A Review of the Relationship between Gut Microbiome and Obesity
Abstract
1. Introduction
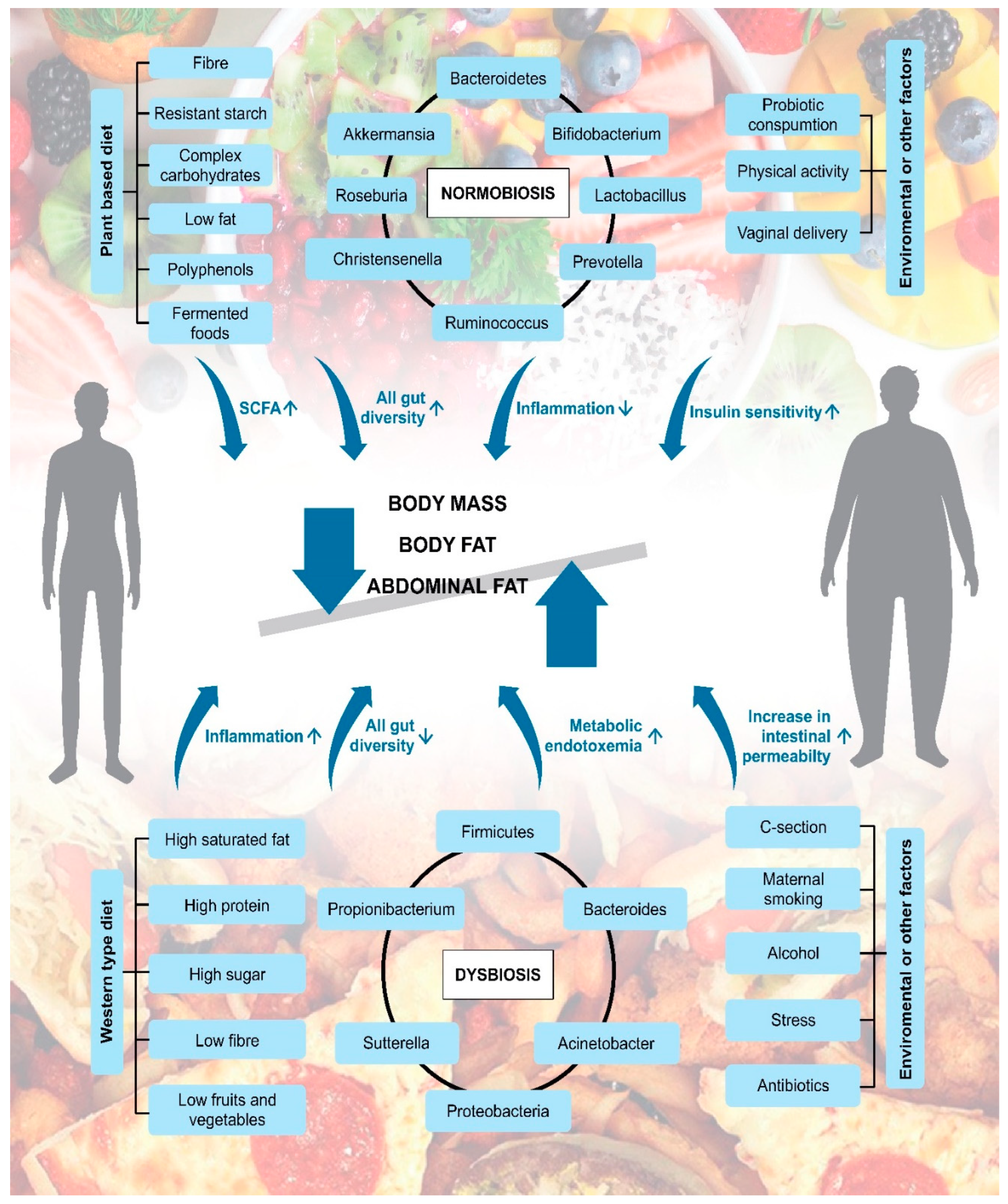
2. The Relationship between Gut Microbiota and Obesity
3. Relationships between Dietary Patterns, Gut Microbiota Composition, and Obesity in Certain Populations
4. The Role of Certain Bacterial Phylum and Species
5. Effect of Diet or Dietary Components on the Gut Microbiota
6. Impact of Lifestyle and Environmental Factors on the Gut Microbiome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, W.; Sahu, S. The Human Microbiome: History and Future. J. Pharm. Pharm. Sci. 2020, 23, 406–411. [Google Scholar] [CrossRef]
- Tseng, C.H.; Wu, C.Y. The gut microbiome in obesity. J. Formos. Med Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef]
- O′Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- WHO Obesity. Available online: https://www.who.int/news-room/facts-in-pictures/detail/6-facts-on-obesity (accessed on 30 October 2020).
