Wall Shear Stress Measurement in Carotid Artery Phantoms with Variation in Degree of Stenosis Using Plane Wave Vector Doppler
Abstract
1. Introduction
2. Materials and Methods
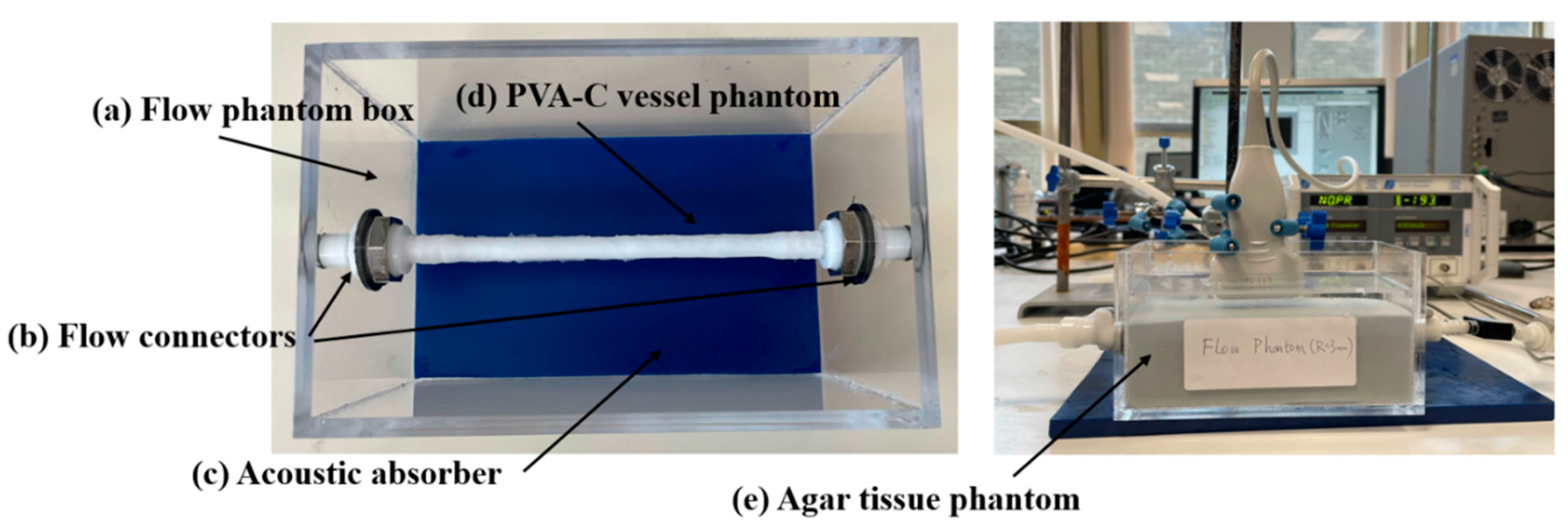
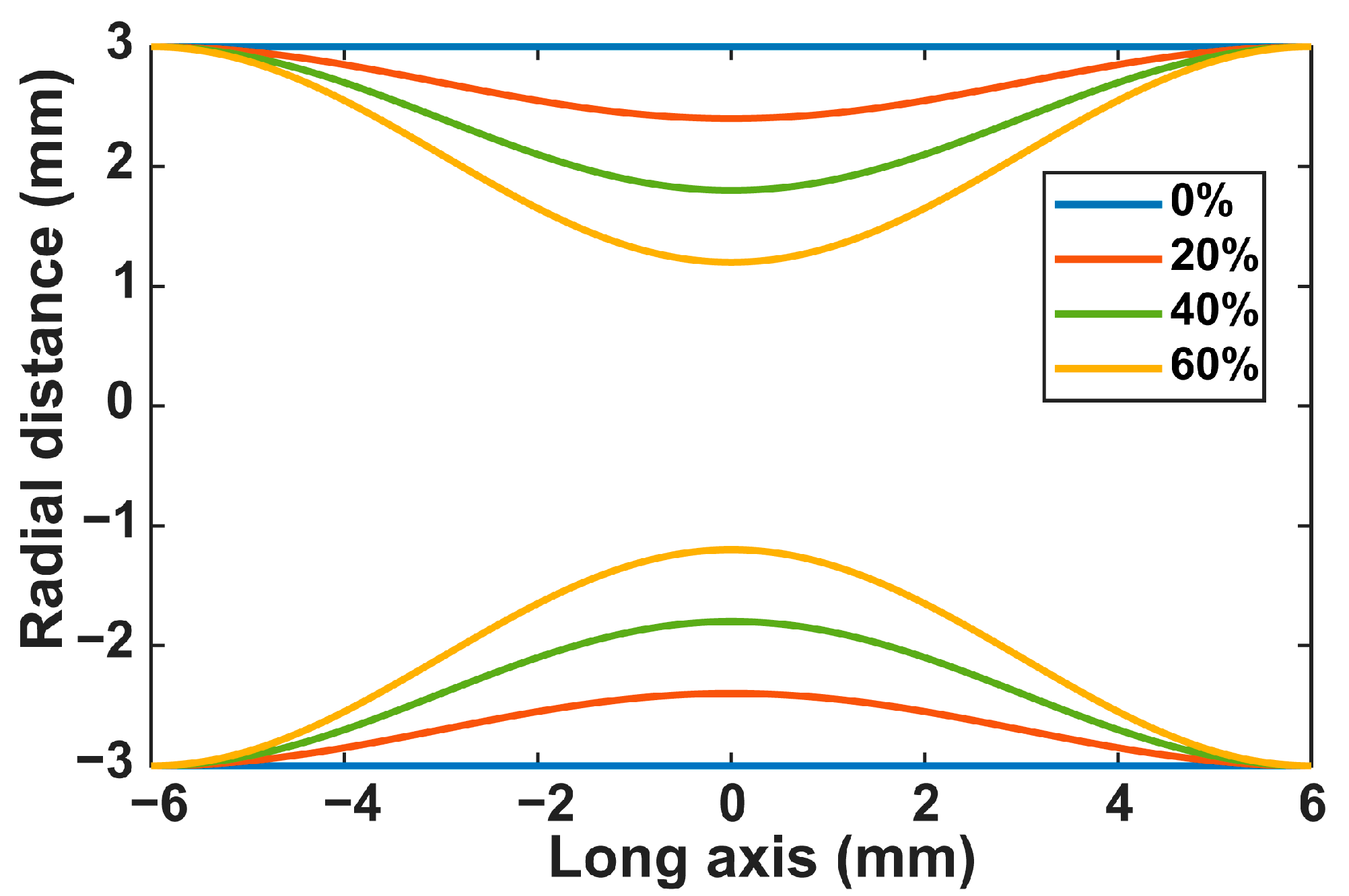
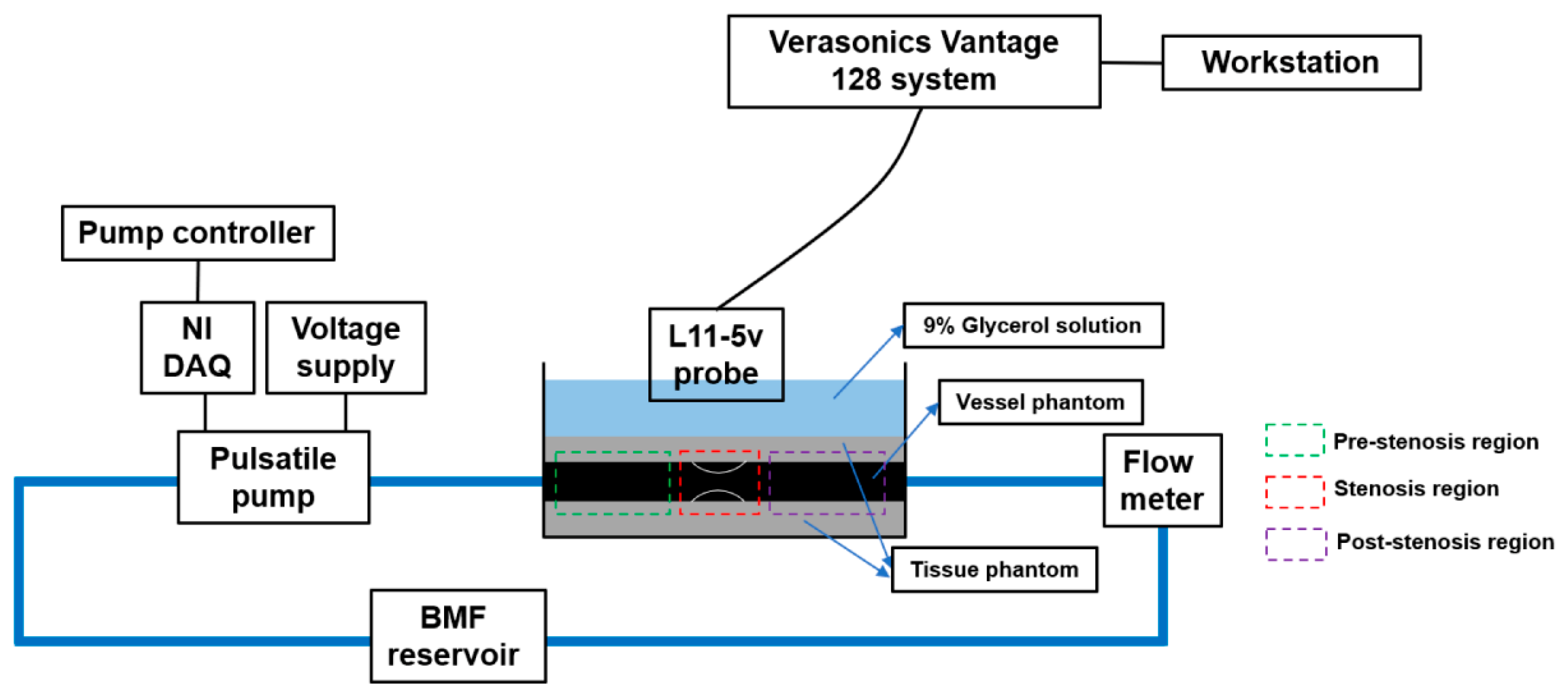
2.1. Flow Circuit Setup
2.2. Ultrasound Simulation Setup
2.3. Ultrasound Experimental Setup
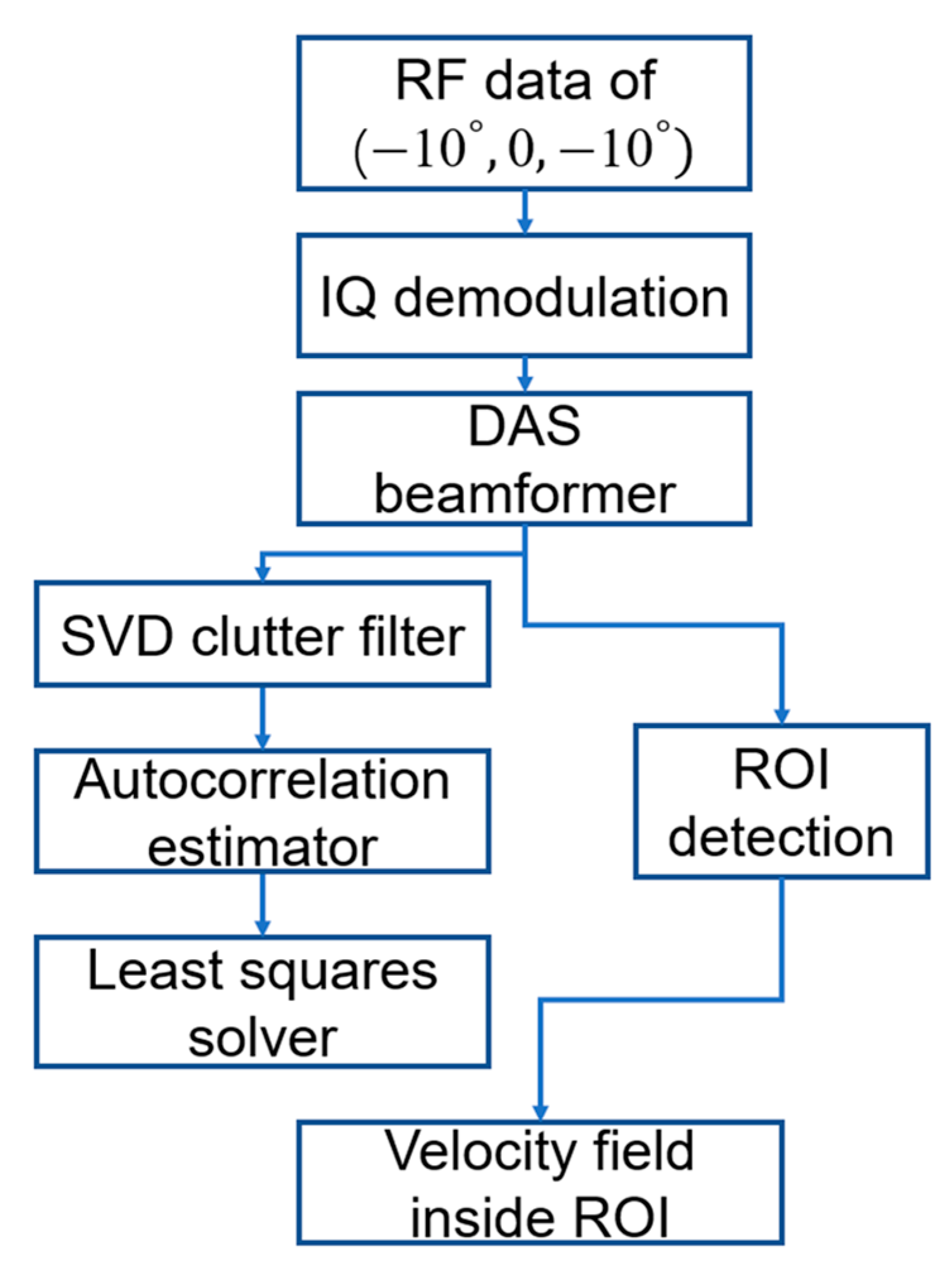
2.4. Ultrasound Signal Processing
2.5. Wall Shear Stress Estimation
2.6. Performance Evaluation
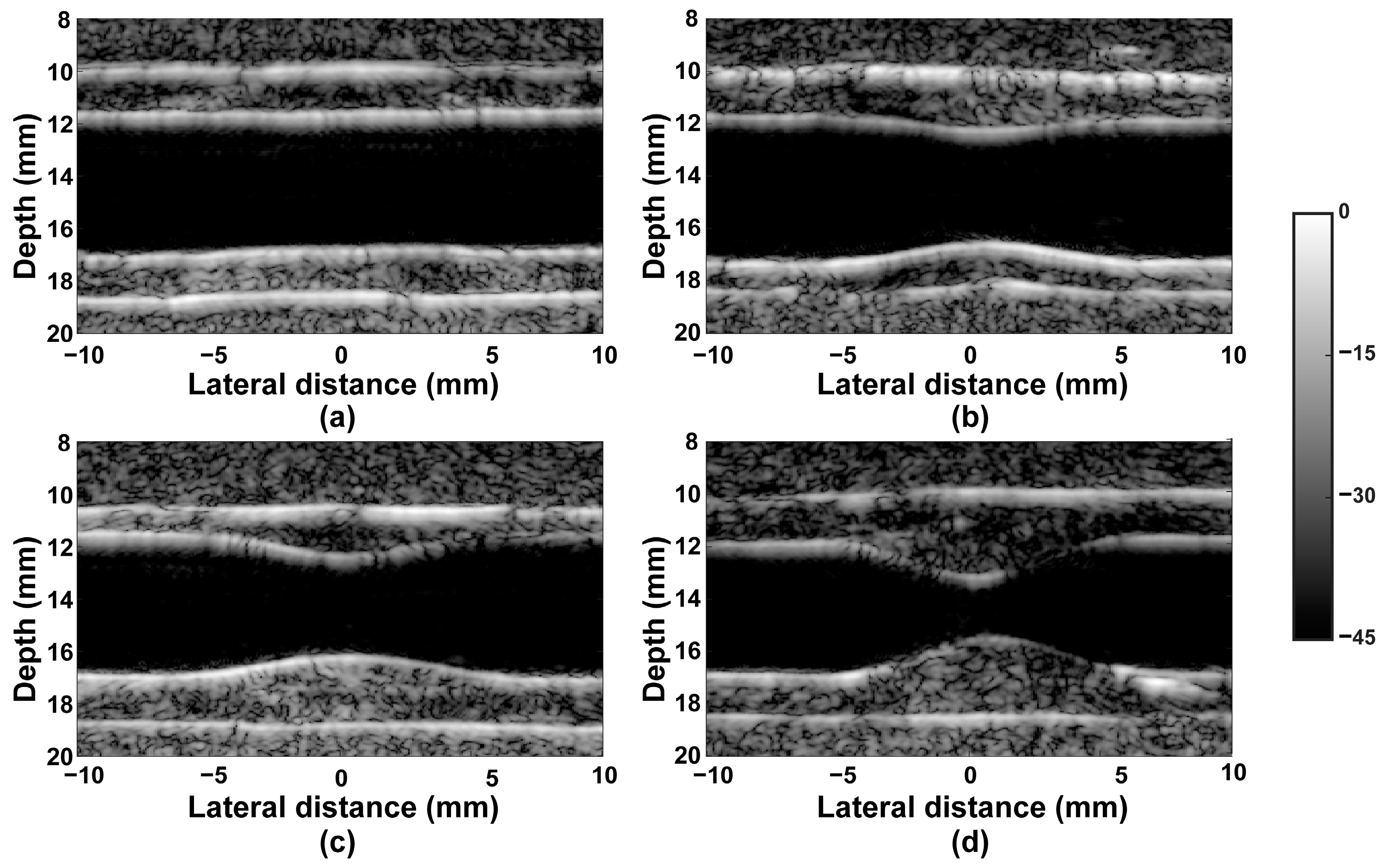
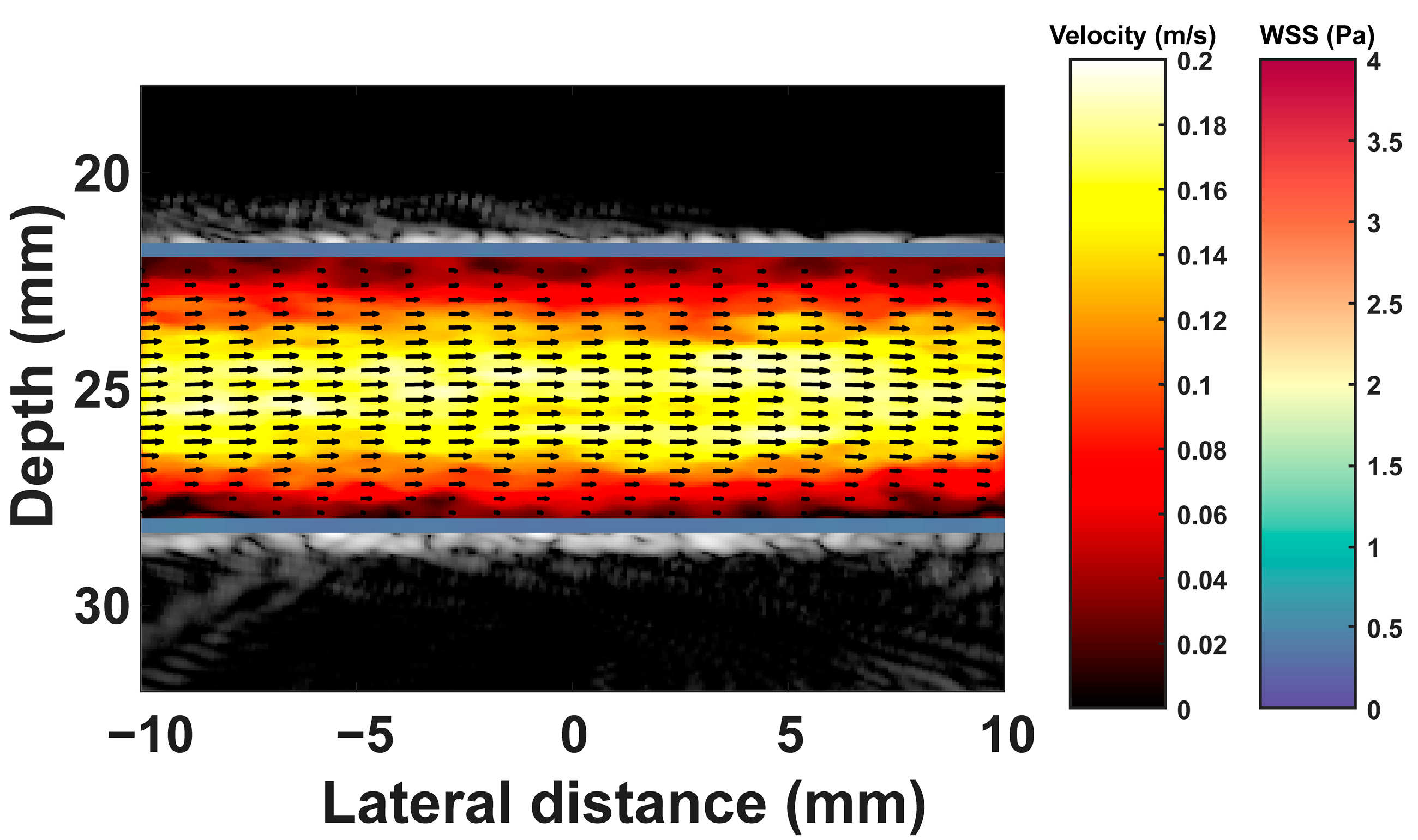
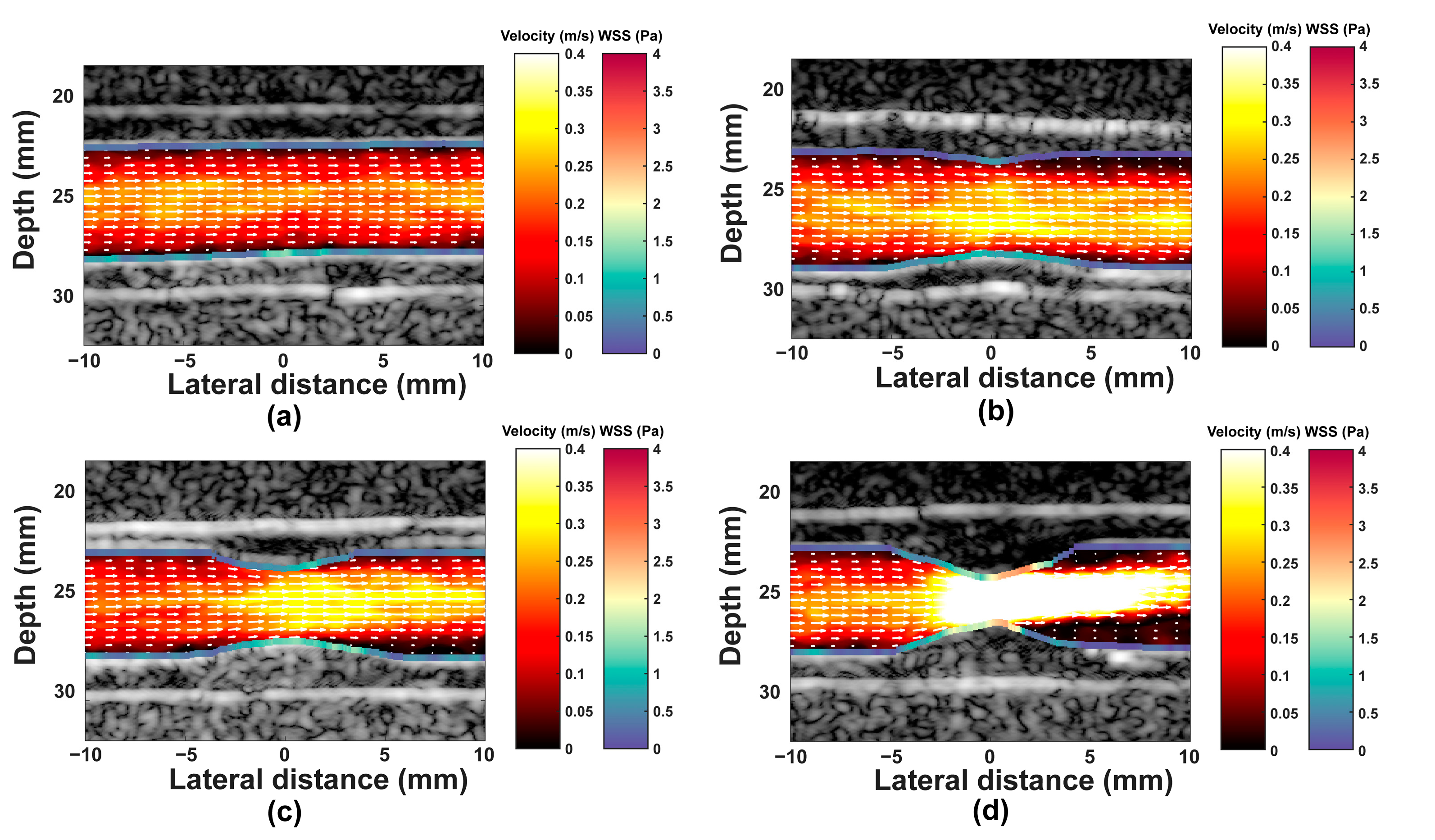
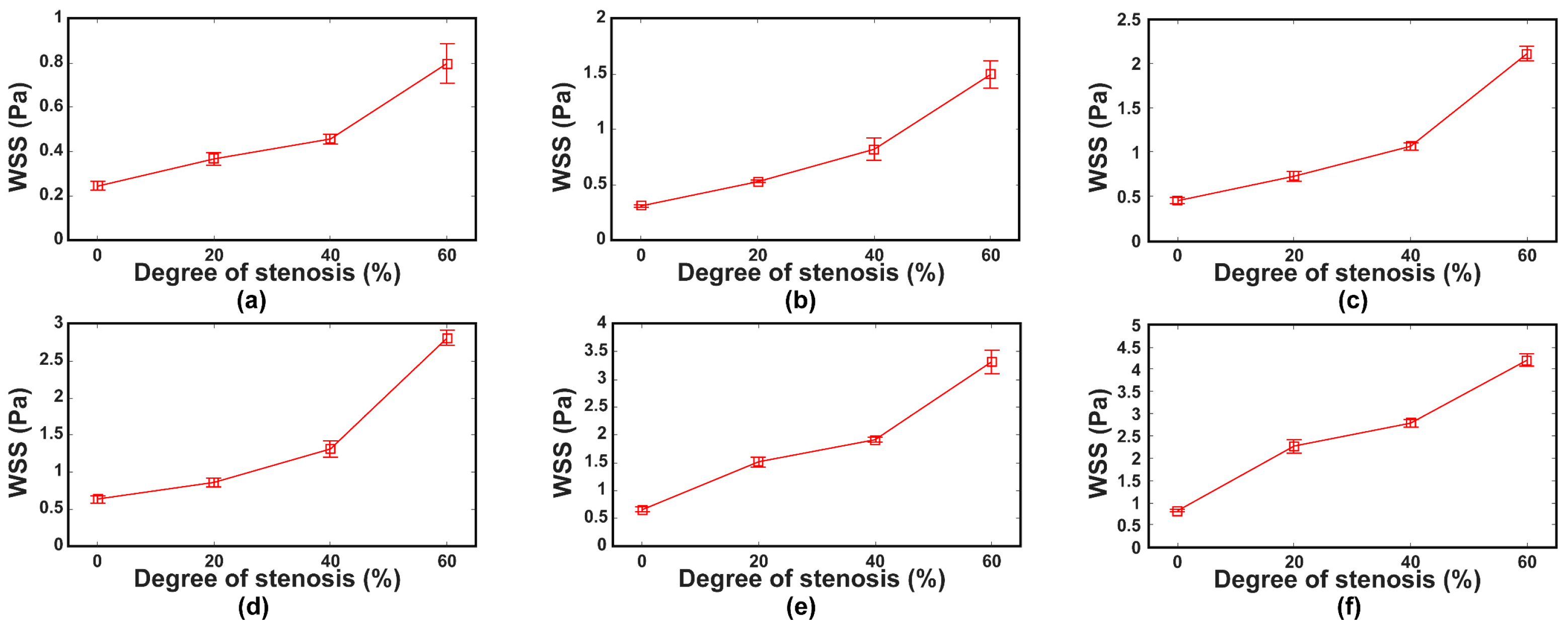
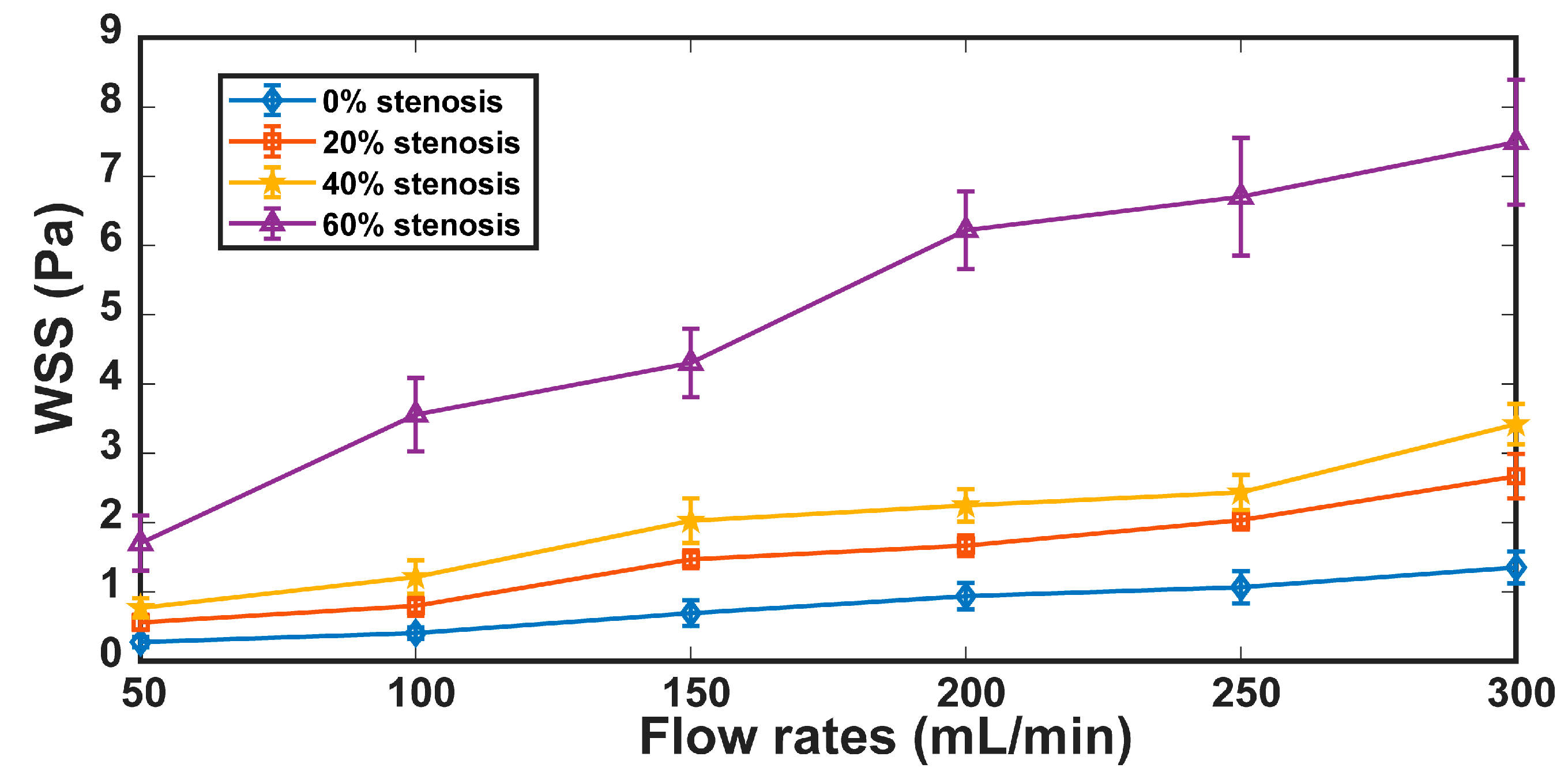
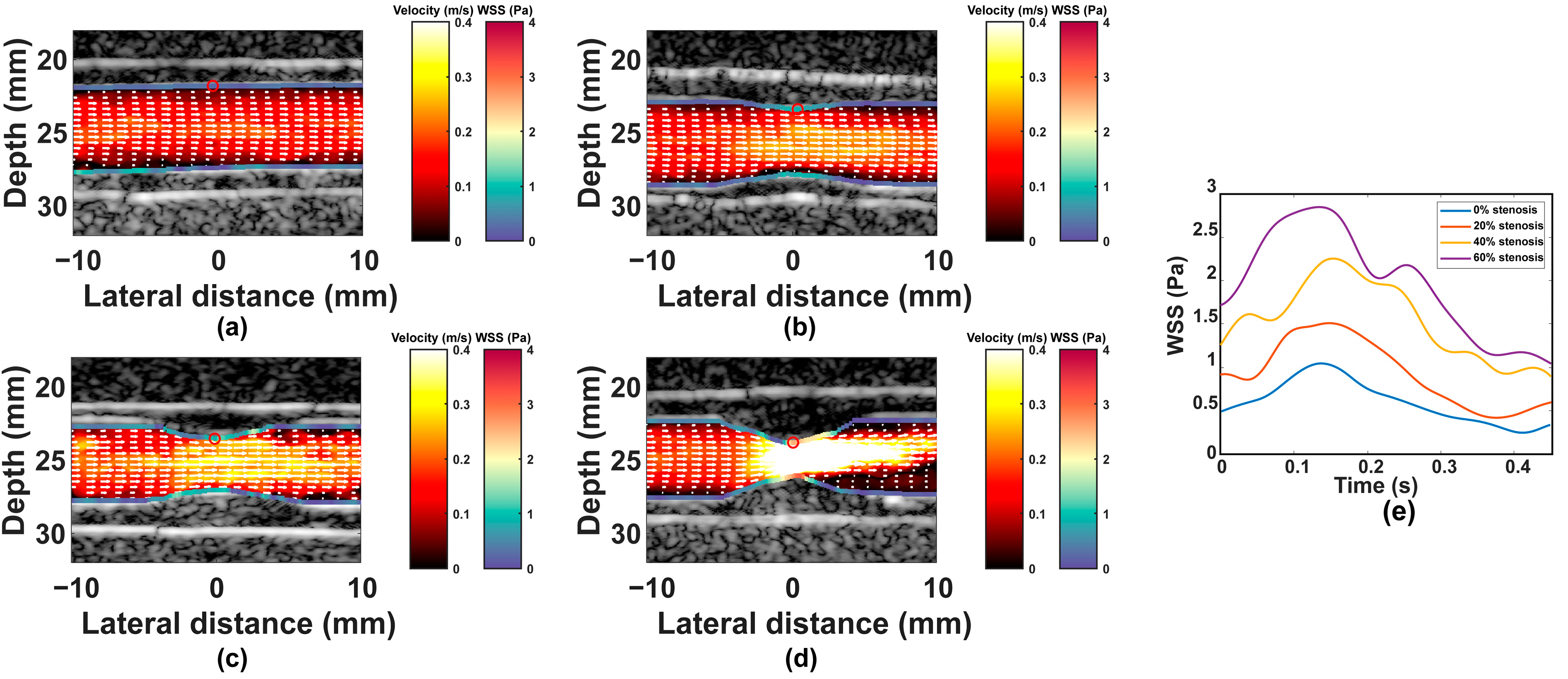
3. Results
3.1. Results
3.2. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.R.; Lawford, P.V.; Doyle, B.J. Cardiovascular Biomechanics; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Chaer, R.A.; Abularrage, C.J.; Coleman, D.M.; Eslami, M.H.; Kashyap, V.S.; Rockman, C.; Murad, M.H. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J. Vasc. Surg. 2020, 72, 3S–39S. [Google Scholar] [CrossRef] [PubMed]
- Oates, C.P.; Naylor, A.R.; Hartshorne, T.; Charles, S.M.; Fail, T.; Humphries, K.; Aslam, M.; Khodabakhsh, P. Joint Recommendations for Reporting Carotid Ultrasound Investigations in the United Kingdom. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 251–261. [Google Scholar] [CrossRef]
- Slager, C.J.; Wentzel, J.J.; Gijsen, F.J.; Schuurbiers, J.C.; van der Wal, A.C.; van der Steen, A.F.; Serruys, P.W. The role of shear stress in the generation of rupture-prone vulnerable plaques. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Slager, C.J.; Wentzel, J.J.; Gijsen, F.J.; Thury, A.; van der Wal, A.C.; Schaar, J.A.; Serruys, P.W. The role of shear stress in the destabilization of vulnerable plaques and related therapeutic implications. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 456–464. [Google Scholar] [CrossRef]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Steinman, D.A.; Thomas, J.B.; Ladak, H.M.; Milner, J.S.; Rutt, B.K.; Spence, J.D. Reconstruction of carotid bifurcation hemodynamics and wall thickness using computational fluid dynamics and MRI. Magn. Reson. Med. 2002, 47, 149–159. [Google Scholar] [CrossRef]
- Köhler, U.; Marshall, I.; Robertson, M.B.; Long, Q.; Xu, X.Y.; Hoskins, P.R. MRI measurement of wall shear stress vectors in bifurcation models and comparison with CFD predictions. J. Magn. Reson. Imaging 2001, 14, 563–573. [Google Scholar] [CrossRef]
- Ricci, S.; Swillens, A.; Ramalli, A.; Segers, P.; Tortoli, P. Wall shear rate measurement: Validation of a new method through multiphysics simulations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2017, 64, 66–77. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, C.; Stephen, G.; Khan, F.; Corner, G.A.; Hoskins, P.R.; Huang, Z. Investigation of Ultrasound-Measured Flow Velocity, Flow Rate and Wall Shear Rate in Radial and Ulnar Arteries Using Simulation. Ultrasound Med. Biol. 2017, 43, 981–992. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brands, P.J.; Hoeks, A.P.; Hofstra, L.; Reneman, R.S. Reneman. A noninvasive method to estimate wall shear rate using ultrasound. Ultrasound Med. Biol. 1995, 21, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Montaldo, G.; Loupas, T.; Savery, D.; Mézière, F.; Fink, M.; Tanter, M. Ultrafast compound Doppler imaging: Providing full blood flow characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2011, 58, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Ekroll, I.K.; Swillens, A.; Segers, P.; Dahl, T.; Torp, H.; Lovstakken, L. Simultaneous quantification of flow and tissue velocities based on multi-angle plane wave imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013, 60, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Yiu, B.Y.; Lai, S.S.; Yu, A.C. Vector projectile imaging: Time-resolved dynamic visualization of complex flow patterns. Ultrasound Med. Biol. 2014, 40, 2295–2309. [Google Scholar] [CrossRef]
- Karageorgos, G.M.; Apostolakis, I.Z.; Nauleau, P.; Gatti, V.; Weber, R.; Kemper, P.; Konofagou, E.E. Pulse wave imaging coupled with vector flow mapping: A phantom, simulation, and in vivo study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2021, 68, 2516–2531. [Google Scholar] [CrossRef]
- Yang, K.; Hoskins, P.R.; Corner, G.A.; Xia, C.; Huang, Z. Vector flow imaging by plane wave speckle tracking based on different beamformers. In Proceedings of the 2022 7th International Conference on Signal and Image Processing (ICSIP), Nanjing, China, 20–22 July 2022; pp. 563–566. [Google Scholar]
- Hasegawa, H.; Mozumi, M.; Omura, M.; Nagaoka, R.; Saito, K. Measurement of flow velocity vectors in carotid artery using plane wave imaging with repeated transmit sequence. J. Med. Ultrason. 2021, 48, 417–427. [Google Scholar] [CrossRef]
- Kenwright, D.A.; Anderson, T.; Moran, C.M.; Hoskins, P.R. Assessment of spectral Doppler for an array-based preclinical ultrasound scanner using a rotating phantom. Ultrasound Med. Biol. 2015, 41, 2232–2239. [Google Scholar] [CrossRef]
- Chee, A.J.; Ho, C.K.; Yiu, B.Y.; Yu, A.C. Walled carotid bifurcation phantoms for imaging investigations of vessel wall motion and blood flow dynamics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 1852–1864. [Google Scholar] [CrossRef]
