A CNN-Based Strategy to Classify MRI-Based Brain Tumors Using Deep Convolutional Network
Abstract
1. Introduction
2. Related Works
3. Materials and Methods
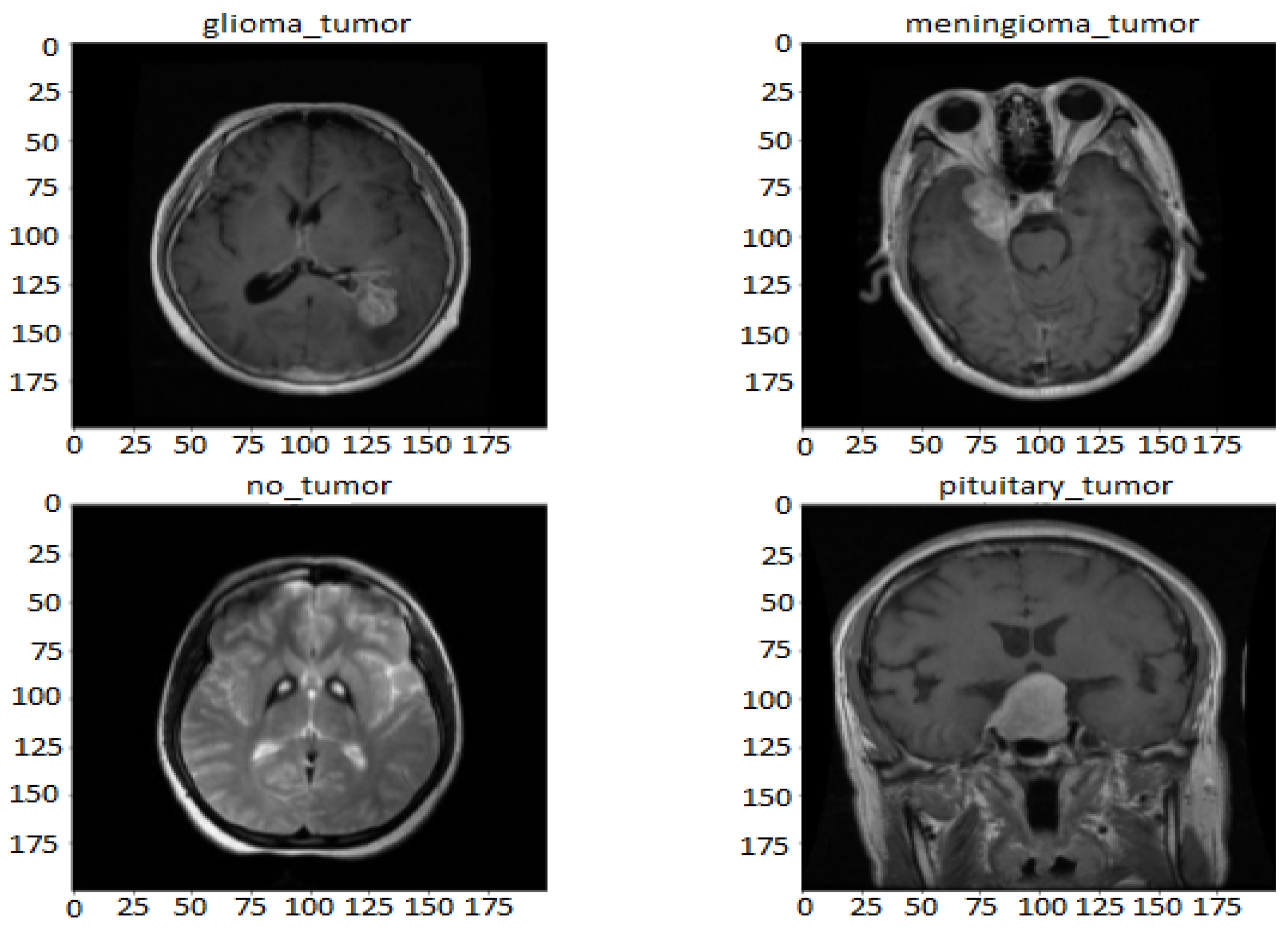
3.1. Dataset
3.2. Preprocessing
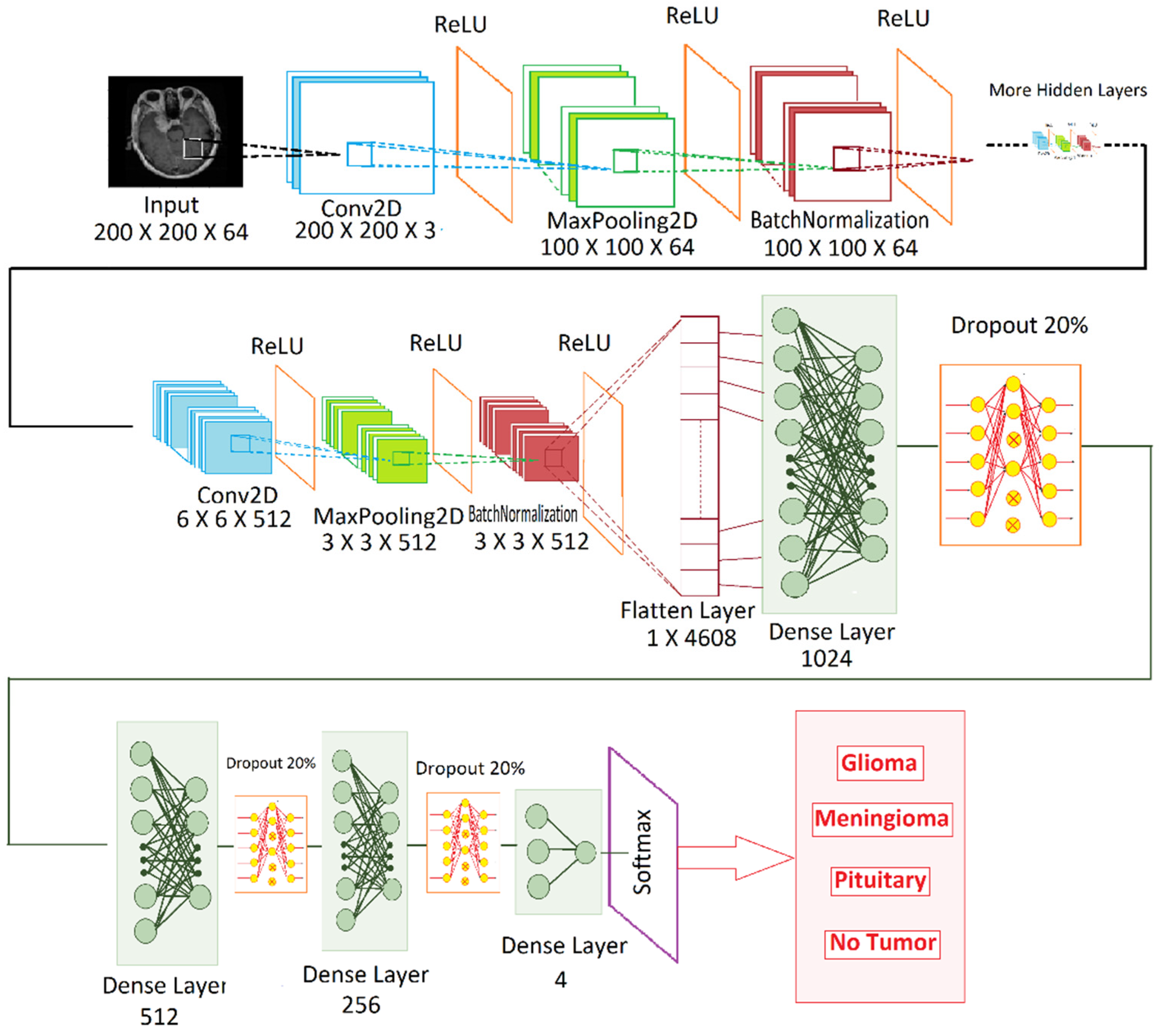
3.3. Proposed Model
3.4. Pseudocode
| Input: |
| Xt: Brain tumour pre-processed train dataset; |
| Xv: Brain tumour pre-processed test dataset; |
| ε: Number of epochs; |
| η: Learning Rate; |
| Β: Batch Size; |
| Output: |
| Assessment Metrics (accuracy etc.) calculation on test dataset. |
| Start Procedure |
| Add_Conv2D (filters, kernel_size, padding, activation) |
| Add_MaxPool2D (pool_size) |
| Add_BatchNormalization () |
| Add_Flatten () |
| Add_Dense () |
| Add_Dropout (0.2) |
| Optimizing with Stochastic gradient descent (η) |
| for all epochs in 1 to ε do |
| for Β ∈ a random batch from Xt do |
| model_fit with test data (Xv) |
| append (Accuracy) |
| Endfor |
| Endfor |
| Evaluate trained Model dataset -> totalAccuracy |
| return totalAccuracy |
| EndProcedure |
4. Results
4.1. Performance Analysis
4.2. Confusion Matrix
5. Discussion
6. Conclusion and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeAngelis, L.M. Brain Tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ricci, P.E.; Dungan, D.H. Imaging of low- and intermediate-grade gliomas. Semin. Radiat. Oncol. 2001, 11, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Day, M.R.; Lacarra, E.D.; Sacks, J.M.; Sadraei, A. Theranostics of Glioblastoma Multiforme: In Vitro Characterization of Targeted Nanoemulsions and Creation of a 3D Statistical Heatmap to Visualize Nanoemulsion Uptake. 2014. Available online: https://web.wpi.edu/Pubs/E-project/Available/E-project-043015-050001/ (accessed on 13 July 2022).
