Microencapsulation of Lactobacillus plantarum 299v Strain with Whey Proteins by Lyophilization and Its Application in Production of Probiotic Apple Juices
Abstract
Featured Application
Abstract
1. Introduction
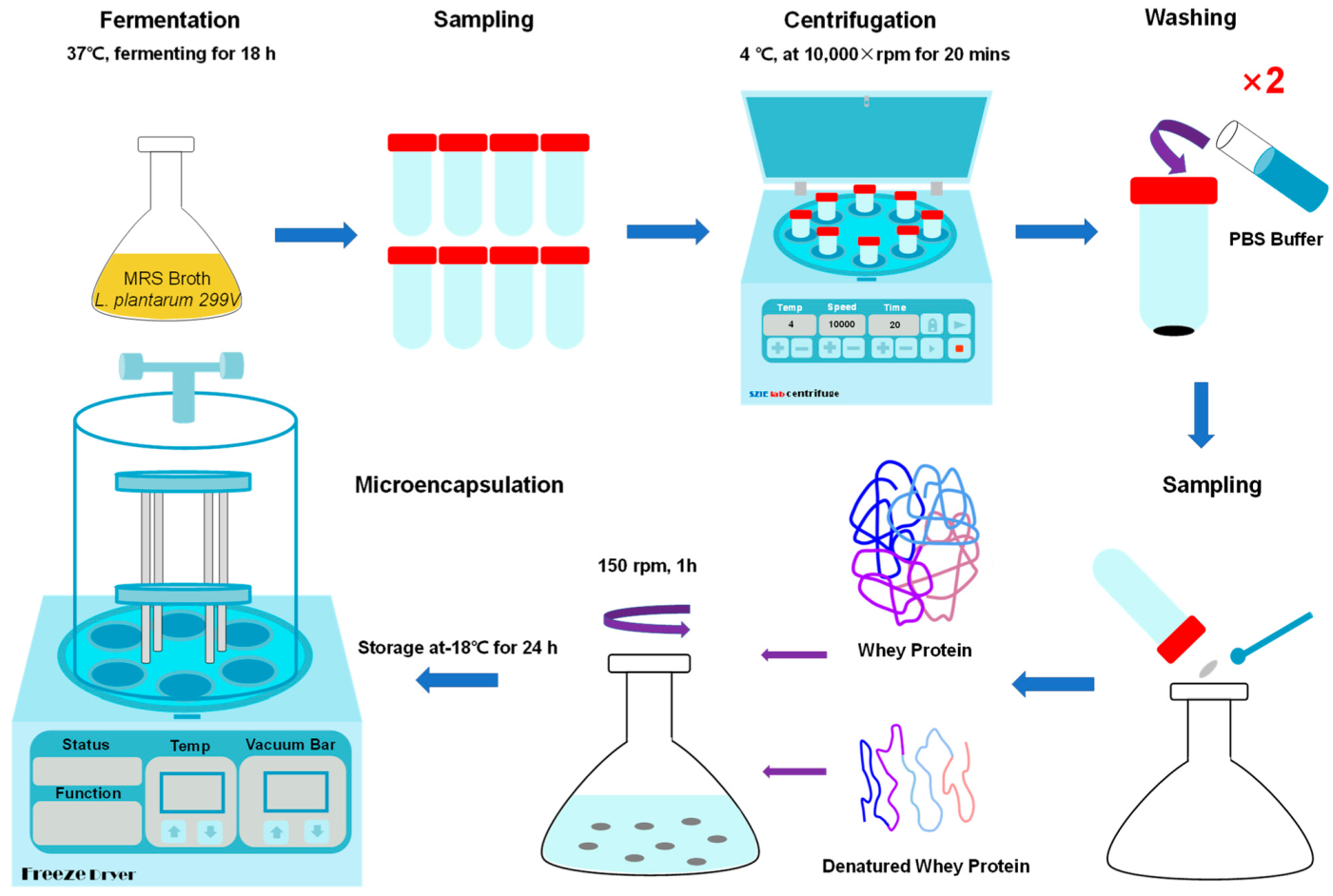
2. Materials and Methods
2.1. Preparation of Probiotics Culture
2.2. Preparation of Sample Solutions for Microencapsulation
2.3. Lyophilization
2.4. Determination Encapsulation Efficiency
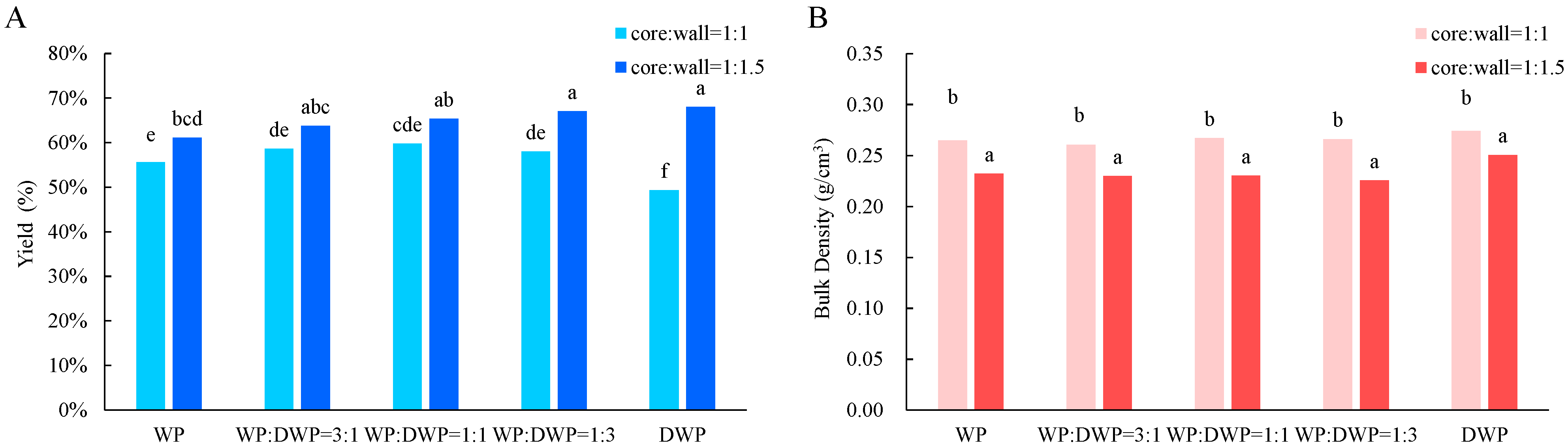
2.5. Determination of Yield
2.6. Determination of Bulk Density
2.7. Scanning Electron Microscopy
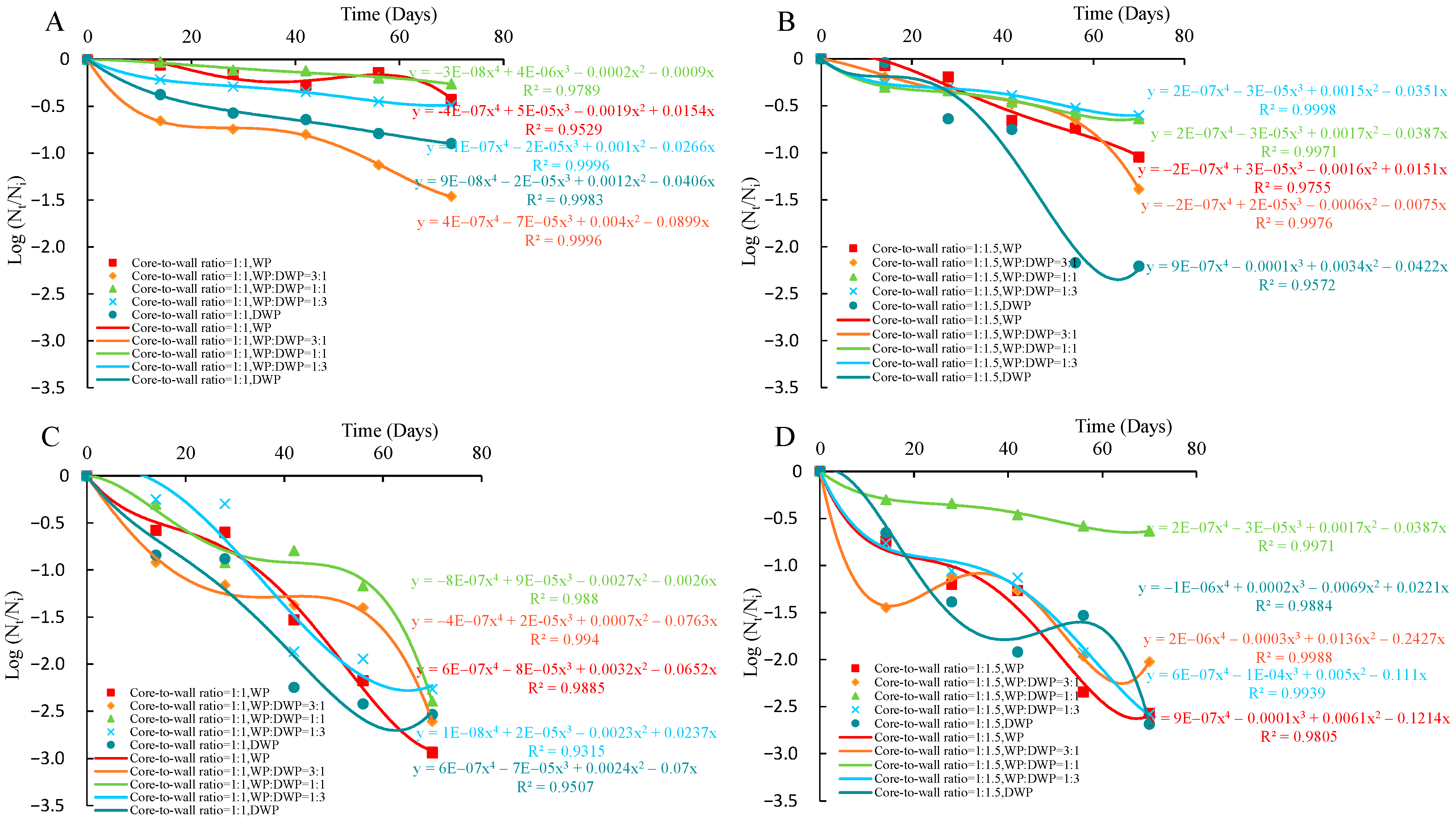
2.8. Viability of Microencapsulated Probiotics during Storage
2.9. Viability of Microencapsulated Probiotics In Vitro Digestion Process
2.10. Application of Microencapsulated Probiotics in Fermented and Fortified Apple Juice
2.11. Statistical Analysis
3. Results and Discussion
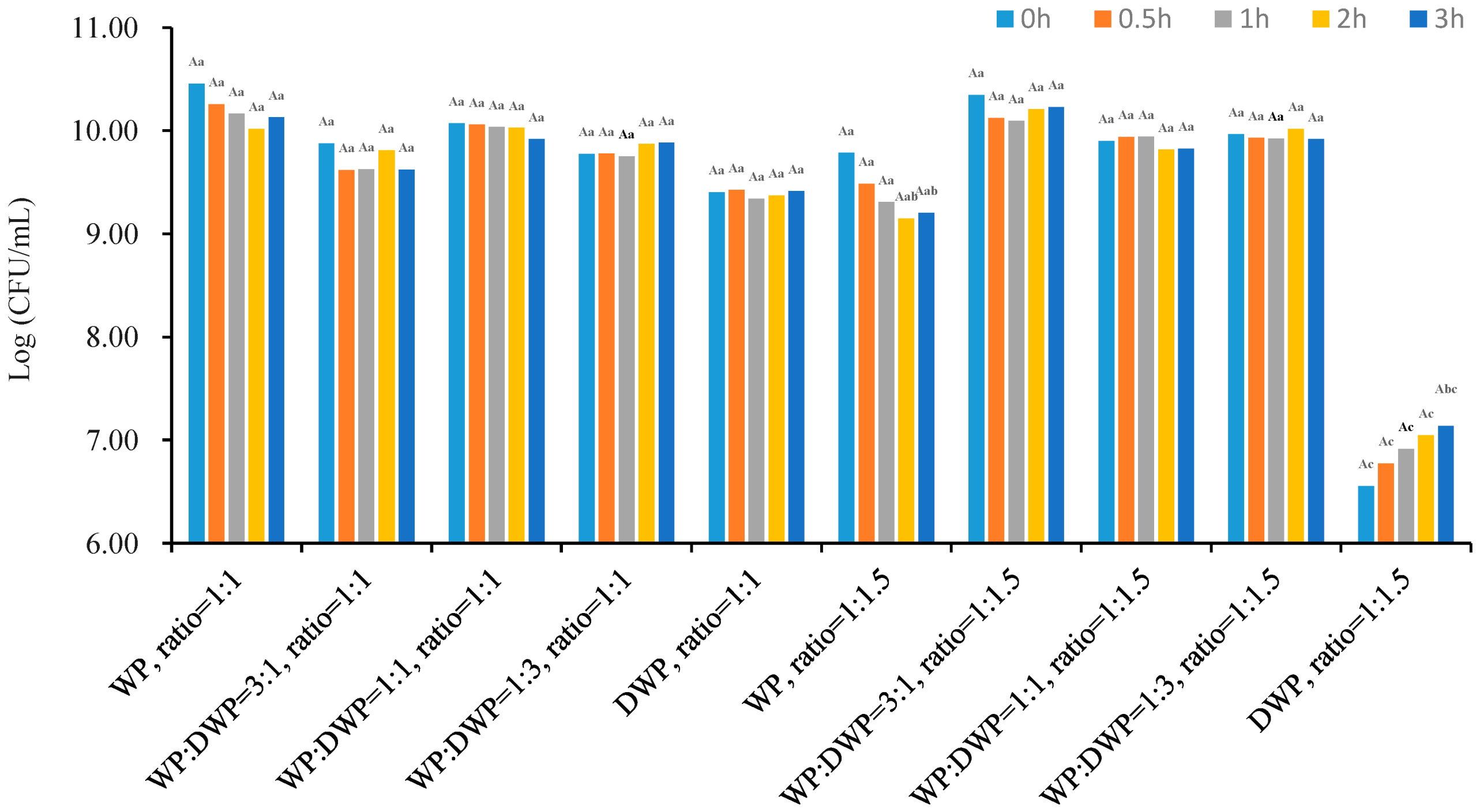
3.1. Cell Number and Encapsulation Efficiency of Microcapsules
3.2. Yield and Bulk Density of Microcapsules
3.3. Morphology of Microcapsules of L. plantarum 299v Strain
3.4. Viability of Microencapsulated Probiotics during Storage
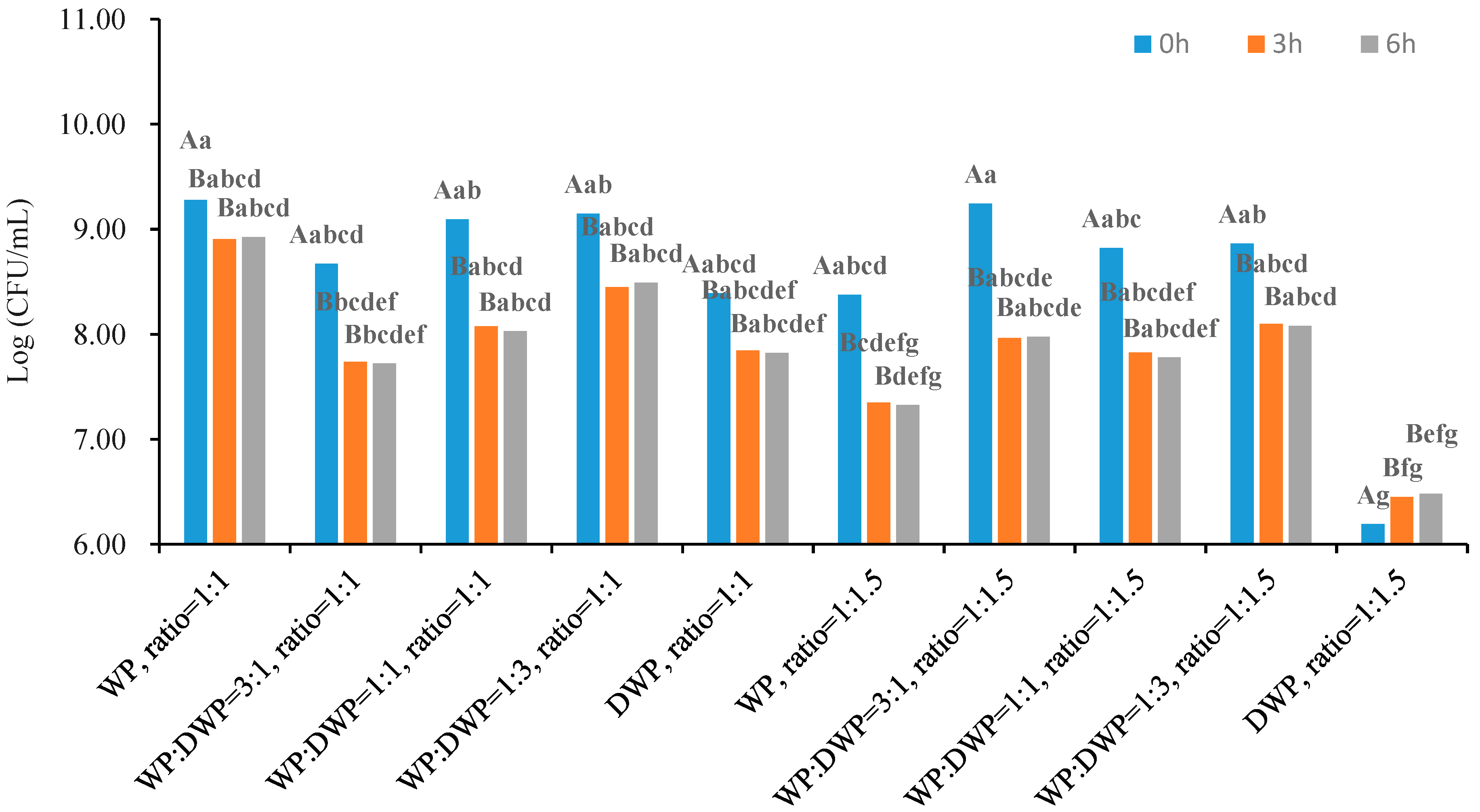
3.5. Viability of Microencapsulated Probiotics In Vitro Digestion Process
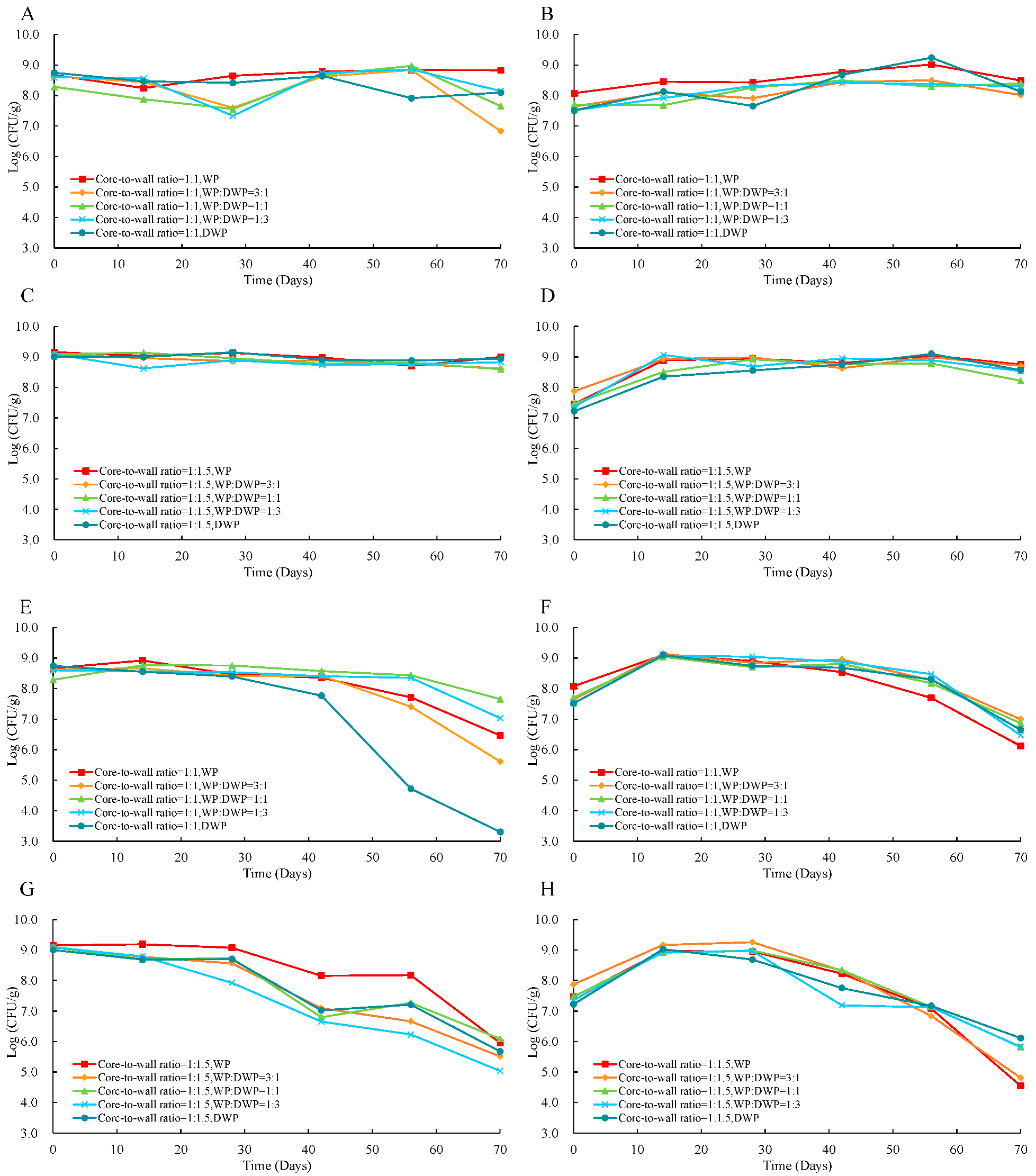
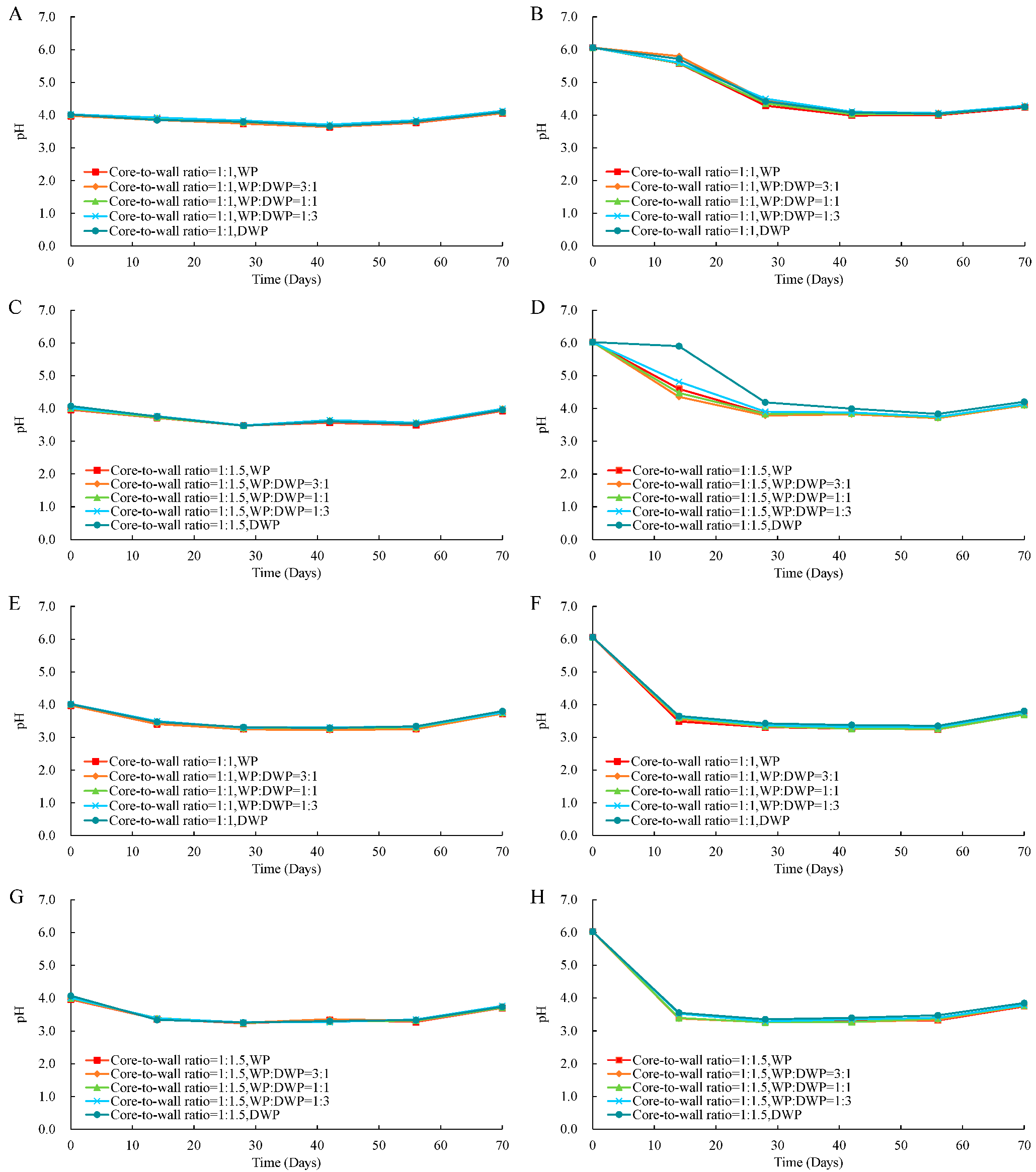
3.6. Application of Microencapsulated Probiotics in Apple Juice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO/WHO: Cordoba, Argentina, 2001. [Google Scholar]
