The San Giovanni Baptistery in Florence (Italy): Assessment of the State of Conservation of Surfaces and Characterization of Stone Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Evaluation of the State of Conservation: Main Decay Phenomena
3.2. Marbles
- Carrara marble (Italy), white and veined, with a typical homeoblastic fabric, a mosaic-polygonal microstructure, with straight grain boundaries, MGS ranging from 150 to 500 micron, and isotopic ratios of C from 1.63 to 2.35 and O from −3.25 to −1.16, is used in the original and substituted portions of monument (Figure 8a,b);
- Hymettus marble (Greece), likely coming from the spolia of Fiesole Roman Theatre, Capitolium, and Thermae [9] is a white bluish marble characterized by grey/blue veins. The fabric is heteroblastic, with mosaic, lineated microstructure, curved grain boundaries, and an MGS ranging from 1 mm and 2 mm. The isotopic ratio varies from 3.35 to 2.33 for C and from −3.15 to −1.97 for O (Figure 8c,d);
- Lasa marble, used in the 20th century for the substitution of the most weathered slabs, comes from Laas (Lasa, South Tyrol, Northern Italy), and was selected by restorers for the macroscopic similarities to Hymettus marble (Figure 8e) [34]. It is a pure calcitic marble, containing small amounts of quartz, with a homeoblastic fabric and mosaic microstructure. The grain boundaries are prevalently curved (Figure 8f). The isotopic ratios of C and O fall in the range of data published on Lasa marbles [35];
- Other ancient marbles are most probably re-used marbles (i.e., Paros, Pentelicum) for some decorative elements (Figure 4).
3.3. Mortars
3.4. Bricks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morolli, G. L’architettura del Battistero e l’ordine antico. In Il Battistero di San Giovanni; Paolucci, A., Ed.; Franco Cosimo Panini: Modena, Italy, 1994; pp. 33–132. [Google Scholar]
- Cardini, M. L’ipotesi tardo antica del Battistero. In Il bel San Giovanni e Santa Maria del Fiore; Cardini, D., Ed.; Le Lettere: Firenze, Italy, 1996; pp. 62–93. [Google Scholar]
