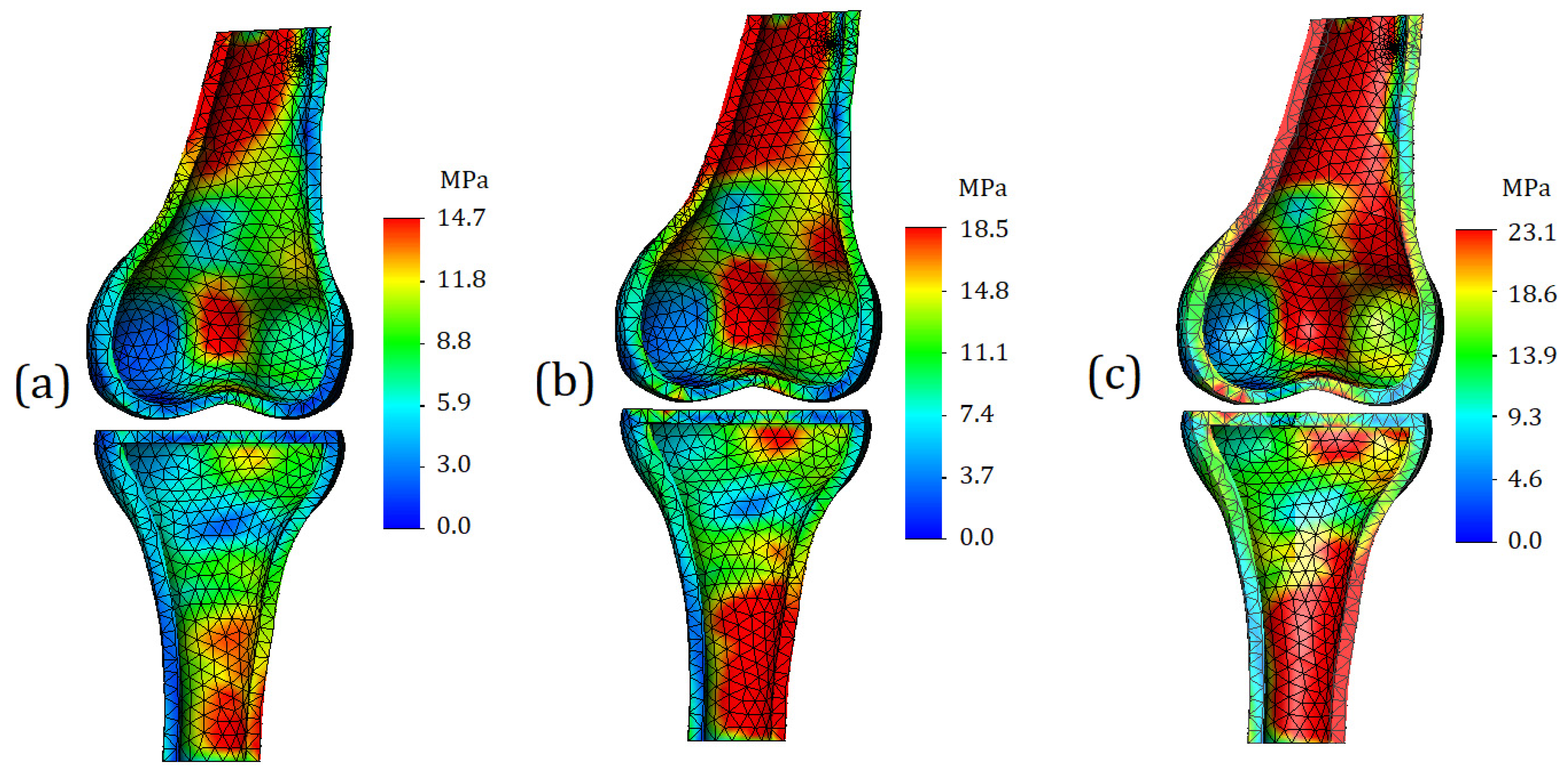
The section view of the finite element model is shown in
Figure 11. The area of zones with high stress values increased as expected. As mentioned earlier, such a distribution of stresses is caused by the external moment. However, from a strength point of view, the areas of the “red” zones (especially in the case of severe cartilage,
Figure 11c), does not come close to critical stress values. Comparing the obtained values with reported yield strength of the bone (140 MPa), we find that the strength capacity under this type of loading exceeds 80 percent.