- Mao, L.; Pan, Q.; Hou, Z.; Yuan, F.; Gao, Y. Development of soy protein isolate-carrageenan conjugates through Maillard reaction for the microencapsulation of Bifidobacterium longum. Food Hydrocoll. 2018, 84, 489–497. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Cai, D.; Zheng, M.; Xiu, L.; Liu, J. Advances in wall materials and methods of probiotic microencapsulation. China Dairy Ind. 2016, 44, 31–37. [Google Scholar]
- Eckert, C.; Serpa, V.G.; Felipe dos Santos, A.C.; Marinês da Costa, S.; Dalpubel, V.; Lehn, D.N.; Volken de Souza, C.F. Microencapsulation of Lactobacillus plantarum ATCC 8014 through spray drying and using dairy whey as wall materials. LWT-Food Sci. Technol. 2017, 82, 176–183. [Google Scholar] [CrossRef]
- Dias, C.O.; dos Santos Opuski de Almeida, J.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; de Mello Castanho Amboni, R.D. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A.; Hamed, F.; Maqsood, S. Comparative study on utilization of micro and nano sized starch particles for encapsulation of camel milk derived probiotics (Pediococcus acidolactici). LWT-Food Sci. Technol. 2019, 110, 231–238. [Google Scholar] [CrossRef]
- Ying, D.Y.; Schwander, S.; Weerakkody, R.; Sanguansri, L.; Gantenbein-Demarchi, C.; Augustin, M.A. Microencapsulated Lactobacillus rhamnosus GG in whey protein and resistant starch matrices: Probiotic survival in fruit juice. J. Funct. Foods. 2013, 5, 98–105. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT-Food Sci. Technol. 2015, 60, 773–780. [Google Scholar] [CrossRef]