- Rocchi Coopmans de Yoldi, G. Il Battistero di San Giovanni: Lo svolgimento della fabbrica. In Santa Maria del Fiore—Piazza, Battistero, Campanile; Rocchi Coopmans de Yoldi, G., Ed.; Università Degli Studi di Firenze, Dipartimento di Architettura, Il Torchio: Firenze, Italy, 1996; pp. 27–72. [Google Scholar]
- Degl’Innocenti, P. Misurare, disegnare, conoscere: Dai rilievi del San Giovanni alle ipotesi storico-costruttive. In Il Battistero di San Giovanni, Conoscenza, Diagnostica, Conservazione; Gurrieri, F., Ed.; Mandragora: Firenze, Italy, 2017; pp. 87–103. [Google Scholar]
- Siegesmund, S.; Torok, A. Building stones. In Stone in Architectures. Properties, Durability; Siegesmund, E., Snethlage, R., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2014. [Google Scholar]
- Antonelli, F.; Lazzarini, L. An updated petrographic and isotopic reference database for white marbles used in antiquity. Rend. Fis. Acc. Lincei 2015, 26, 399–413. [Google Scholar] [CrossRef]
- Moens, L.; Roos, P.; Rudder, J.D.; Paepe, P.D.; Hende, J.V.; Waelkens, M. A multi-method approach to the identification of white marbles used in antique artifacts. In Classical Marble: Geochemistry, Technology, Trade; Herz, N., Waelkens, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 243–250. [Google Scholar] [CrossRef]
- Lazzarini, L. Archaeometric aspects of white and coloured marbles used in antiquity: The state of the art. Per. Mineral. 2004, 73, 113–125. [Google Scholar]
- Coli, M.; Ciuffreda, A.L.; Donigaglia, T.; Bencaster, A.; Caciagli, S.; Agostini, B.; Iandelli, N. Saint John Baptistery in Florence (Italy): Studies for Conservation of the External Marble Cladding. Appl. Sci. 2021, 11, 6329. [Google Scholar] [CrossRef]
- Fratini, F.; Rescic, S.; Pittaluga, D. Serpentinite and ophicalcite in the architecture of eastern Liguria and as decoration of Tuscan religious buildings. Resour. Polic 2022, 75, 102505. [Google Scholar] [CrossRef]