The distribution of stresses on the contact surfaces of the bone is shown in
Figure 12. In the case of mild cartilage (
Figure 12a), small differences in values occur on the contact surface of the femur; however, they are not too intense, so it is not advisable to consider them as stress concentrators. On the contact surface of tibia, the distribution of stresses is even more uniform, which may indicate a good functionality of the cartilage: in comparison with the first case of loading, the displacement was doubled; however, this did not cause stress concentrators.
In the case of cartilage of moderate stiffness, with a supplied displacement of 2 mm, stress concentrators appear on both contact surfaces of the bones. They have a different area and are dispersed over the entire articular surface (
Figure 12b).
In the case of severe cartilage stiffness, stress concentrators already occupy a significant part of the contact area (
Figure 12c). Again, from the strength point of view, the values themselves are safe; however, such pronounced differences in values in the long term may contribute to more intense wear of tissue.
The distribution of stresses on the trabecular component of the model is shown in
Figure 13. The trends described in the previous subsection are reflected, but it should be noted that in the case of higher displacement (2 mm), the resulting stress values on the trabeculae are percentage closer to the stress values on the cortical bone. The obtained values of stress are equal to 13.6 MPa, 17.4 MPa, and 22.3 MPa for calculations with mild, moderate, and severe cartilage stiffness, respectively.
3.3. Comparison of Stress Values and Discussion
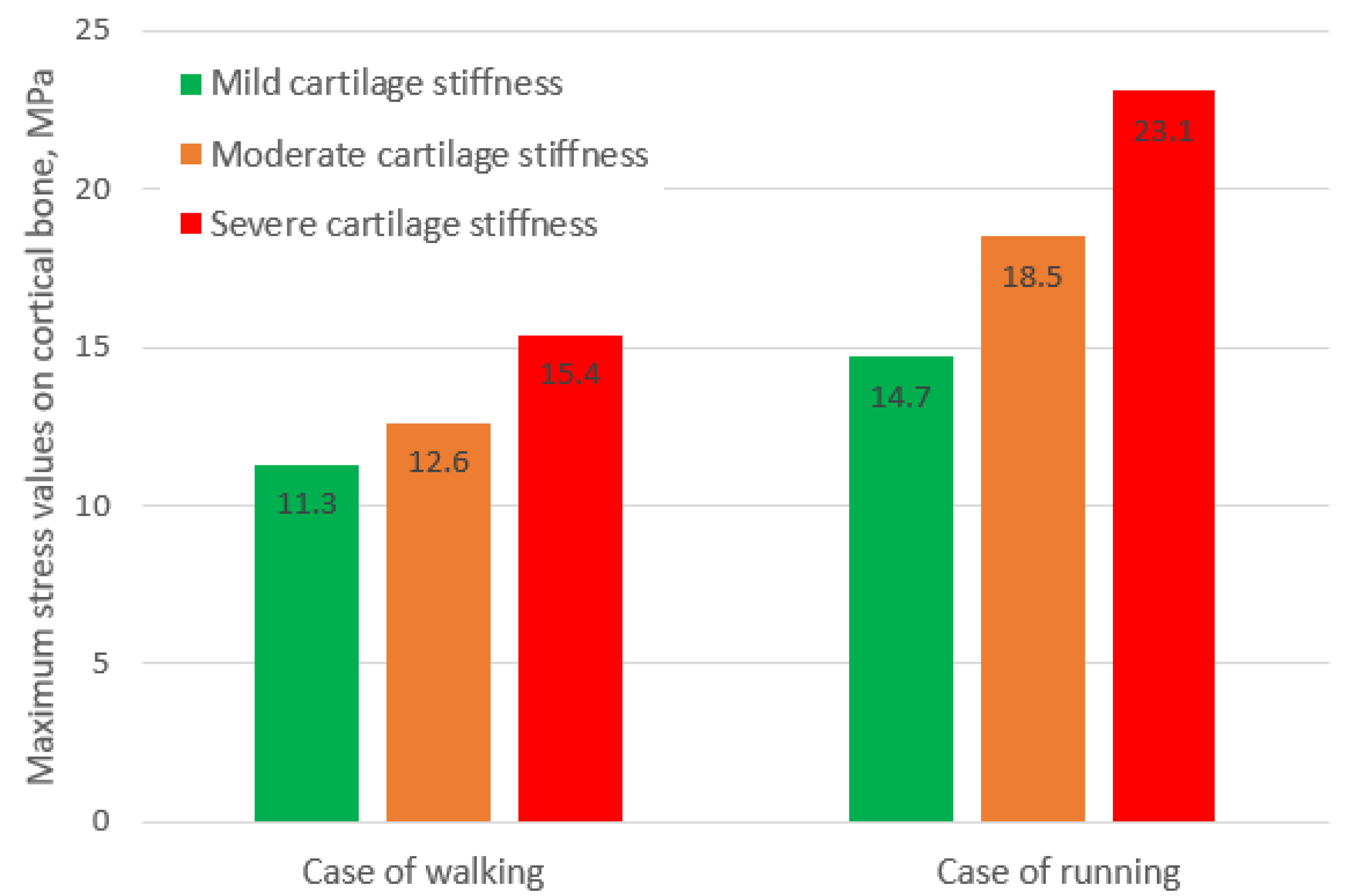
The diagram representing the obtained values of maximum stresses under different loading conditions is shown in
Figure 14. As can be seen from the values, in the case of running, the stresses on bone caused by mild cartilage are lower than those in case of walking caused by moderate and severe cartilage, obtained at loading typical of normal walking.
The percentage increase in stress in cases of running is shown in
Figure 15. Interestingly, in the case of soft cartilage, the growth of stress reached 31%, whereas in case of medium and high cartilage stiffness, the percentage increase in stress values was approximately the same and reached 50%.