- Suryabhan, P.; Lohith, K.; Anu-Appaiah, K.A. Sucrose and sorbitol supplementation on maltodextrin encapsulation enhance the potential probiotic yeast survival by spray drying. LWT-Food Sci. Technol. 2019, 107, 243–248. [Google Scholar] [CrossRef]
- Liu, H.; Gong, J.; Chabot, D.; Miller, S.S.; Cui, S.W.; Ma, J.; Zhong, F.; Wang, Q. Incorporation of polysaccharides into sodium caseinate-low melting point fat microparticles improves probiotic bacterial survival during simulated gastrointestinal digestion and storage. Food Hydrocoll. 2016, 54, 328–337. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Petrović, T.; Dimitrijević-Branković, S.; Lević, S.; Nedović, V.; Kourkoutas, Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT-Food Sci. Technol. 2016, 71, 169–174. [Google Scholar] [CrossRef]
- Alfaro-Galarza, O.; López-Villegas, E.O.; Rivero-Perez, N.; Tapia- Maruri, D.; Jiménez-Aparicio, A.R.; Palma-Rodríguez, H.M.; Vargas-Torres, A. Protective effects of the use of taro and rice starch as wall material on the viability of encapsulated Lactobacillus paracasei subsp. Paracasei. LWT-Food Sci. Technol. 2020, 117, 108686. [Google Scholar] [CrossRef]
- Vanden Braber, N.L.; Díaz Vergara, L.I.; Rossi, Y.E.; Aminahuel, C.A.; Mauri, A.N.; Cavaglieri, L.R.; Montenegro, M.A. Effect of microencapsulation in whey protein and water-soluble chitosan derivative on the viability of the probiotic Kluyveromyces marxianus VM004 during storage and in simulated gastrointestinal conditions. LWT-Food Sci. Technol. 2020, 118, 108844. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Mohamed Ahmed, I.A.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT-Food Sci. Technol. 