- Pecchioni, E.; Magrini, D.; Cantisani, E.; Fratini, F.; Garzonio, C.A.; Nosengo, C.; Santo, A.P.; Vettori, S.A. Non-Invasive Approach for the Identification of “Red Marbles” from Santa Maria Del Fiore Cathedral (Firenze, Italy). Int. J. Archit. Herit. 2021, 15, 494–504. [Google Scholar] [CrossRef]
- Pecchioni, E.; Cantisani, E.; Fratini, F. La città di Firenze: Un museo di litologia all’aperto. In Il Museo di Storia Naturale dell’Università degli Studi di Firenze; Le collezioni mineralogiche e litologiche; Firenze University Press: Firenze, Italy, 2012; Volume IV, pp. 245–269. [Google Scholar]
- Malesani, P.; Pecchioni, E.; Cantisani, E.; Fratini, F. Geolithology and provenance of materials of some historical buildings and monuments in the centre of Florence (Italy). Episodes 2003, 26, 250–255. [Google Scholar] [CrossRef]
- Garzonio, C.A.; Cantisani, E.; Coli, M.; Cuzman, O.; Del Luca, D.; Lubrito, C.; Ricci, M.; Vettori, S.; Sibilia, E. I materiali costitutivi del Battistero. In Il Battistero di San Giovanni, Conoscenza, Diagnostica, Conservazione; Gurrieri, F., Ed.; Mandragora: Firenze, Italy, 2017; pp. 179–191. [Google Scholar]
- Elsen, J. Microscopy of historic mortars—A review. Cem. Concr. Res. 2006, 36, 1416–1424. [Google Scholar] [CrossRef]
- Ingham, J.P. Geomaterials under the Microscope. A Colour Guide; CRC Press: London, UK, 2010. [Google Scholar]
- Pecchioni, E.; Fratini, F.; Cantisani, E. Atlas of the Ancient Mortars in Thin Section under Optical Microscope, 2nd ed.; Nardini: Florence, Italy, 2020. [Google Scholar]
- Reedy, C.L. Thin-Section Petrography of Stone and Ceramic Cultural Materials; Archetype Publications: London, UK, 2008; p. 256. ISBN 978-1-9049-8233-3. [Google Scholar]
- Cantisani, E.; Fratini, F.; Pecchioni, E. Optical and Electronic Microscope for Minero-Petrographic and Microchemical Studies of Lime Binders of Ancient Mortars. Minerals 2022, 12, 41. [Google Scholar] [CrossRef]
- Arizzi, A.; Cultrone, G. Mortars and plasters-how to characterize hydraulic mortars. Archaeol. Anthropol. Sci. 2021, 13, 2–22. [Google Scholar] [CrossRef]