The analysis of the obtained results allows us to state that taking into account the non-linear properties of soft tissues is extremely important for assessing the stress state of the entire biological subsystem. The main difficulty, however, is still the lack of data regarding the mechanical behavior of tissues in certain diseases. Despite the fact that general trends, such as an increase in the stiffness of cartilage in osteoarthritis, are known, modern science either does not have information on the specific values of the required constants or parameters, or this information is contradictory.
The advantages of in silico studies were emphasized in the work; however, for their full-scale application, an information base based on experimental data is necessary.
As for this specific study and the proposed methodology, its improvement can be performed in several directions. Firstly, the numerical model geometry can be improved. To more thoroughly study the processes occurring in the knee joint, the model can be supplemented with important anatomical components, such as ligaments. The presence of ligaments will allow the verification of more complex loading cases, such as flexion. Secondly, a more complex mathematical apparatus can be applied. Accounting for properties such as viscoelasticity and damping will allow for research on impact loads that the knee joint is subjected to during normal life cycles. To obtain more reliable results, it would be desirable to use more advanced material models that take into account the anisotropy of mechanical properties. The mathematical models of such materials themselves have been developed and mathematically defined, but the main difficulty is that each bone is strictly individual, and the absence of a template severely limits the application of more accurate models.
4. Conclusions
In this study, a numerical model was developed to assess the stress state of components within the knee joint, taking into account both the applied load and mechanical properties. The proposed novel model offers several advantages, including parameterization features that allow for the geometrical variables control, such as the porosity of trabecular tissue, the thickness of cortical bone, and the thickness of cartilage.
The performed research helped to determine some new patterns related to the non-linear behavior of soft tissues, particularly the increase in bone stress caused by the initial stiffness of cartilage, the appearance of stress concentrators on articular surfaces of the bone, and the dislocation tendencies of stress on spongious bone trabeculae. For a more thorough implementation of the model, as well as the proposed method in medical practice, extensive experimental studies of biological samples are required, the aim of which would be to compare the mechanical properties of knee joint components to the properties inherent in degenerative diseases.