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Sihag, M.K.; Tomar, S.K.; Arora, S.; Sabikhi, L.; Singh, A.K. Development and physico-chemical characterization of microencapsulated flaxseed oil powder: A functional ingredient for omega-3 fortification. Powder Technol. 2015, 286, 527–537. [Google Scholar] [CrossRef]
- Liao, L.K.; Wei, X.Y.; Gong, X.; Li, J.H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus casei LK-1 by spray drying related to its stability and in vitro digestion. LWT-Food Sci. Technol. 2017, 82, 82–89. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Wang, Y.; Wei, X.; Liao, M.; Rong, X.; Chen, J. Development of a new protocol for freeze-drying preservation of Pseudoalteromonas nigrifaciens and its protective effect on other marine bacteria. Electron. J. Biotechnol. 2020, 44, 1–5. [Google Scholar] [CrossRef]
- Otero, M.C.; Espeche, M.C.; Nader-Macías, M.E. Optimization of the freeze-drying media and survival throughout storage of freeze-dried Lactobacillus gasseri and Lactobacillus delbrueckii subsp. delbrueckii for veterinarian probiotic applications. Process Biochem. 2007, 42, 1406–1411. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Wang, W.; Liu, G.; Ge, W.; Zhang, M.; Yue, B.; Kong, M. Microencapsulation of Lactobacillus casei BNCC 134415 under lyophilization enhances cell viability during cold storage and pasteurization, and in simulated gastrointestinal fluids. LWT-Food Sci. Technol. 2019, 116, 108521. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Rama, G.R.; Kuhn, D.; Beux, S.; Maciel, M.J.; Volken de Souza, C.F. Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int. Dairy J. 2019, 98, 25–37. [Google Scholar] [CrossRef]