- Maggetti, M. Phase analysis and its significance for technology and origin. In Archaeological Ceramics; Olin, J.S., Franklin, A., Eds.; Smithsonian Institution Press: Washigton, DC, USA, 1982; pp. 121–133. [Google Scholar]
- Pavía, S. The determination of brick provenance and technology using analytical techniques from the physical sciences. Archaeometry 2006, 48, 201–218. [Google Scholar] [CrossRef] [Green Version]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Characterization of ancient, byzantine and later historic mortars by thermal and X-ray diffraction techniques. Thermochim. Acta 1995, 269–270, 779–795. [Google Scholar] [CrossRef]
- Bakolas, A.; Biscontin, G.; Contardi, V.; Franceschi, E.; Moropoulou, A.; Palazzi, D.; Zendri, E. Thermoanalytical research on traditional mortars in Venice. Thermochim. Acta 1995, 269–270, 817–828. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Bakolas, A.; Moropoulou, A. Physico-chemical study of Cretan ancient mortars. Cem. Concr. Res. 2003, 33, 651–661. [Google Scholar] [CrossRef]
- Cantisani, E.; Cuzman, O.A.; Vettori, S.; Chelazzi, L.; Ciattini, S.; Ricci, M.; Garzonio, C.A. A multi-analytical approach for the study of red stains on heritage marble. Analyst 2019, 144, 2375–2386. [Google Scholar] [CrossRef]
- Pensabene, P.; Antonelli, F.; Lazzarini, L.; Cancelliere, S. Provenance of marble sculptures and artifacts from the so-called Canopus and other buildings of ‘‘Villa Adriana’’ (Hadrian’s villa—Tivoli, Italy). J. Archaeol. Sci. 2012, 37, 994–1005. [Google Scholar] [CrossRef]
- Columbu, S.; Antonelli, F.; Lezzerini, M.; Miriello, D.; Adembri, B.; Blanco, A. Provenance of marbles used in the Heliocaminus Baths of Hadrian’s Villa (Tivoli, Italy). J. Archaeol. Sci. 2014, 49, 332–342. [Google Scholar] [CrossRef]
- Antonelli, F.; Columbu, S.; Lezzerini, M.; Miriello, D. Petrographic characterization and provenance determination of the white marbles used in the Roman sculptures of Forum Sempronii (Fossombrone, Marche, Italy). Appl. Phys. A 2014, 115, 1033–1040. [Google Scholar] [CrossRef]