- Shimizu, M.; Tanaka, T.; Okada, T.; Seki, Y.; Nishiyama, T. Wall shear stress measurement method based on parallel flow model near vascular wall in echography. Jpn. J. Appl. Phys. 2017, 56, 07JF08. [Google Scholar] [CrossRef][Green Version]
- Nagaoka, R.; Ishikawa, K.; Mozumi, M.; Cinthio, M.; Hasegawa, H. Basic study on estimation method of wall shear stress in common carotid artery using blood flow imaging. Jpn. J. Appl. Phys. 2020, 59, SKKE16. [Google Scholar] [CrossRef]
- Chayer, B.; van den Hoven, M.; Cardinal, M.R.; Li, H.; Swillens, A.; Lopata, R.; Cloutier, G. Atherosclerotic carotid bifurcation phantoms with stenotic soft inclusions for ultrasound flow and vessel wall elastography imaging. Phys. Med. Biol. 2019, 64, 095025. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.R.; Easson, W.J.; Hoskins, P.R. A dual-phantom system for validation of velocity measurements in stenosis models under steady flow. Ultrasound Med. Biol. 2009, 35, 1510–1524. [Google Scholar] [CrossRef]
- Zhou, X.; Kenwright, D.A.; Wang, S.; Hossack, J.A.; Hoskins, P.R. Fabrication of two flow phantoms for Doppler ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2017, 64, 53–65. [Google Scholar] [CrossRef]
- Goudot, G.; Poree, J.; Pedreira, O.; Khider, L.; Julia, P.; Alsac, J.M.; Laborie, E.; Mirault, T.; Tanter, M.; Messas, E.; et al. Wall shear stress measurement by ultrafast vector flow imaging for atherosclerotic carotid stenosis. Ultraschall Med. 2021, 42, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.C.; Huang, H.; Chang, W.T.; Huang, C.C. Wall shear stress mapping for human femoral artery based on ultrafast ultrasound vector Doppler estimations. Med. Phys. 2021, 48, 6755–6764. [Google Scholar] [CrossRef] [PubMed]