- Işin, A.; Direko, C.; Şah, M. Review of MRI-based Brain Tumor Image Segmentation Using Deep Learning Methods. Procedia Comput. Sci. 2016, 102, 317–324. [Google Scholar] [CrossRef]
- Drevelegas, A. Imaging of Brain Tumors with Histological Correlations; Springer: Berlin/Heidelberg, Germany, 2011; p. 432. [Google Scholar]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; Van Der Laak, J.A.; Van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef]
- Kaus, M.R.; Warfield, S.K.; Nabavi, A.; Black, P.M.; Jolesz, F.A.; Kikinis, R. Automated segmentation of MR images of brain tumors. Radiology 2001, 218, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Mohan, G.; Subashini, M.M. MRI based medical image analysis: Survey on brain tumor grade classification. Biomed. Signal Process. Control 2018, 39, 139–161. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, W.; Cao, S.; Yang, R.; Yang, W.; Yun, Z.; Wang, Z.; Feng, Q. Enhanced performance of brain tumor classification via tumor region augmentation and partition. PLoS ONE 2015, 10, e0140381. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, W.; Huang, M.; Huang, W.; Jiang, J.; Zhou, Y.; Yang, R.; Zhao, J.; Feng, Y.; Feng, Q.; et al. Retrieval of Brain Tumors by Adaptive Spatial Pooling and Fisher Vector Representation. PLoS ONE 2016, 11, e0157112. [Google Scholar] [CrossRef]
- Swati, Z.N.K.; Zhao, Q.; Kabir, M.; Ali, F.; Ali, Z.; Ahmed, S.; Lu, J. Brain tumor classification for MR images using transfer learning and fine-tuning. Comput. Med. Imaging Graph. 2019, 75, 34–46. [Google Scholar] [CrossRef]
- Rajesh, T.; Malar, R.S.M. Rough set theory and feed forward neural network based brain tumor detection in magnetic resonance images. In Proceedings of the International Conference on “Advanced Nanomaterials and Emerging Engineering Technologies”, ICANMEET 2013, Chennai, India, 24–26 July 2013; pp. 240–244. [Google Scholar] [CrossRef]
- Machhale, K.; Nandpuru, H.B.; Kapur, V.; Kosta, L. MRI brain cancer classification using hybrid classifier (SVM-KNN). In Proceedings of the 2015 International Conference on Industrial Instrumentation and Control, ICIC 2015, Pune, India, 28–30 May 2015; pp. 60–65. [Google Scholar] [CrossRef]
- Shasidhar, M.; Raja, V.S.; Kumar, B.V. MRI brain image segmentation using modified fuzzy c-means clustering algorithm. In Proceedings of the 2011 International Conference on Communication Systems and Network Technologies, CSNT 2011, Katra, India, 3–5 June 2011; pp. 473–478. [Google Scholar] [CrossRef]
- Goswami, M.S.; Bhaiya, M.L.K.P. Brain tumour detection using unsupervised learning based neural network. In Proceedings of the 2013 International Conference on Communication Systems and Network Technologies, CSNT 2013, Gwalior, India, 6–8 April 2013; pp. 573–577. [Google Scholar] [CrossRef]
- Sadad, T.; Rehman, A.; Munir, A.; Saba, T.; Tariq, U.; Ayesha, N.; Abbasi, R. Brain tumor detection and multi-classification using advanced deep learning techniques. Microsc. Res. Tech. 2021, 84, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.I.; Khan, M.A.; Alhussein, M.; Aurangzeb, K.; Raza, M. A decision support system for multimodal brain tumor classification using deep learning. Complex Intell. Syst. 2021, 8, 3007–3020. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nizamani, F.; Nürnberger, A.; Speck, O. Classifcation of brain tumours in MR images using deep spatiospatial models. Sci. Rep. 2022, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, C.; Gensheimer, M.; Padda, S.; Kato, F.; Shirato, H.; Wei, Y.; Schönlieb, C.B.; Price, S.J.; Jaffray, D.; et al. Radiological tumour classification across imaging modality and histology. Nat. Mach. Intell. 2021, 3, 787–798. [Google Scholar] [CrossRef]