- Damsgaard, C.T.; Michaelsen, K.F.; Molbo, D.; Mortensen, E.L.; Sørensen, T.I. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Silvestre, M.P.; Middleton, D.; Korpela, K.; Jalo, E.; Broderick, D.; de Vos, W.M.; Fogelholm, M.; Taylor, M.W.; Raben, A.; et al. Gut microbiota predicts body fat change following a low-energy diet: A PREVIEW intervention study. Genome Med. 2022, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Winnike, J.H.; Crowell, J.M.; O’Connor, A.; Bennett, B.J. Microbial modulation of host body composition and plasma metabolic profile. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Rampelli, S.; Schnorr, S.L.; Consolandi, C.; Turroni, S.; Severgnini, M.; Peano, C.; Brigidi, P.; Crittenden, A.N.; Henry, A.G.; Candela, M. Metagenome Sequencing of the Hadza Hunter-Gatherer Gut Microbiota. Curr. Biol. 2015, 25, 1682–1693. [Google Scholar] [CrossRef]
- Young, T.K.; Bjerregaard, P.; Dewailly, E.; Risica, P.M.; Jørgensen, M.E.; Ebbesson, S.E. Prevalence of Obesity and Its Metabolic Correlates Among the Circumpolar Inuit in 3 Countries. Am. J. Public Heal. 2007, 97, 691–695. [Google Scholar] [CrossRef]
- Girard, C.; Tromas, N.; Amyot, M.; Shapiro, B.J. Gut Microbiome of the Canadian Arctic Inuit. Msphere 2017, 2, e00297-16. [Google Scholar] [CrossRef]
- Prasoodanan PK, V.; Sharma, A.K.; Mahajan, S.; Dhakan, D.B.; Maji, A.; Scaria, J.; Sharma, V.K. Western and non-western gut microbiomes reveal new roles of Prevotella in carbohydrate metabolism and mouth–gut axis. npj Biofilms Microbiomes 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. [Google Scholar] [CrossRef] [PubMed]
- Woolcott, O.O.; Gutierrez, C.; Castillo, O.A.; Elashoff, R.M.; Stefanovski, D.; Bergman, R.N. Inverse association between altitude and obesity: A prevalence study among andean and low-altitude adult individuals of Peru. Obesity 2016, 24, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Amugsi, D.A.; Dimbuene, Z.T.; Mberu, B.; Muthuri, S.; Ezeh, A.C. Prevalence and time trends in overweight and obesity among urban women: An analysis of demographic and health surveys data from 24 African countries, 1991–2014. BMJ Open 2017, 7, e017344. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Estaki, M.; Gibson, D.L. Clinical Consequences of Diet-Induced Dysbiosis. Ann. Nutr. Metab. 2013, 63 (Suppl. 2), 28–40. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Christensen, L.; Vuholm, S.; Roager, H.M.; Nielsen, D.S.; Krych, L.; Kristensen, M.; Astrup, A.; Hjorth, M.F. Prevotella Abundance Predicts Weight Loss Success in Healthy, Overweight Adults Consuming a Whole-Grain Diet Ad Libitum: A Post Hoc Analysis of a 6-Wk Randomized Controlled Trial. J. Nutr. 2019, 149, 2174–2181. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Xin, S.-S.; Ding, L.-N.; Ding, W.-Y.; Hou, Y.-L.; Liu, C.-Q.; Zhang, X.-D. The Potential Role of Probiotics in Controlling Overweight/Obesity and Associated Metabolic Parameters in Adults: A Systematic Review and Meta-Analysis. Evidence-Based Complement. Altern. Med. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Rodriguez, J.; Zhang, Z.; Seethaler, B.; Sánchez, C.R.; Roumain, M.; Hiel, S.; Bindels, L.B.; Cani, P.D.; Paquot, N.; et al. Prebiotic dietary fibre intervention improves fecal markers related to inflammation in obese patients: Results from the Food4Gut randomized placebo-controlled trial. Eur. J. Nutr. 2021, 60, 3159–3170. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef] [PubMed]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef] [PubMed]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, G.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Nguyen, N.K.; Seethaler, B.; Beisner, J.; Kügler, P.; Stefan, T. Gut Microbiota Patterns Predicting Long-Term Weight Loss Success in Individuals with Obesity Undergoing Nonsurgical Therapy. Nutrients 2022, 14, 3182. [Google Scholar] [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2011, 36, 817–825. [Google Scholar] [CrossRef]