- Ekroll, I.K.; Perrot, V.; Liebgott, H.; Avdal, J. Tapered vector Doppler for improved quantification of low velocity blood flow. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2021, 68, 1017–1031. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Giddens, D.P. Flow disturbance measurements through a constricted tube at moderate Reynolds numbers. J. Biomech. 1983, 16, 955–963. [Google Scholar] [CrossRef]
- Li, M.X.; Beech-Brandt, J.J.; John, L.R.; Hoskins, P.R.; Easson, W.J. Numerical analysis of pulsatile blood flow and vessel wall mechanics in different degrees of stenoses. J. Biomech. 2007, 40, 3715–3724. [Google Scholar] [CrossRef]
- Zell, K.; Sperl, J.I.; Vogel, M.W.; Niessner, R.; Haisch, C. Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys. Med. Biol. 2007, 52, N475–N484. [Google Scholar] [CrossRef]
- Ramnarine, K.V.; Nassiri, D.K.; Hoskins, P.R.; Lubbers, J. Validation of a new blood-mimicking fluid for use in Doppler flow test objects. Ultrasound Med. Biol. 1998, 24, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Svendsen, N.B. Calculation of pressure fields from arbitrarily shaped, apodized, and excited ultrasound transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1992, 39, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A. Field: A program for simulating ultrasound systems. In Proceedings of the 10th Nordic-Baltic Conference on Biomedical Imaging, Medical and Biological Engineering and Computing, Tampere, Finland, 9–13 June 1996; pp. 351–353. [Google Scholar]