- De Bellis, P.; Sisto, A.; Lavermicocca, P. Probiotic bacteria and plant-based matrices: An association with improved health-promoting features. J. Funct. Foods. 2021, 87, 104821. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Chakkaravarthi, S.; Aravind, S.M. Fruit Juice Added with Prebiotics and Probiotics. In Probiotics and Prebiotics in Foods, 1st ed.; Gomes da Cruz, A., Senaka Ranadheera, C., Nazzaro, F., Mortazavian, A., Eds.; Academic Press: London, UK, 2021; pp. 219–232. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, J.; Fan, L.; Qin, Z.; Chen, Q.; Zhao, L. Antioxidant properties of a vegetable–fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotechnol. 2018, 27, 1719–1726. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Novel Fermented Fruit and Vegetable-Based Products. In Novel Food Fermentation Technologies. Food Engineering Series; Springer: Cham, Switzerland, 2016; pp. 279–291. [Google Scholar] [CrossRef]
- Ishii, M.; Matsumoto, Y.; Nishida, S.; Sekimizu, K. Decreased sugar concentration in vegetable and fruit juices by growth of functional lactic acid bacteria. Drug Discov. Ther. 2017, 11, 30–34. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Bujna, E.; Fekete, N.; Tran, A.T.M.; Rezessy-Szabo, J.M.; Prasad, R.; Nguyen, Q.D. Probiotic beverage from pineapple juice fermented with Lactobacillus and Bifidobacterium strains. Front. Nutr. 2019, 6, 54. [Google Scholar] [CrossRef]
