Effects of Life-Long Supplementation of Potassium Nitrate on Male Mice Longevity and Organs Pathology
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Feeding Assay
2.3. Preparation
2.4. Statistical Analysis
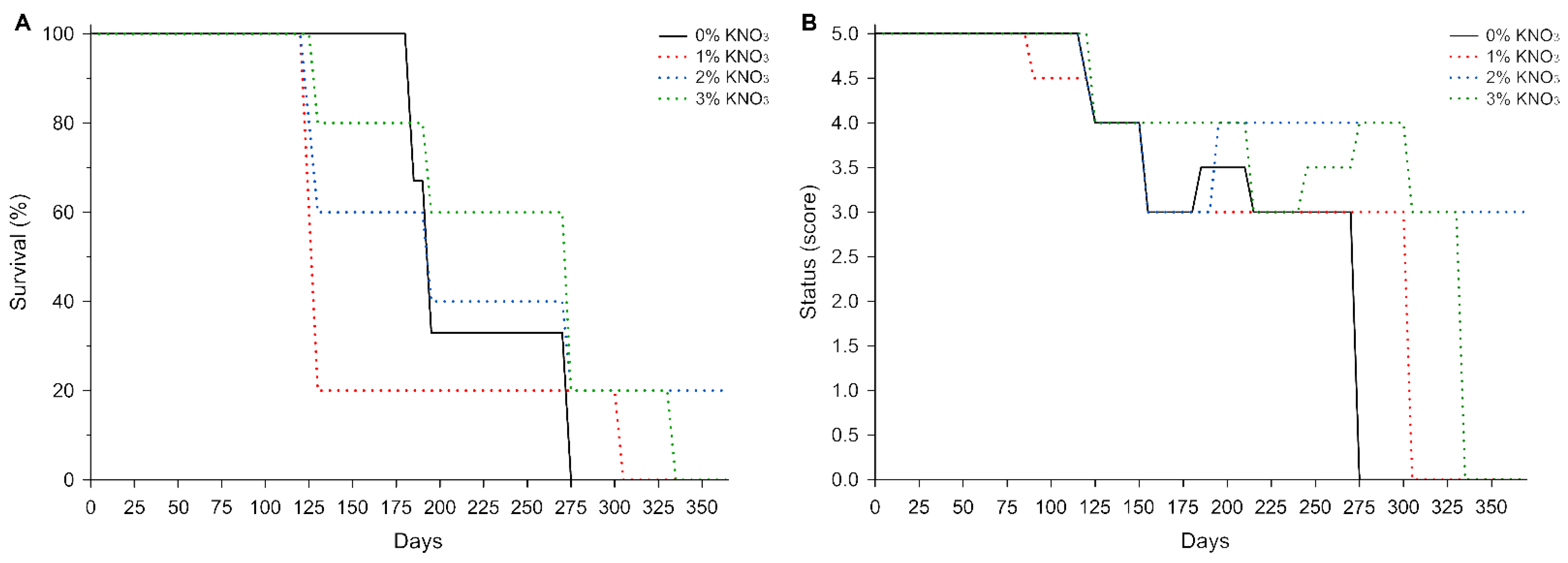
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent to Participate
Consent for Publication
References
- Lidder, S.; Webb, A.J. Vascular Effects of Dietary Nitrate (as Found in Green Leafy Vegetables and Beetroot) via the Nitrate-Nitrite-Nitric Oxide Pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eisinaite, V.; Vinauskiene, R.; Viskelis, P.; Leskauskaite, D. Effects of Freeze-Dried Vegetable Products on the Technological Process and the Quality of Dry Fermented Sausages. J. Food Sci. 2016, 81, C2175–C2182. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Evaluation of certain food additives (Fifty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Tech. Rep. Ser. 2002, 913, 1–153. [Google Scholar]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. Genet. Toxicol. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Agency for Toxic Substances & Disease Registry. Nitrate/nitrite toxicity. In What are the Health Effects from Exposure to Nitrates and Nitrites; Agency for Toxic Substances & Disease Registry: Atlanta, GA, USA, 2013; 135p. [Google Scholar]
- Honikel, K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Nitrate in vegetables. Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 689, 1–79. [Google Scholar]
- Walker, R. Nitrates, nitrites and N-nitrosocompounds: A review of the occurrence in food and diet and the toxicological implications. Food Addit. Contam. 1990, 7, 717–768. [Google Scholar] [CrossRef]
- WHO. Diet, nutrition and the prevention of chronic diseases. Report of the joint WHO/FAO expert consultation. Tech. Rep. Ser. 2003, 916, 1–160. [Google Scholar]
- Weitzberg, E.; Lundberg, J.O. Novel Aspects of Dietary Nitrate and Human Health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Hendgen-Cotta, U.B.; Luedike, P.; Berenbrink, M.; Klare, J.P.; Steinhoff, H.-J.; Semmler, D.; Shiva, S.; Williams, D.; Kipar, A.; et al. Nitrite Regulates Hypoxic Vasodilation via Myoglobin-Dependent Nitric Oxide Generation. Circulation 2012, 126, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Dreißigacker, U.; Wendt, M.; Wittke, T.; Tsikas, D.; Maassen, N. Positive correlation between plasma nitrite and performance during high-intensive exercise but not oxidative stress in healthy men. Nitric Oxide 2010, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Carlstrom, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary Inorganic Nitrate Improves Mitochondrial Efficiency in Humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef]
- Liubertas, T.; Kairaitis, R.; Stasiule, L.; Capkauskiene, S.; Stasiulis, A.; Viskelis, P.; Viškelis, J.; Urbonaviciene, D. The influence of amaranth (Amaranthus hypochondriacus) dietary nitrates on the aerobic capacity of physically active young persons. J. Int. Soc. Sports Nutr. 2020, 17, 37. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef]