- Antonelli, F.; Lazzarini, L. The use of white marble in the central and upper adriatic between Greece and Rome: Hellenistic Stelae from the necropolis of Ancona (Italy). Camb. Archaeol. J. 2013, 23, 149–162. [Google Scholar] [CrossRef]
- Attanasio, D.; Boschi, C.; Bracci, S.; Cantisani, E.; Paolucci, F. Provenance Studies of the Marble of Ancient Sculptures in the Tribune of the Uffizi Gallery, Florence. Archaeometry 2015, 57, 74–89. [Google Scholar] [CrossRef]
- Attanasio, D.; Boschi, C.; Bracci, S.; Cantisani, E.; Paolucci, F. The Greek and Asiatic marbles of the Florentine Niobids. J. Archaeol. Sci. 2016, 66, 103–111. [Google Scholar] [CrossRef]
- Attanasio, D.; Brilli, M.; Ogle, N. The Isotopic Signatures of Classical Marbles; L’erma di Bretschneider: Rome, Italy, 2006; p. 279. [Google Scholar]
- Bianchini, P. I Paramenti esterni. I materiali, i restauri degli anni 1938–1944 e cenni sullo stato di conservazione attuale. In Santa Maria del Fiore—Piazza, Battistero, Campanile; Rocchi Coopmans de Yoldi, G., Ed.; Università Degli Studi di Firenze, Dipartimento di Architettura; Il Torchio: Firenze, Italy, 1996; pp. 97–98. [Google Scholar]
- Unterwurzacher, M.; Obojes, U. White marble from Laas (Lasa), south Tyrol-its occurrence, use and petrographic-isotopical characterisation. Austrian J. Earth Sci. 2012, 105, 26–37. [Google Scholar]
- Iandelli, N.; Coli, M.; Donigaglia, T.; Ciuffreda, A.L. An Unconventional Field Mapping Application: A Complete Opensource Workflow Solution Applied to Lithological Mapping of the Coatings of Cultural Heritage. ISPRS Int. J. Geo. Inf. 2021, 10, 357. [Google Scholar] [CrossRef]
- Cantisani, E.; Calandra, S.; Barone, S.; Caciagli, S.; Fedi, M.; Garzonio, C.A.; Liccioli, L.; Salvadori, B.; Salvatici, T.; Vettori, S. The mortars of Giotto’s Bell Tower (Florence, Italy): Raw materials and technologies. Constr. Build. Mater 2021, 267, 120801. [Google Scholar] [CrossRef]