- Montaldo, G.; Tanter, M.; Bercoff, J.; Benech, N.; Fink, M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2009, 56, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Baranger, J.; Arnal, B.; Perren, F.; Baud, O.; Tanter, M.; Demene, C. Adaptive spatiotemporal svd clutter filtering for ultrafast Doppler imaging using similarity of spatial singular vectors. IEEE Trans. Med. Imaging 2018, 37, 1574–1586. [Google Scholar] [CrossRef]
- Kasai, C.; Namekawa, K.; Koyano, A.; Omoto, R. Real-time two-dimensional blood flow imaging using an autocorrelation technique. IEEE Trans. Sonics Ultrason. 1985, 32, 458–464. [Google Scholar] [CrossRef]
- Blake, J.R.; Meagher, S.; Fraser, K.H.; Easson, W.J.; Hoskins, P.R. A method to estimate wall shear rate with a clinical ultrasound scanner. Ultrasound Med. Biol. 2008, 34, 760–774. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Stefanadis, C.J.H.J.C. Vascular wall shear stress: Basic principles and methods. Hell. J. Cardiol. 2005, 46, 9–15. [Google Scholar]
- Leow, C.H.; Tang, M.X. Spatio-temporal flow and wall shear stress mapping based on incoherent ensemble-correlation of ultrafast contrast enhanced ultrasound images. Ultrasound Med. Biol. 2018, 44, 134–152. [Google Scholar] [CrossRef]
- Chee, A.J.Y.; Yiu, B.Y.S.; Ho, C.K.; Yu, A.C.H. Arterial phantoms with regional variations in wall stiffness and thickness. Ultrasound Med. Biol. 2018, 44, 872–883. [Google Scholar] [CrossRef]
- Jensen, J.; Villagomez Hoyos, C.A.; Stuart, M.B.; Ewertsen, C.; Nielsen, M.B.; Jensen, J.A. Fast plane wave 2-d vector flow imaging using transverse oscillation and directional beamforming. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2017, 64, 1050–1062. [Google Scholar] [CrossRef]