- Loyeau, P.A.; Spotti, M.J.; Vanden Braber, N.L.; Rossi, Y.E.; Montenegro, M.A.; Vinderola, G.; Carrara, C.R. Microencapsulation of Bifidobacterium animalis subsp. lactis INL1 using whey proteins and dextrans conjugates as wall materials. Food Hydrocoll. 2018, 85, 129–135. [Google Scholar] [CrossRef]
- Savedboworn, W.; Noisumdang, C.; Arunyakanon, C.; Kongcharoen, P.; Phungamngoen, C.; Rittisak, S.; Charoen, R.; Phattayakorn, K. Potential of protein-prebiotic as protective matrices on the storage stability of vacuum-dried probiotic Lactobacillus casei. LWT-Food Sci. Technol. 2020, 131, 109578. [Google Scholar] [CrossRef]
- Halim, M.; Mohd Mustafa, N.A.; Othman, M.; Wasoh, H.; Kapri, M.R.; Ariff, A.B. Effect of encapsulant and cryoprotectant on the viability of probiotic Pediococcus acidilactici ATCC 8042 during freeze-drying and exposure to high acidity, bile salts and heat. LWT-Food Sci. Technol. 2017, 81, 210–216. [Google Scholar] [CrossRef]
- Muhammad, Z.; Ramzan, R.; Huo, G.C.; Tian, H.; Bian, X. Integration of polysaccharide-thermoprotectant formulations for microencapsulation of Lactobacillus plantarum, appraisal of survivability and physico-biochemical properties during storage of spray dried powders. Food Hydrocoll. 2017, 66, 286–295. [Google Scholar] [CrossRef]
- Guerin, J.; Petit, J.; Burgain, J.; Borges, F.; Bhandari, B.; Perroud, C.; Desobry, S.; Scher, J.; Gaiani, C. Lactobacillus rhamnosus GG encapsulation by spray-drying: Milk proteins clotting control to produce innovative matrices. J. Food Eng. 2017, 193, 10–19. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Development of a spray-dried conjugated whey protein hydrolysate powder with entrapped probiotics. J. Dairy Sci. 2022, 105, 2038–2048. [Google Scholar] [CrossRef]