- Bogaert, M.G. Clinical pharmacokinetics of nitrates. Cardiovasc. Drugs Ther. 1994, 8, 693–699. [Google Scholar] [CrossRef]
- Brender, J.D.; Olive, J.M.; Felkner, M.; Suarez, L.; Marckwardt, W.; Hendricks, K.A. Dietary Nitrites and Nitrates, Nitrosatable Drugs, and Neural Tube Defects. Epidemiology 2004, 15, 330–336. [Google Scholar] [CrossRef]
- Liubertas, T.; Poderys, J.; Vilma, Z.; Capkauskiene, S.; Viskelis, P. Impact of Dietary Potassium Nitrate on the Life Span of Drosophila melanogaster. Processes 2021, 9, 1270. [Google Scholar] [CrossRef]
- Moretti, C.H.; Schiffer, T.A.; Montenegro, M.F.; Larsen, F.J.; Tsarouhas, V.; Carlström, M.; Samakovlis, C.; Weitzberg, E.; Lundberg, J.O. Dietary nitrite extends lifespan and prevents age-related locomotor decline in the fruit fly. Free. Radic. Biol. Med. 2020, 160, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, T.; Foltz, C.; Karlsson, E.; Linton, C.G.; Smith, J.M. Health Evaluation of Experimental Laboratory Mice. Curr. Protoc. Mouse Biol. 2012, 2, 145–165. [Google Scholar] [CrossRef]
- Scudamore, C.L.; Busk, N.; Vowell, K. A simplified necropsy technique for mice: Making the most of unscheduled deaths. Lab. Anim. 2014, 48, 342–344. [Google Scholar] [CrossRef]
- Pettan-Brewer, C.; Treuting, P.M.M. Practical pathology of aging mice. Pathobiol. Aging Age-Related Dis. 2011, 1, 7202. [Google Scholar] [CrossRef]
- Mensinga, T.T.; Speijers, G.J.A.; Meulenbelt, J. Health implications of exposure to environmental nitrogenous compounds. Toxicol. Rev. 2003, 22, 41–51. [Google Scholar] [CrossRef]
- Jeffrey, J.S.; Andrew, L.M. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide. 2012, 26, 259–266. [Google Scholar] [CrossRef]
- Chow, C.; Chen, C.; Gairola, C. Effect of nitrate and nitrite in drinking water on rats. Toxicol. Lett. 1980, 6, 199–206. [Google Scholar] [CrossRef]
- Maekawa, A.; Ogiu, T.; Onodera, H.; Furuta, K.; Matsuoka, C.; Ohno, Y.; Odashima, S. Carcinogenicity studies of sodium nitrite and sodium nitrate in F-344 rats. Food Chem. Toxicol. 1982, 20, 25–33. [Google Scholar] [CrossRef]
- Hezel, M.P.; Liu, M.; Schiffer, T.A.; Larsen, F.J.; Checa, A.; Wheelock, C.E.; Carlström, M.; Lundberg, J.O.; Weitzberg, E. Effects of long-term dietary nitrate supplementation in mice. Redox Biol. 2015, 5, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.R.R.; Guimarães, D.D.; Flôr, A.F.L.; Leite, E.G.; Ruiz, C.R.; de Andrade, J.T.; Monteiro, M.M.; Balarini, C.M.; de Lucena, R.B.; Sandrim, V.C.; et al. Effects of chronic dietary nitrate supplementation on longevity, vascular function and cancer incidence in rats. Redox Biol. 2021, 48, 102209. [Google Scholar] [CrossRef] [PubMed]
- Roger, F.; Picazo, C.; Reiter, W.; Libiad, M.; Asami, C.; Hanzén, S.; Gao, C.; Lagniel, G.; Welkenhuysen, N.; Labarre, J.; et al. Peroxiredoxin promotes longevity and H2O2-resistance in yeast through redox-modulation of protein kinase A. eLife 2021, 9, e60346. [Google Scholar] [CrossRef] [PubMed]