- Rong, B.; Wu, Q.; Saeed, M.; Sun, C. Gut microbiota—A positive contributor in the process of intermittent fasting-mediated obesity control. Anim. Nutr. 2021, 7, 1283–1295. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 682. [Google Scholar] [CrossRef]
- Mazier, W.; Le Corf, K.; Martinez, C.; Tudela, H.; Kissi, D.; Kropp, C.; Coubard, C.; Soto, M.; Elustondo, F.; Rawadi, G.; et al. A New Strain of Christensenella minuta as a Potential Biotherapy for Obesity and Associated Metabolic Diseases. Cells 2021, 10, 823. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Martinez, K.B.; Leone, V.; Chang, E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.R.; Ahire, J.J.; Jayanthi, N.; Tripathi, A.; Nanal, S. Effect of multi-strain probiotic (UB0316) in weight management in overweight/obese adults: A 12-week double blind, randomised, placebo-controlled study. Benef. Microbes 2019, 10, 855–866. [Google Scholar] [CrossRef]
- Czajeczny, D.; Kabzińska, K.; Wójciak, R.W. Does probiotic supplementation aid weight loss? A randomized, single-blind, placebo-controlled study with Bifidobacterium lactis BS01 and Lactobacillus acidophilus LA02 supplementation. Eat Weight Disord. 2021, 26, 1719–1727. [Google Scholar] [CrossRef]
- Cabral, L.Q.T.; Ximenez, J.A.; Moreno, K.G.T.; Fernandes, R. Probiotics have minimal effects on appetite-related hormones in overweight or obese individuals: A systematic review of randomized controlled trials. Clin. Nutr. 2020, 40, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front Nutr 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Lange, B.J.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; A Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2011, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Pagliai, G.; Russo, E.; Niccolai, E.; Dinu, M.; Di Pilato, V.; Magrini, A.; Bartolucci, G.; Baldi, S.; Menicatti, M.; Giusti, B.; et al. Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: The CARDIVEG Study. Eur. J. Nutr. 2020, 59, 2011–2024. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef]
- Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Leyrolle, Q.; Bindels, L.B.; da Silveria Cauduro, C.G.; Mulders, M.D.; Zamariola, G.; Azzi, A.-S.; et al. Link between gut microbiota and health outcomes in inulin -treated obese patients: Lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin. Nutr. 2020, 39, 3618–3628. [Google Scholar] [CrossRef] [PubMed]
- Mayengbam, S.; Lambert, J.E.; Parnell, J.A.; Tunnicliffe, J.M.; Nicolucci, A.C.; Han, J.; Sturzenegger, T.; Shearer, J.; Mickiewicz, B.; Vogel, H.J.; et al. Impact of dietary fiber supplementation on modulating microbiota–host–metabolic axes in obesity. J. Nutr. Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef]
- Li, Z.; Henning, S.M.; Lee, R.-P.; Lu, Q.-Y.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.-H.; Finegold, S.M.; et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef]