- Hernández-López, Z.; Rangel-Vargas, E.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Cadena-Ramírez, A.; Acevedo-Sandoval, O.A.; Gordillo-Martínez, A.J.; Falfán-Cortés, R.N. Optimization of a spray-drying process for the production of maximally viable microencapsulated Lactobacillus pentosus using a mixture of starch-pulque as wall material. LWT-Food Sci. Technol. 2018, 95, 216–222. [Google Scholar] [CrossRef]
- Kumar, R.; Grover, S.; Kaushik, J.K.; Batish, V.K. IS30-related transposon mediated insertional inactivation of bile salt hydrolase (bsh1) gene of Lactobacillus plantarum strain Lp20. Microbiol. Res. 2014, 169, 553–560. [Google Scholar] [CrossRef]
- Maciel, G.M.; Chaves, K.S.; Grosso, C.R.F.; Gigante, M.L. Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J. Dairy Sci. 2014, 97, 1991–1998. [Google Scholar] [CrossRef]
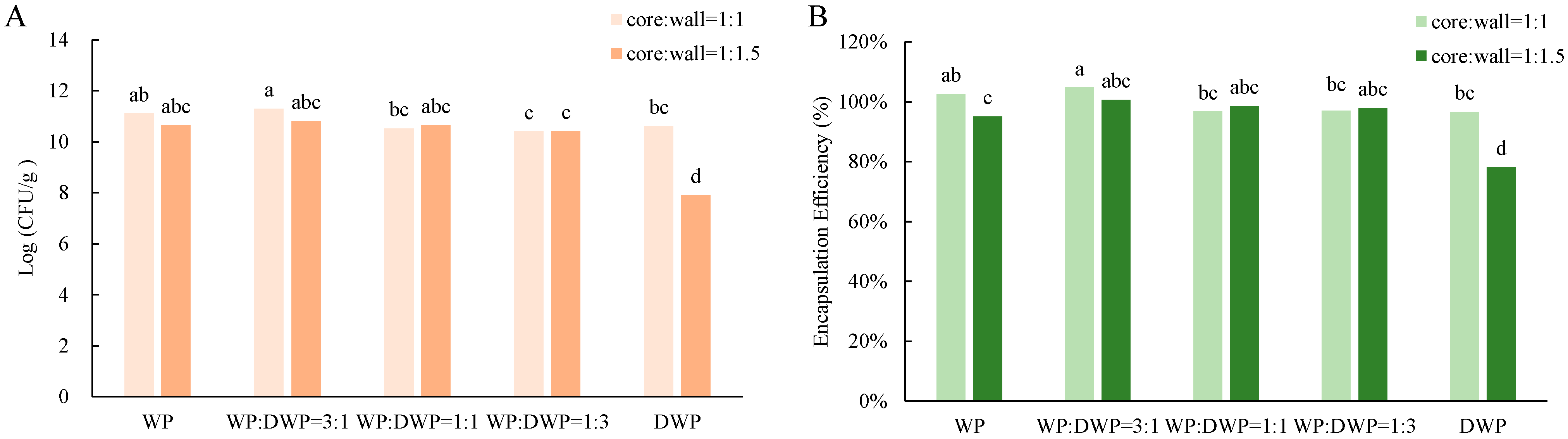

| Ratio | Wall Materials Formulation | Wall Materials Ratio (w/w) | WP (g) | DWP (g) | L. plantarum 299v (Wet Weight, g) | Microcapsule Solution Concentration (% w/w) | Total Weight (g) |
|---|---|---|---|---|---|---|---|
| 1:1 | WP | - | 20 | 0 | 20 | 20 | 200 |
| WP:DWP | 3:1 | 15 | 5 | 20 | 20 | 200 | |
| WP:DWP | 1:1 | 10 | 10 | 20 | 20 | 200 | |
| WP:DWP | 1:3 | 5 | 15 | 20 | 20 | 200 | |
| DWP | - | 0 | 20 | 20 | 20 | 200 | |
| 1:1.5 | WP | - | 30 | 0 | 20 | 20 | 250 |
| WP:DWP | 3:1 | 22.5 | 7.5 | 20 | 20 | 250 | |
| WP:DWP | 1:1 | 15 | 15 | 20 | 20 | 250 | |