- Polak, J.F.; Alessi-Chinetti, J.M.; Kremkau, F.W. Doppler velocity estimates of internal carotid artery stenosis: Angle correction parallel to the color Doppler lumen versus parallel to the artery wall. J. Ultrasound Med. 2019, 38, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Poree, J.; Pellissier, A.; Chayer, B.; Tournoux, F.; Cloutier, G.; Garcia, D. Staggered multiple-prf ultrafast color Doppler. IEEE Trans. Med. Imaging 2016, 35, 1510–1521. [Google Scholar] [CrossRef]
- Poree, J.; Goudot, G.; Pedreira, O.; Laborie, E.; Khider, L.; Mirault, T.; Messas, E.; Julia, P.; Alsac, J.M.; Tanter, M.; et al. Dealiasing high-frame-rate color Doppler using dual-wavelength processing. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2021, 68, 2117–2128. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, C.; Khan, F.; Corner, G.A.; Huang, Z.; Hoskins, P.R. Investigation of ultrasound-measured flow rate and wall shear rate in wrist arteries using flow phantoms. Ultrasound Med. Biol. 2016, 42, 815–823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fekkes, S.; Saris, A.; Nillesen, M.M.; Menssen, J.; Hansen, H.H.G.; de Korte, C.L. Simultaneous vascular strain and blood vector velocity imaging using high-frequency versus conventional-frequency plane wave ultrasound: A phantom study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2018, 65, 1166–1181. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.J. Ultrasound deep learning for wall segmentation and near-wall blood flow measurement. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2020, 67, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Dorazil, J.; Repp, R.; Kropfreiter, T.; Pruller, R.; Riha, K.; Hlawatsch, F. Tracking carotid artery wall motion using an unscented Kalman filter and data fusion. IEEE Access 2020, 8, 222506–222519. [Google Scholar] [CrossRef]
- Tang, M.X.; Mulvana, H.; Gauthier, T.; Lim, A.K.; Cosgrove, D.O.; Eckersley, R.J.; Stride, E. Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability. Interface Focus 2011, 1, 520–539. [Google Scholar] [CrossRef]