- Cantisani, E.; Falabella, A.; Fratini, F.; Pecchioni, E.; Vettori, S.; Antonelli, F.; Giamello, M.; Lezzerini, M. Production of the Roman Cement in Italy: Characterization of a raw material used in Tuscany between 19th and 20th century and its comparison with a commercialized French stone material. Int. J. Archit. Herit. 2018, 12, 1038–1050. [Google Scholar] [CrossRef]
- Lezzerini, M.; Ramacciotti, M.; Cantini, F.; Fatighenti, B.; Antonelli, F.; Pecchioni, E.; Fratini, F.; Cantisani, E.; Giamello, M. Archaeometric study of natural hydraulic mortars: The case of the Late Roman Villa dell’Oratorio (Florence, Italy). Archaeol. Anthrop. Sci. 2017, 9, 603–615. [Google Scholar] [CrossRef]
- Megna, B.; Rizzo, G.; Ercoli, L. The mortars and plasters under the mosaics and the wall paintings of the Roman villa at Piazza Armerina, Sicily. In Proceedings of the 2nd Conference on Historic Mortars—HMC 2010 and RILEM TC 203-RHM Final Workshop, Prague, Czech Republic, 22–24 September 2010; pp. 275–283. [Google Scholar]
- Riccardi, M.P.; Messiga, B.; Duminuco, P. An approach to the dynamics of clay firing. Appl. Clay Sci. 1999, 15, 393–409. [Google Scholar] [CrossRef]
- Cultrone, G.; Rodriguez-Navarro, C.; Sebastian, E.; Cazalla, O.; De la Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Mineral. 2001, 13, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Galli, A.; Martini, M.; Maspero, F.; Panzeri, L.; Sibilia, E. Surface dating of bricks, an application of luminescence techniques. Eur. Phys. J. Plus 2014, 129, 101. [Google Scholar] [CrossRef]





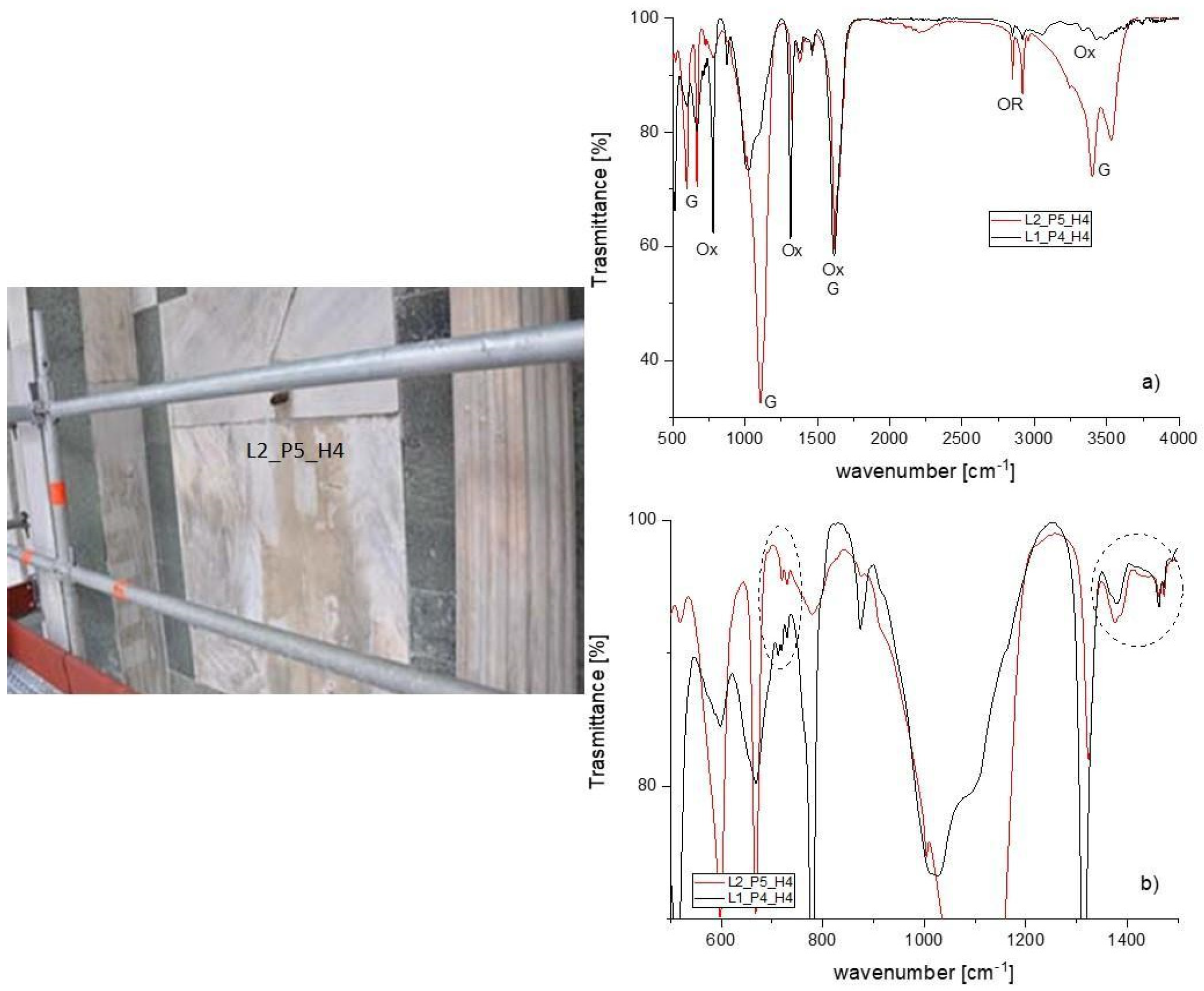
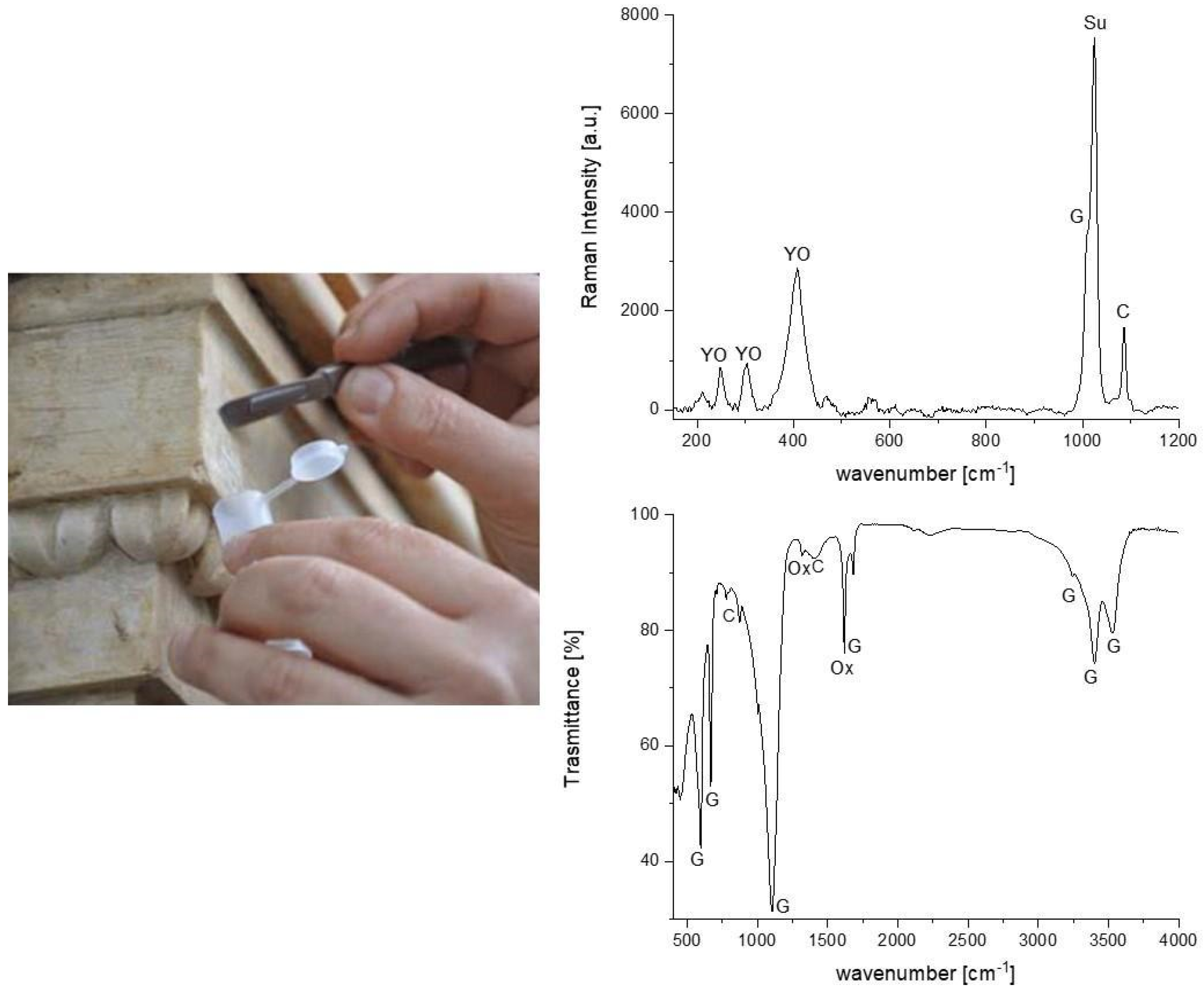


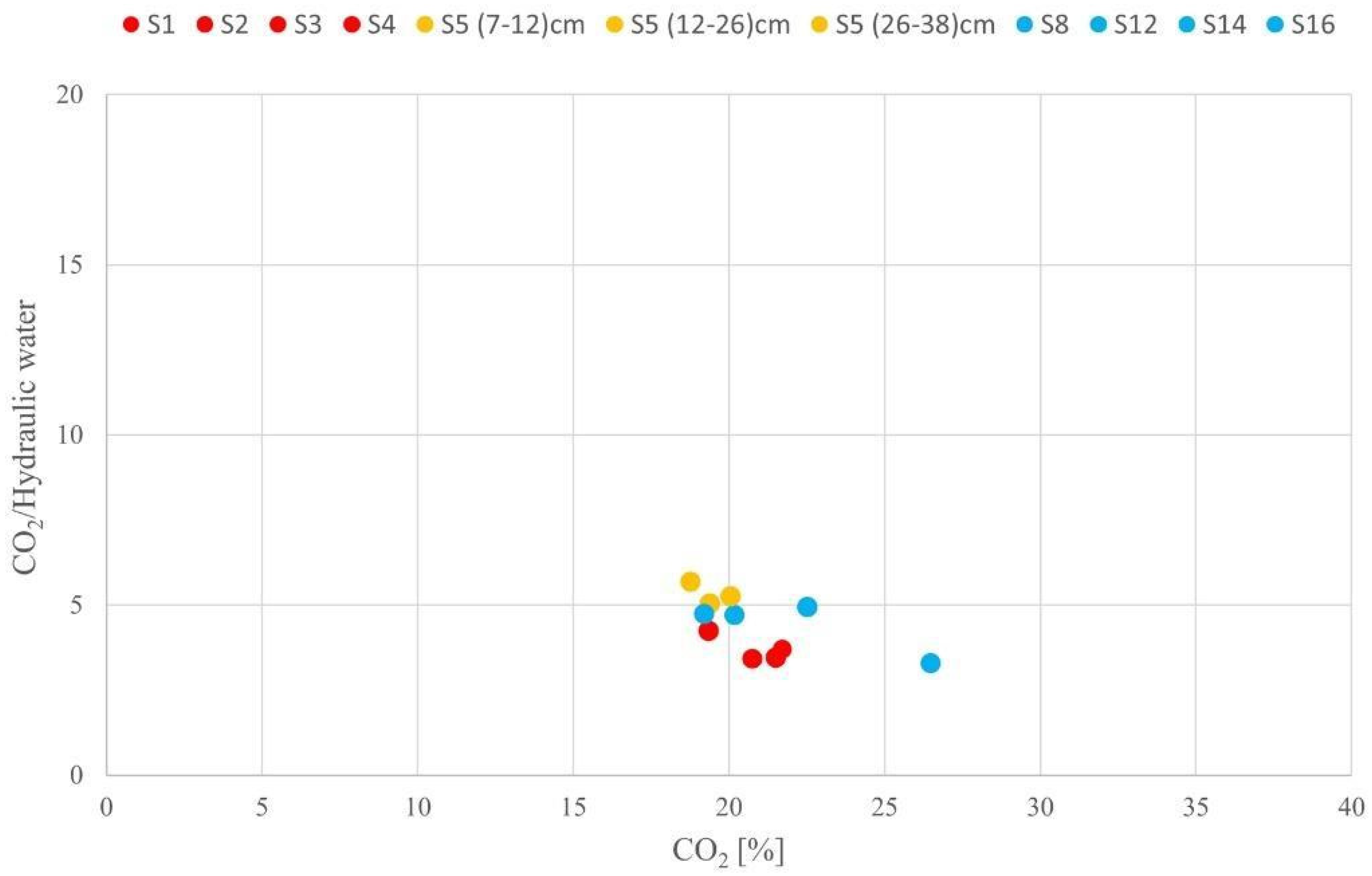

| Type of Materials | Samples Number | Type of Analyzes | Position | Notes |
|---|---|---|---|---|
| Patina, encrustations, and other decay phenomena | 25 | XRPD, FT-IR, OM, Raman | sides 1, 3, 5, 6, 8, 9, 10 | for the evaluation of the state of conservation and the identification of past treatments |
| White and veined marbles | 27 | XRPD, OM, C and O Isotopes | side 2 (5 samples) side 3 (2 samples) side 4 (5 samples) side 6 (9 samples) side 7 (2 samples) side 8 (1 sample) side 9 (2 samples) | for provenance assessment |