- Fan, M.; Xia, P.; Clarke, R.; Wang, Y.; Li, L. Radiogenomic signatures reveal multiscale intratumour heterogeneity associated with biological functions and survival in breast cancer. Nat. Commun. 2020, 11, 4861. [Google Scholar] [CrossRef] [PubMed]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef]
- Wu, J. Introduction to Convolutional Neural Networks. Natl. Key Lab Nov. Softw. Technol. 2017, 5, 495. Available online: https://web.archive.org/web/20180928011532/https://cs.nju.edu.cn/wujx/teaching/15_CNN.pdf (accessed on 28 June 2022).
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Nair, V.; Hinton, G.E. Rectified linear units improve restricted boltzmann machines. Procedings of the 27th International Conference on Machine Learning, ICML 2010, Haifa, Israel, 21–June 2010; Omnipress: Madison, WI, USA, 2010; pp. 807–814. [Google Scholar]
- Qiu, S.; Xu, X.; Cai, B. FReLU: Flexible Rectified Linear Units for Improving Convolutional Neural Networks. Proc.-Int. Conf. Pattern Recognit. 2018, 2018, 1223–1228. [Google Scholar] [CrossRef]
- Xu, B.; Wang, N.; Chen, T.; Li, M. Empirical Evaluation of Rectified Activations in Convolutional Network. 2015. Available online: http://arxiv.org/abs/1505.00853 (accessed on 28 June 2022).
- Ramachandran, P.; Zoph, B.; Le Google Brain, Q. Swish: A Self-Gated Activation Function. In Proceedings of the 6th International Conference on Learning Representations, ICLR 2018—Workshop Track Proceedings, Vancouver, BC, Canada, 30 April–3 May 2018. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, X.; Liu, M.; Sun, J. Activate or Not: Learning Customized Activation. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Virtual, 10 September 2020; pp. 8028–8038. [Google Scholar] [CrossRef]
- Bridle, J.S. Training Stochastic Model Recognition Algorithms Training Stochastic Model Recognition Algorithms as Networks can lead to Maximum Mutual Information Estimation of Parameters. In Proceedings of the 2nd International Conference on Neural Information Processing Systems, Denver, CO, USA, 27–30 November 1989. [Google Scholar]
- Pan, Y.; Huang, W.; Lin, Z.; Zhu, W.; Zhou, J.; Wong, J.; Ding, Z. Brain tumor grading based on Neural Networks and Convolutional Neural Networks. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Milan, Italy, 25–29 August 2015; Volume 2015, pp. 699–702. [Google Scholar] [CrossRef]
- Othman, M.F.; Basri, M.A.M. Probabilistic Neural Network for brain tumor classification. In Proceedings of the 2011 Second International Conference on Intelligent Systems, Modelling and Simulation, Phnom Penh, Cambodia, 25–27 January 2011; pp. 136–138. [Google Scholar] [CrossRef]
- Sultan, H.H.; Salem, N.M.; Al-Atabany, W. Multi-Classification of Brain Tumor Images Using Deep Neural Network. IEEE Access 2019, 7, 69215–69225. [Google Scholar] [CrossRef]