- Timofeeva, M.; Ooi, A.; Poon, E.K.W.; Barlis, P. Numerical simulation of the blood flow through the coronary artery stenosis: Effects of varying eccentricity. Comput. Biol. Med. 2022, 146, 105672. [Google Scholar] [CrossRef]
| Parameters | Simulation | Experiment |
|---|---|---|
| Probe | N/A | L11-5v |
| Steering angle | −10°, 0, 10° | −10°, 0, 10° |
| Transmission frequency | 6.25 MHz | 6.25 MHz |
| Number of elements | 128 | 128 |
| Number of transmit/receive elements | 128 | 128 |
| PRF | 10 kHz | 10 kHz |
| Sampling frequency | 50 MHz | 25 MHz |
| Pulse length | 2.5 cycles | 2.5 cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Hoskins, P.R.; Corner, G.A.; Xia, C.; Huang, Z. Wall Shear Stress Measurement in Carotid Artery Phantoms with Variation in Degree of Stenosis Using Plane Wave Vector Doppler. Appl. Sci. 2023, 13, 617. https://doi.org/10.3390/app13010617
Yang K, Hoskins PR, Corner GA, Xia C, Huang Z. Wall Shear Stress Measurement in Carotid Artery Phantoms with Variation in Degree of Stenosis Using Plane Wave Vector Doppler. Applied Sciences. 2023; 13(1):617. https://doi.org/10.3390/app13010617
Chicago/Turabian StyleYang, Ke, Peter R. Hoskins, George A. Corner, Chunming Xia, and Zhihong Huang. 2023. "Wall Shear Stress Measurement in Carotid Artery Phantoms with Variation in Degree of Stenosis Using Plane Wave Vector Doppler" Applied Sciences 13, no. 1: 617. https://doi.org/10.3390/app13010617
APA StyleYang, K., Hoskins, P. R., Corner, G. A., Xia, C., & Huang, Z. (2023). Wall Shear Stress Measurement in Carotid Artery Phantoms with Variation in Degree of Stenosis Using Plane Wave Vector Doppler. Applied Sciences, 13(1), 617. https://doi.org/10.3390/app13010617