| WP:DWP | 1:3 | 7.5 | 22.5 | 20 | 20 | 250 | |
| DWP | - | 0 | 30 | 20 | 20 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Nguyen, Q.D.; Sipiczki, G.; Ziane, S.R.; Hristovski, K.; Friedrich, L.; Visy, A.; Hitka, G.; Gere, A.; Bujna, E. Microencapsulation of Lactobacillus plantarum 299v Strain with Whey Proteins by Lyophilization and Its Application in Production of Probiotic Apple Juices. Appl. Sci. 2023, 13, 318. https://doi.org/10.3390/app13010318
Sun W, Nguyen QD, Sipiczki G, Ziane SR, Hristovski K, Friedrich L, Visy A, Hitka G, Gere A, Bujna E. Microencapsulation of Lactobacillus plantarum 299v Strain with Whey Proteins by Lyophilization and Its Application in Production of Probiotic Apple Juices. Applied Sciences. 2023; 13(1):318. https://doi.org/10.3390/app13010318
Chicago/Turabian StyleSun, Weizhe, Quang D. Nguyen, Gizella Sipiczki, Sofia Radja Ziane, Kristijan Hristovski, László Friedrich, Anna Visy, Géza Hitka, Attila Gere, and Erika Bujna. 2023. "Microencapsulation of Lactobacillus plantarum 299v Strain with Whey Proteins by Lyophilization and Its Application in Production of Probiotic Apple Juices" Applied Sciences 13, no. 1: 318. https://doi.org/10.3390/app13010318
APA StyleSun, W., Nguyen, Q. D., Sipiczki, G., Ziane, S. R., Hristovski, K., Friedrich, L., Visy, A., Hitka, G., Gere, A., & Bujna, E. (2023). Microencapsulation of Lactobacillus plantarum 299v Strain with Whey Proteins by Lyophilization and Its Application in Production of Probiotic Apple Juices. Applied Sciences, 13(1), 318. https://doi.org/10.3390/app13010318