- Reider, S.; Watschinger, C.; Längle, J.; Pachmann, U.; Przysiecki, N.; Pfister, A.; Zollner, A.; Tilg, H.; Plattner, S.; Moschen, A.R. Short- and Long-Term Effects of a Prebiotic Intervention with Polyphenols Extracted from European Black Elderberry—Sustained Expansion of Akkermansia spp. J. Pers. Med. 2022, 12, 1479. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Lominchar, M.G.M.; Juan, C.S.; Larrosa, M. Microbiota Features Associated With a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.M.; Milan, A.M.; Mitchell, C.J.; Gillies, N.A.; D’Souza, R.F.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; et al. Protein Intake at Twice the RDA in Older Men Increases Circulatory Concentrations of the Microbiome Metabolite Trimethylamine-N-Oxide (TMAO). Nutrients 2019, 11, 2207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Luukkonen, P.; Sädevirta, S.; Yki-Järvinen, H.; Salonen, A. Impact of short-term overfeeding of saturated or unsaturated fat or sugars on the gut microbiota in relation to liver fat in obese and overweight adults. Clin. Nutr. 2020, 40, 207–216. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Akyon, Y.; Aydin-Kose, F.; Karagozlu, C.; Ozgen, A.G.; Brinkmann, A.; Nitsche, A.; Ergunay, K.; Yilmaz, E.; et al. Effects of Regular Kefir Consumption on Gut Microbiota in Patients with Metabolic Syndrome: A Parallel-Group, Randomized, Controlled Study. Nutrients 2019, 11, 2089. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2020, 106, 64–79. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Selma-Royo, M.; Arboleya, S.; Martínez-Costa, C.; Solís, G.; Suárez, M.; Fernández, N.; de los Reyes-Gavilán, C.G.; Díaz-Coto, S.; Martínez-Camblor, P.; et al. Levels of Predominant Intestinal Microorganisms in 1 Month-Old Full-Term Babies and Weight Gain during the First Year of Life. Nutrients 2021, 13, 2412. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 2013, 63, 559–566. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.; Jun, S.; Kozyrskyj, A. Impact of maternal smoking on the infant gut microbiota and its association with child overweight: A scoping review. World J. Pediatr. 2019, 15, 341–349. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kekkonen, R.A.; de Vos, W.M. Association of Early-Life Antibiotic Use and Protective Effects of Breastfeeding: Role of the Intestinal Microbiota. JAMA Pediatr. 2016, 170, 750–757. [Google Scholar] [CrossRef]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G559–G571. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise training modulates gut microbiota profile and improves endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of Gut Microbiota in the Aetiology of Obesity: Proposed Mechanisms and Review of the Literature. J. Obes. 2016, 2016, 7353642. [Google Scholar] [CrossRef]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.-L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. A High Protein Calorie Restriction Diet Alters the Gut Microbiome in Obesity. Nutrients 2020, 12, 3221. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.C.; Wang, Z.; Usyk, M.; Sotres-Alvarez, D.; Daviglus, M.L.; Schneiderman, N.; Talavera, G.A.; Gellman, M.D.; Thyagarajan, B.; Moon, J.-Y.; et al. Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity. Genome Biol 2019, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Raskine, L.; Simoneau, G.; Vicaut, E.; Neut, C.; Flourié, B.; Brouns, F.; Bornet, F.R. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: A double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am. J. Clin. Nutr. 2004, 80, 1658–1664. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jang, C.; Liu, J.; Uehara, K.; Gilbert, M.; Izzo, L.; Zeng, X.; Trefely, S.; Fernandez, S.; Carrer, A.; et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020, 579, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Dig. Liver Dis. 2016, 48, e268. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Prebiotic Capacity of Inulin-Type Fructans. J. Nutr. 2007, 137, 2503S–2506S. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Olivares, M.; Neyrinck, A.M.; Beaumont, M.; Kjølbæk, L.; Larsen, T.M.; Benítez-Páez, A.; Romaní-Pérez, M.; Garcia-Campayo, V.; Bosscher, D.; et al. Nutritional interest of dietary fiber and prebiotics in obesity: Lessons from the MyNewGut consortium. Clin. Nutr. 2019, 39, 414–424. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2018, 63, 27–34. [Google Scholar] [CrossRef]