- Ari, A.; Hanbay, D. Deep learning based brain tumor classification and detection system. Turk. J. Electr. Eng. Comput. Sci. 2018, 26, 2275–2286. [Google Scholar] [CrossRef]
- Ghassemi, N.; Shoeibi, A.; Rouhani, M. Deep neural network with generative adversarial networks pre-training for brain tumor classification based on MR images. Biomed. Signal Process. Control 2020, 57, 101678. [Google Scholar] [CrossRef]
- Pushpa, R.; Palani, S. Brain Tumor Detection and Classification Using Deep Learning Classifier on MRI Images. Appl. Sci. Eng. Technol. 2015, 10, 177–187. [Google Scholar] [CrossRef]
- Seetha, J.; Raja, S.S. Brain Tumor Classification Using Convolutional Neural Networks. Biomed. Pharmacol. J. 2018, 11, 1457–1461. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, S.; Muhammad, K.; Wu, W.; Ullah, A.; Baik, S.W. Multi-grade brain tumor classification using deep CNN with extensive data augmentation. J. Comput. Sci. 2019, 30, 174–182. [Google Scholar] [CrossRef]
- Mohsen, H.; El-Dahshan, E.-S.A.; El-Horbaty, E.-S.M.; Salem, A.-B.M. Classification using deep learning neural networks for brain tumors. Futur. Comput. Inform. J. 2018, 3, 68–71. [Google Scholar] [CrossRef]
- Khan, M.A.; Ashraf, I.; Alhaisoni, M.; Damaševičius, R.; Scherer, R.; Rehman, A.; Bukhari, S.A.C. Multimodal brain tumor classification using deep learning and robust feature selection: A machine learning application for radiologists. Diagnostics 2020, 10, 565. [Google Scholar] [CrossRef]
- Deepak, S.; Ameer, P.M. Brain tumor classification using deep CNN features via transfer learning. Comput. Biol. Med. 2019, 111, 103345. [Google Scholar] [CrossRef]
- Li, J.P.; Kumar, R.; Ali, Z.; Khan, I.; Uddin, M.I.; Agbley, B.L.Y. MCNN: A multi-level CNN model for the classification of brain tumors in IoT-healthcare system. J. Ambient Intell. Humaniz. Comput. 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Raza, A.; Ayub, H.; Khan, J.A.; Ahmad, I.; SSalama, A.; Daradkeh, Y.I.; Javeed, D.; Ur Rehman, A.; Hamam, H. A Hybrid Deep Learning-Based Approach for Brain Tumor Classification. Electronics 2022, 11, 1146. [Google Scholar] [CrossRef]
- El Kader, I.A.; Xu, G.; Shuai, Z.; Saminu, S.; Javaid, I.; Ahmad, I.S. Differential Deep Convolutional Neural Network Model for Brain Tumor Classification. Brain Sci. 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.F.; Ali, M.U.; Hussain, S.J.; Zafar, A.; Mohatram, M.; Irfan, M.; AlRuwaili, R.; Alruwaili, M.; Ali, N.H.; Albarrak, A.M. Brain Tumor/Mass Classification Framework Using Magnetic-Resonance-Imaging-Based Isolated and Developed Transfer Deep-Learning Model. Sensors 2022, 22, 372. [Google Scholar] [CrossRef] [PubMed]
- Brain Tumor Dataset | Kaggle. Available online: https://www.kaggle.com/datasets/nniisshhaann/braintumors (accessed on 5 October 2022).
- Brain Tumor Dataset. Available online: https://figshare.com/articles/dataset/brain_tumor_dataset/1512427?fbclid=IwAR3VaHmuktRYQKmRLMYpDVD2DUxFWQqMlm4fqe5voJuNidukZD6WzC-kf0U (accessed on 5 October 2022).
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2014, arXiv:1409.1556. Available online: http://arxiv.org/abs/1409.1556 (accessed on 13 July 2022).
- Keskar, N.S.; Socher, R. Improving Generalization Performance by Switching from Adam to SGD. arXiv 2017, arXiv:1712.07628. Available online: http://arxiv.org/abs/1712.07628 (accessed on 20 September 2022).