- van Dam, E.; van Leeuwen, L.A.G.; dos Santos, E.; James, J.; Best, L.; Lennicke, C.; Vincent, A.J.; Marinos, G.; Foley, A.; Buricova, M.; et al. Sugar-Induced Obesity and Insulin Resistance Are Uncoupled from Shortened Survival in Drosophila. Cell Metab. 2020, 31, 710–725.e7. [Google Scholar] [CrossRef]
- Duranski, M.R.; Greer, J.J.; Dejam, A.; Jaganmohan, S.; Hogg, N.; Langston, W.; Patel, R.P.; Yet, S.-F.; Wang, X.; Kevil, C.G.; et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Investig. 2005, 115, 1232–1240. [Google Scholar] [CrossRef]
- Vitturi, D.A.; Patel, R.P. Current perspectives and challenges in understanding the role of nitrite as an integral player in nitric oxide biology and therapy. Free Radic. Biol. Med. 2011, 51, 805–812. [Google Scholar] [CrossRef]
- Li, W.; Meng, Z.; Liu, Y.; Patel, R.P.; Lang, J.D. The Hepatoprotective Effect of Sodium Nitrite on Cold Ischemia-Reperfusion Injury. J. Transplant. 2012, 2012, 635179. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef]
- Carlström, M.; Lundberg, J.O.; Weitzberg, E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol. 2018, 224, e13080. [Google Scholar] [CrossRef]
- Hunault, C.C.; van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Meulenbelt, J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol. Lett. 2009, 190, 48–53. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, X.; Sun, Y.; Yang, X. Effects of spinach nitrate on insulin resistance, endothelial dysfunction markers and inflammation in mice with high-fat and high-fructose consumption. Food Nutr. Res. 2016, 60, 32010. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Bakhtiarzadeh, F.; Jeddi, S.; Farrokhfall, K.; Zardooz, H.; Ghasemi, A. Nitrite increases glucose-stimulated insulin secretion and islet insulin content in obese type 2 diabetic male rats. Nitric Oxide 2017, 64, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Pennypacker, J.K. Drugs that modulate aging: The promising yet difficult path ahead. Transl. Res. 2014, 163, 456–465. [Google Scholar] [CrossRef]
- Harper, S. Economic and social implications of aging societies. Science 2014, 346, 587–591. [Google Scholar] [CrossRef]
- Bloom, D.E.; Chatterji, S.; Kowal, P.; Lloyd-Sherlock, P.; McKee, M.; Rechel, B.; Rosenberg, L.; Smith, J.P. Macroeconomic implications of population ageing and selected policy responses. Lancet 2015, 385, 649–657. [Google Scholar] [CrossRef]
- Petsko, G.A. A seat at the table. Genome Biol. 2008, 9, 113. [Google Scholar] [CrossRef]
- Hayflick, L. Biological Aging Is No Longer an Unsolved Problem. Ann. N. Y. Acad. Sci. 2007, 1100, 1–13. [Google Scholar] [CrossRef]
| Score | Animal Status | Agility | Coat | Body Posture | Health Status | Appetite |
|---|---|---|---|---|---|---|
| 5 | Excellent | Moves fast and a lot, climbs the cage, burrows in the beddings, curious | White, soft, shiny | Characteristic to species, movements are comfortable, body physiologically bent | No changes | Good, feeds constantly, drinks after eating |