- Kopf, J.C.; Suhr, M.J.; Clarke, J.; Eyun, S.-I.; Riethoven, J.-J.M.; Ramer-Tait, A.E.; Rose, D.J. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: A randomized controlled trial. Nutr. J. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Deehan, E.C.; Zhang, Z.; Riva, A.; Armet, A.M.; Perez-Muñoz, M.E.; Nguyen, N.K.; Krysa, J.A.; Seethaler, B.; Zhao, Y.-Y.; Cole, J.; et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 2022, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef]
- Han, K.; Bose, S.; Wang, J.-H.; Kim, B.-S.; Kim, M.J.; Kim, E.-J.; Kim, H. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, K.-Y. Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. J. Funct. Foods 2018, 47, 325–333. [Google Scholar] [CrossRef]
- Hric, I.; Ugrayová, S.; Penesová, A.; Rádiková, Ž.; Kubáňová, L.; Šardzíková, S.; Baranovičová, E.; Klučár, Ľ.; Beke, G.; Grendar, M.; et al. The Efficacy of Short-Term Weight Loss Programs and Consumption of Natural Probiotic Bryndza Cheese on Gut Microbiota Composition in Women. Nutrients 2021, 13, 1753. [Google Scholar] [CrossRef] [PubMed]
- Laster, J.; Bonnes, S.L.; Rocha, J. Increased Use of Emulsifiers in Processed Foods and the Links to Obesity. Curr. Gastroenterol. Rep. 2019, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Van De Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Halmos, E.P.; Mack, A.; Gibson, P.R. Review article: Emulsifiers in the food supply and implications for gastrointestinal disease. Aliment. Pharmacol. Ther. 2018, 49, 41–50. [Google Scholar] [CrossRef]
- Cox, L.M.; Blaser, M.J. Antibiotics in early life and obesity. Nat. Rev. Endocrinol. 2015, 11, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Million, M.; Kankoe, S.; Lagier, J.-C.; Armougom, F.; Giorgi, R.; Raoult, D. Abnormal Weight Gain and Gut Microbiota Modifications Are Side Effects of Long-Term Doxycycline and Hydroxychloroquine Treatment. Antimicrob. Agents Chemother. 2014, 58, 3342–3347. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Murphy, E.F.; O′Sullivan, O.; Lucey, A.; Humphreys, M.; Hogan, A.; Hayes, P.; O′Reilly, M.; Jeffery, I.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Willyard, C. Could baby′s first bacteria take root before birth? Nature 2018, 553, 264–266. [Google Scholar] [CrossRef]
- Martin, R.; Makino, H.; Cetinyurek Yavuz, A.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef]
- Reyman, M.; van Houten, M.A.; van Baarle, D.; Bosch, A.; Man, W.H.; Chu, M.; Arp, K.; Watson, R.L.; Sanders, E.A.M.; Fuentes, S.; et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef] [PubMed]
- Lavin, T.; Preen, D.B. Investigating Caesarean Section Birth as a Risk Factor for Childhood Overweight. Child Obes. 2018, 14, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, S.; Tong, O.S.; Woolcott, C.G. Association between caesarean section and childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 295–303. [Google Scholar] [CrossRef]
- Mueller, N.T.; Mao, G.; Bennet, W.L.; Hourigan, S.K.; Dominguez-Bello, M.G.; Appel, L.J.; Wang, X. Does vaginal delivery mitigate or strengthen the intergenerational association of overweight and obesity? Findings from the Boston Birth Cohort. Int. J. Obes. 2017, 41, 497–501. [Google Scholar] [CrossRef]