- Thakkar, V.; Tewary, S.; Chakraborty, C. Batch Normalization in Convolutional Neural Networks—A comparative study with CIFAR-10 data. In Proceedings of the 5th International Conference on Emerging Applications of Information Technology, EAIT 2018, Kolkata, India, 12–13 January 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Ul Haq, A.; Li, J.P.; Agbley, B.L.Y.; Mawuli, C.B.; Ali, Z.; Nazir, S.; Din, S.U. A survey of deep learning techniques based Parkinson’s disease recognition methods employing clinical data. Expert Syst. Appl. 2022, 208, 118045. [Google Scholar] [CrossRef]
- Paul, J.S.; Plassard, A.J.; Landman, B.A.; Fabbri, D. Deep learning for brain tumor classification. In Medical Imaging 2017: Biomedical Applications in Molecular, Structural, and Functional Imaging; SPIE: Bellingham, EA, USA, 2017; Volume 10137. [Google Scholar] [CrossRef]
- Afshar, P.; Plataniotis, K.N.; Mohammadi, A. Capsule Networks for Brain Tumor Classification based on MRI Images and Course Tumor Boundaries. In Proceedings of the ICASSP 2019–2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Brighton, UK, 12–17 May 2019; Volume 2019, pp. 1368–1372. [Google Scholar] [CrossRef]
- Anaraki, A.K.; Ayati, M.; Kazemi, F. Magnetic resonance imaging-based brain tumor grades classification and grading via convolutional neural networks and genetic algorithms. Biocybern. Biomed. Eng. 2019, 39, 63–74. [Google Scholar] [CrossRef]
- Tan, M.; Le, Q.V. EfficientNet: Rethinking Model Scaling for Convolutional Neural Networks. In Proceedings of the 36th International Conference on Machine Learning, PMLR, Long Beach, CA, USA, 9–15 June 2019; Volume 97, pp. 10691–10700. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015; pp. 770–778. [Google Scholar] [CrossRef]




| Author(s) | Concept | Method | Findings | Gaps |
|---|---|---|---|---|
| Deepak, Ameer [41] | Designed a model to classify three pathological types of brain tumor. | Using deep transfer learning and a pre-trained GoogLeNet to extract features, a classifier to classify the types. | For a small dataset, higher classification accuracy was observed. | Higher misclassification in the confusion matrix, overfitting because of a small dataset. |
| Emrah Irmak [45] | Three types of classification tasks have been performed. | Three CNN models perform three classification tasks, in which hyperparameters have been manually optimized using a grid. | Using the grid optimizer is effective as it could find the best model for classification types. | Three classification systems for all three types, a joint multi-classification system can decline its necessity. |
| Sharif, Attique, Musaed, Khursheed, Mudassar [18] | Brain tumor classifications on four types of MRI images, such as T1W, T1CE, T2W, and Flair. | Selection of the most optimal features using Modified Genetic Algorithm (MGA) and entropy-Kurtosis-based techniques and trained by a fine-tuned pre-trained DenseNet201 | Using a feature selection technique improved the result of a publicly available dataset. | Reducing certain key features could have a great impact, as it could help the system achieve accuracy. |
| Layers | Output Size | Parameters |
|---|---|---|
| Conv2D | None,200,200,3 | 1664 |
| MaxPooling 2D | None,100,100,64 | 0 |
| BatchNormalization | None,100,100,64 | 256 |
| Conv2D | None,100,100,128 | 204,928 |
| MaxPooling 2D | None,50,50,128 | 0 |
| BatchNormalization | None,50,50,128 | 512 |
| Conv2D | None,50,50,128 | 409,728 |
| MaxPooling 2D | None,25,25,128 | 0 |
| BatchNormalization | None,25,25,128 | 512 |
| Conv2D | None,25,25,256 | 819,456 |
| MaxPooling 2D | None,12,12,256 | 0 |
| BatchNormalization | None,12,12,256 | 1024 |
| Convo2D | None,12,12,256 | 1,638,656 |
| MaxPooling | None,6,6,256 | 0 |