| 4 | Very good | Moves fast and a lot, burrows in the beddings, doesn’t climb the cage mesh and walls, curious | White, sometimes matted, looks like wet | Characteristic to species, movements comfortable, body physiologically bent | No changes | Good, feeds constantly, drinks after eating |
| 3 | Good | Movements characteristic to species but agility is reduced, avoids interacting with other animals | The coat „wet “, „matted “, „sticky“ | Characteristic to species, the back curved, legs under the body | No changes | Appetite is reduced, shows less interest in food and water |
| 2 | Satisfactory | Moves less, burrows in the beddings, spends time laying down, lethargic | Coat yellowish, matted, looks wet | The body snuggled down, the spine bent in a hump, all feet under the body | No changes | The appetite is bad, no evident interest in food or water |
| 1 | Bad | Almost no movement, laying snuggled down on the bottom, the head down in the beddings, no interest in surrounding | Sticking, yellowish, with bald spots | The body snuggled down, the spine bent in a hump, all feet under the body | Possible discharge from the eyes, nose, and signs of diarrhoea, in some cases sniffing and coughing. Must additionally indicate when evaluating. | The appetite is bad, no evident interest in food or water. On the touch feels skinny, the ribs sticking out. |
| Organ Pathology | Survival Days: Mean ± SD (Number of Cases) | |||
|---|---|---|---|---|
| 0% KNO3 | 1% KNO3 | 2% KNO3 | 3% KNO3 | |
| Pulmonary hyperemia | - | 267 (1) | 340 ± 96 (2) | 301 ± 38 (2) |
| Pulmonary edema and hyperemia | 267 (1) | 137 ± 0 (2) | 152 ± 35 (3) | 202 ± 70 (3) |
| Pulmonary edema and hyperemia, liver failure | 224 ± 59 (2) | 126 ± 0 (2) | - | - |
| Pulmonary edema, hyperemia, liver and kidney failure | 189 ± 6 (3) | - | - | - |
| Number of organ pathologies: mean ± SD (number of cases) | 3.3 ± 0.8 (6) | 2.2 ± 0.8 (5) | 1.6 ± 0.6 (5) | 1.6 ± 0.6 (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liubertas, T.; Poderys, L.J.; Zigmantaite, V.; Capkauskiene, S.; Trakimas, G.; Pukenas, K.; Viskelis, P. Effects of Life-Long Supplementation of Potassium Nitrate on Male Mice Longevity and Organs Pathology. Appl. Sci. 2023, 13, 177. https://doi.org/10.3390/app13010177
Liubertas T, Poderys LJ, Zigmantaite V, Capkauskiene S, Trakimas G, Pukenas K, Viskelis P. Effects of Life-Long Supplementation of Potassium Nitrate on Male Mice Longevity and Organs Pathology. Applied Sciences. 2023; 13(1):177. https://doi.org/10.3390/app13010177
Chicago/Turabian StyleLiubertas, Tomas, Liudas Jonas Poderys, Vilma Zigmantaite, Sandrija Capkauskiene, Giedrius Trakimas, Kazimieras Pukenas, and Pranas Viskelis. 2023. "Effects of Life-Long Supplementation of Potassium Nitrate on Male Mice Longevity and Organs Pathology" Applied Sciences 13, no. 1: 177. https://doi.org/10.3390/app13010177
APA StyleLiubertas, T., Poderys, L. J., Zigmantaite, V., Capkauskiene, S., Trakimas, G., Pukenas, K., & Viskelis, P. (2023). Effects of Life-Long Supplementation of Potassium Nitrate on Male Mice Longevity and Organs Pathology. Applied Sciences, 13(1), 177. https://doi.org/10.3390/app13010177