- Tun, H.M.; Bridgman, S.L.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Sears, M.R.; et al. Roles of Birth Mode and Infant Gut Microbiota in Intergenerational Transmission of Overweight and Obesity From Mother to Offspring. JAMA Pediatr. 2018, 172, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.D.P.; Ayres, L.F.A.; Barreto, D.S.; Henriques, B.D.; Prado, M.R.M.C.; Dos Passos, C.M. Acquisition of microbiota according to the type of birth: An integrative review. Rev. Latino-Americana de Enferm. 2021, 29, e3446. [Google Scholar] [CrossRef]
- Masukume, G.; McCarthy, F.P.; Baker, P.N.; Kenny, L.C.; Morton, S.M.; Murray, D.M.; Hourihane, J.O.B.; Khashan, A.S. Association between caesarean section delivery and obesity in childhood: A longitudinal cohort study in Ireland. BMJ Open 2019, 9, e025051. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Azad, M.B.; Vehling, L.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Field, C.J.; Lefebvre, D.; Sears, M.R.; Becker, A.B.; et al. Association of Exposure to Formula in the Hospital and Subsequent Infant Feeding Practices With Gut Microbiota and Risk of Overweight in the First Year of Life. JAMA Pediatr. 2018, 172, e181161. [Google Scholar] [CrossRef] [PubMed]
- Qamar, N.; Castano, D.; Patt, C.; Chu, T.; Cottrell, J.; Chang, S.L. Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact. Behav. Brain Res. 2019, 376, 112196. [Google Scholar] [CrossRef]
| Population | Dietary Pattern | Microbiome Diversity | Obesity Prevalence | Reference |
|---|---|---|---|---|
| Hadza tribe | Predominantly plant-based diet | ↑Prevotella ↑Bacteroidetes ↑Treponema | <5% | [18] |
| Inuit | High in animal fat and protein, low in dietary fibre | ↓Prevotella ↓Akkermansia muciniphila | 20.6% | [19,20] |
| Western population US Netherlands Italy Spain | Western diet (high in fat, sugar, sodium, animal protein, processed food; low in fruits, vegetables, whole grains, and dietary fibre) | ↑Bacteroides ↓Prevotella | 38.2% (US) 12.8% (Netherlands) 9.8% (Italy) 16.7% (Spain) | [21,22] |
| Non-western populations parts of central and northern India Peru Madagascar | Agricultural diets, predominantly containing plant-based components with the presence of animal-based components | ↑Prevotella ↓Bacteroides | 5% (India) 26.3% (Peru) 4% (Madagascar) | [22,23,24] |
| Diet or Dietary Pattern | Impact on Microbiome | Impact on Host | Reference |
|---|---|---|---|
| Vegan/vegetarian diet | ↑Prevotella | ↓Visceral fat ↓Body mass ↓Inflammation Promote gut barrier integrity via anti-tumorigenesis | [48,49,50,51,52] |
| ↑Roseburia | |||
| ↑Ruminococcus | |||
| ↑Bifidobacterium | |||
| ↓E. coli | |||
| ↓Firmicutes | |||
| ↑E. rectale | |||
| ↑F. prausnitzii | |||
| ↑Anaerostipes | |||
| ↑Streptococcus | |||
| ↑Odoribacter | |||
| ↑Clostridium sensu stricto | |||
| Vegan diet with low fat | ↑Bacteroidetes | ↓Body mass ↓Body fat ↓Visceral fat ↑Insulin sensitivity | [53] |
| ↑C.clostridioforme | |||
| ↑Faecalibacterium prausnitzii | |||
| ↓Firmicutes | |||
| Dietary fibre | ↑Prevotella | ↑SCFA synthesis ↓Body mass | [49] |
| ↑Lactobacillus | |||
| ↑Ruminococcus bromii | |||
| ↓Firmicutes | |||
| Inulin | ↑Actinobacteria | ↑Insulin sensitivity ↓Body weight ↓BMI ↓Fat mass ↓Visceral fat | [54,55] |
| ↓Clostridia | |||
| ↓B. obeum | |||
| ↓B. luti | |||
| ↓B. faecis | |||
| ↓R. faecis | |||
| ↓Oscillibacter | |||
| ↑Bifidobacterium | |||
| ↑Catenibacterium | |||
| ↓Desulfovibrio | |||
| ↓Roseburia | |||
| Mixed fibre (mixture of soluble and insoluble fiber with a greater proportion of insoluble) | ↑Barnesiellaceae | ↑Acetate production ↓Isovalerate production Moderate effect on microbiota composition | [56,57] |
| ↑Lachnospira | |||
| ↓Actinomycetaceae | |||
| ↓Enterobacteriaceae | |||