| BatchNormalization | None,6,6,256 | 1024 |
| Convo2D | None,6,6,512 | 3,277,312 |
| Maxpooling | None,3,3,512 | 0 |
| BatchNormalization | None,3,3,512 | 2048 |
| Flatten | None, 4608 | 0 |
| Dense layer | None, 1024 | 4,719,616 |
| Dropout 20% | None, 1024 | 0 |
| Dense layer | None, 512 | 524,800 |
| Dropout 20% | None, 512 | 0 |
| Dense layer | None, 256 | 131,328 |
| Dropout 20% | None, 256 | 0 |
| Dense layer | None, 4 | 1024 |
| Softmax | None,4 | 0 |
| Epoch | 10 | 20 | 30 |
|---|---|---|---|
| Learning Rate | Accuracy | ||
| 0.001 | 98.8% | 99% | 99.2% |
| 0.01 | 98.3% | 99.5% | 99.01% |
| 0.05 | 97% | 98.7% | 99.3% |
| Tumors | TP | TN | FP | FN | Precision | Recall | Specificity | Accuracy | F1-Score |
|---|---|---|---|---|---|---|---|---|---|
| Glioma | 529 | 1510 | 3 | 18 | 0.994 | 0.967 | 0.988 | 98.98 | 0.98 |
| Meningi-oma | 511 | 1532 | 17 | 1 | 0.967 | 0.998 | 0.999 | 99.13 | 0.98 |
| No Tumor | 455 | 1601 | 2 | 2 | 0.996 | 0.996 | 0.998 | 99.81 | 0.99 |
| Pituitary | 543 | 1516 | 0 | 1 | 1.00 | 0.998 | 0.999 | 99.95 | 0.99 |
| Serial | Author | Model Used | Dataset Used | Model Accuracy | Our Model Accuracy |
|---|---|---|---|---|---|
| 1 | Paul et al. [52] | Fully Connected Network (FCN), CNN | 3064 T1-weighted contrast-enhanced images with three kinds of brain tumor [44]. | 91.43% | 96.4% |
| 2 | Afshar et al. [53] | CapsNets incorporated with coarse tumor boundary | 3064 T1-weighted contrast-enhanced images with three kinds of brain tumor [44]. | 90.89% | 96.4% |
| 3 | Anaraki et al. [54] | Genetic Algorithm (GA) | 3064 T1-weighted contrast-enhanced images with three kinds of brain tumor, combined with data from other sources | 94.2% | 95.3% |
| Model | Precision | Recall | F1-Score(macro) | Accuracy | Pre-Trained |
|---|---|---|---|---|---|
| EfficientNetB0 [55] | 0.942 | 0.941 | 0.941 | 0.941 | NO |
| EfficientNetB0 [55] | 0.993 | 0.993 | 0.993 | 0.993 | YES |
| Resnet50 [56] | 0.878 | 0.88 | 0.878 | 0.879 | YES |
| Resnet152 [56] | 0.889 | 0.885 | 0.885 | 0.885 | YES |
| VGG16 [48] | 0.980 | 0.980 | 0.980 | 0.980 | NO |
| Modified-VGGNet | 0.997 | 0.988 | 0.985 | 0.995 | NO |
| Tumors | Precision (VGG-16) | Precision (Proposed Model) | Recall (VGG-16) | Recall (Proposed Model) | F1-Score (VGG-16) | F1-Score (Proposed Model) | Accuracy (VGG-16) | Accuracy (Proposed Model) |
|---|---|---|---|---|---|---|---|---|
| Glioma | 0.98 | 0.994 | 0.95 | 0.967 | 0.97 | 0.98 | ||
| Meningioma | 0.97 | 0.967 | 0.98 | 0.998 | 0.97 | 0.98 | 98% | 99.5% |
| No Tumor | 0.98 | 0.996 | 1.00 | 0.996 | 0.99 | 0.99 | ||
| Pituitary | 0.99 | 1.00 | 0.99 | 0.998 | 0.99 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, A.W.; Hossain, M.S.; Wardiful, M.A.; Farzana, M.; Ahmad, S.; Alam, F.; Nandi, R.N.; Siddique, N. A CNN-Based Strategy to Classify MRI-Based Brain Tumors Using Deep Convolutional Network. Appl. Sci. 2023, 13, 312. https://doi.org/10.3390/app13010312
Reza AW, Hossain MS, Wardiful MA, Farzana M, Ahmad S, Alam F, Nandi RN, Siddique N. A CNN-Based Strategy to Classify MRI-Based Brain Tumors Using Deep Convolutional Network. Applied Sciences. 2023; 13(1):312. https://doi.org/10.3390/app13010312
Chicago/Turabian StyleReza, Ahmed Wasif, Muhammad Sazzad Hossain, Moonwar Al Wardiful, Maisha Farzana, Sabrina Ahmad, Farhana Alam, Rabindra Nath Nandi, and Nazmul Siddique. 2023. "A CNN-Based Strategy to Classify MRI-Based Brain Tumors Using Deep Convolutional Network" Applied Sciences 13, no. 1: 312. https://doi.org/10.3390/app13010312
APA StyleReza, A. W., Hossain, M. S., Wardiful, M. A., Farzana, M., Ahmad, S., Alam, F., Nandi, R. N., & Siddique, N. (2023). A CNN-Based Strategy to Classify MRI-Based Brain Tumors Using Deep Convolutional Network. Applied Sciences, 13(1), 312. https://doi.org/10.3390/app13010312