| Resistant starch | ↓Firmicutes | ↓Abdominal fat | [58] |
| ↑Bacteroidetes | |||
| Polyphenols | ↑Lactobacillus | Increase or maintenance of body mass ↓Inflammation | [48,59,60] |
| ↑Bifidobacterium | |||
| ↑Akkermansia muciniphila | |||
| ↓Clostridium | |||
| Western diet | ↑E. coli | ↑Dysbiosis ↑Inflammation ↑Obesity ↑Inflammatory bowel disease | [42,49,51] |
| ↑Firmicutes | |||
| ↑Alistipes | |||
| ↑Bilophila | |||
| ↑Bacteroides | |||
| ↓Roseburia | |||
| ↓Eubacterium rectale | |||
| ↓Ruminococcus bromii | |||
| High saturated fat | ↑Proteobacteria | Correlations with obesity Weight gain ↓Gut microbiome diversity | [6,49,61,62] |
| ↑Firmicutes | |||
| ↓Bacteroidetes | |||
| ↓Akkermansia muciniphilia | |||
| ↑Anaerotruncus genus | |||
| ↑Eisenbergiella | |||
| ↑Lachnospiraceae | |||
| ↑Campylobacter | |||
| ↑Flavonifractor | |||
| ↑Erysipelatoclostridium | |||
| High protein | ↑Bacteroides | ↓SCFA synthesis ↑Formation of nitrogen compounds | [49,63,64,65] |
| ↑Faecalibacterium | |||
| ↑Sutterella | |||
| ↑Clostridium | |||
| ↑Eisenbergiella | |||
| ↓Bifidobacterium | |||
| ↓Roseburia | |||
| High sugar | ↑Acinetobacter | Bacterial overgrowth associated with obesity ↑Production of endogenous ethanol ↑The risk of non-alcoholic fatty liver disease ↑Pro-inflammatory properties promoting metabolic endotoxemia and low-grade inflammation | [66,67,68] |
| ↑Blautia | |||
| ↑Dorea | |||
| ↑Lactococcus | |||
| ↑Escherichia coli | |||
| ↑Proteobacteria | |||
| ↓Bacteroidetes | |||
| Fermented foods | ↑All gut diversity | ↓Inflammation Body mass maintenance | [69,70] |
| Fasting | ↑Akkermansia muciniphilia | ↓Body fat ↑SCFA production ↓Levels of LPS | [38,71,72] |
| ↑Spirochaetes | |||
| ↑Roseburia |
| Lifestyle and Environment Factors | Model | Impact on Microbiome | Impact on Host | Reference |
|---|---|---|---|---|
| Birth by caesarean section | 6–12 month old infants | ↑Staphylococcus ↓Bacteroidetes | ↑Risk of obesity | [73,74] |
| Maternal smoking | 3 month old infants | ↑Firmicutes | ↑Risk of obesity between 0–3 years | [75] |
| Antibiotics consumption | Healthy children | ↓Bifidobacterium ↓Akkermansia muciniphilia | ↑Risk of obesity | [76] |
| Stress | Norwegian soldiers | ↑Firmicutes ↓Bacteroidetes | Increase in intestinal permeability under stress | [77] |
| Physical activity | Subjects with prediabetes and type 2 diabetes | ↓Firmucites ↑Bacteroidetes ↑Clostridium genus | ↓Endotoxemia ↑Insulin sensitivity | [78] |
| Alcohol | Alcohol dependent patients with high or low intestinal permeability | ↓Ruminococcus ↓Faecalibacterium ↓Subdoligranulum ↓Oscillibacter ↓Anaerofilum | ↓In the overall bacterial load lead to dysbiosis | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsálig, D.; Berta, A.; Tóth, V.; Szabó, Z.; Simon, K.; Figler, M.; Pusztafalvi, H.; Polyák, É. A Review of the Relationship between Gut Microbiome and Obesity. Appl. Sci. 2023, 13, 610. https://doi.org/10.3390/app13010610
Zsálig D, Berta A, Tóth V, Szabó Z, Simon K, Figler M, Pusztafalvi H, Polyák É. A Review of the Relationship between Gut Microbiome and Obesity. Applied Sciences. 2023; 13(1):610. https://doi.org/10.3390/app13010610
Chicago/Turabian StyleZsálig, Dorottya, Anikó Berta, Vivien Tóth, Zoltán Szabó, Klára Simon, Mária Figler, Henriette Pusztafalvi, and Éva Polyák. 2023. "A Review of the Relationship between Gut Microbiome and Obesity" Applied Sciences 13, no. 1: 610. https://doi.org/10.3390/app13010610
APA StyleZsálig, D., Berta, A., Tóth, V., Szabó, Z., Simon, K., Figler, M., Pusztafalvi, H., & Polyák, É. (2023). A Review of the Relationship between Gut Microbiome and Obesity. Applied Sciences, 13(1), 610. https://doi.org/10.3390/app13010610