| Mortars | 17 (core samples) | XRPD, OM, TGA | side 1 (1 core samples) side 4 (2 core sample) side 10 (2 core samples) basements (12 core samples) | for identification of raw materials and technologies of production |
| Bricks | 4 | XRPD, OM | side 4 | for identification of differences related to different size and dating |
| Id Sample | Position | Mineralogical Composition | MGS | Type of Fabric | Main Features of Microstructure | Grain Boundaries | δ13C | δ18O | Provenance |
|---|---|---|---|---|---|---|---|---|---|
| M1 | Side 6 | Calcite | 1 mm | HE | Mosaic, lineated with deformed crystals | Curved | 3.35 | −2.56 | Hymettus |
| M2 | Side 6 | Calcite, quartz * | 650 μm | HO | Mosaic | Curved | 1.46 | −5.19 | Lasa |
| M3 | Side 6 | Calcite, quartz * | 800 μm | HE | Mosaic, lineated | Straight/curved | - | - | Uncertain attribution |
| M4 | Side 6 | Calcite | 350 μm | HO | Mosaic, polygonal | Straight | - | - | Carrara |
| M5 | Side 2 | Calcite | 1.5 mm | HE | Mosaic, fine-grained areas and strained crystals | Curved | 2.77 | −0.46 | Greek marble (Paros II) |
| M6 | Side 8 | Calcite | 400 μm | HO | Mosaic, polygonal | Straight | - | - | Carrara |
| M7 | Side 8 | Calcite, mica *, quartz * | 550 μm | HE | Mortar, lineated | Straight/curved | - | - | Uncertain attribution |
| M8 | Side 6 | Calcite, quartz * | 150 μm | HO | Mosaic, polygonal | Straight | 1.63 | −3.25 | Carrara |
| M9 | Side 7 | Calcite | 500 μm | HO | Mosaic, polygonal | Straight | 2.01 | −1.73 | Carrara |
| M10 | Side 8 | Calcite, mica *, quartz * | 550 μm | HE | Mosaic | Straight/curved | 1.32 | −7.77 | Pentelicum |
| M11 | Side 9 | Calcite, dolomite, mica *, quartz * | 550 μm | HE | Mosaic | Straight/curved | 0.15 | −6.56 | Pentelicum |
| M12 | Side 4 | Calcite, mica *, quartz * | 350 μm | HO | Mosaic, polygonal | Straight | 2.10 | −1.51 | Carrara |
| M13 | Side 4 | Calcite, quartz * | 350 μm | HO | Mosaic, polygonal | Straight | 2.15 | −1.67 | Carrara |
| M14 | Side 3 | Calcite, dolomite *, quartz * | 1 mm | HE | Lineated | Curved/embayed | 1.81 | −8.12 | Pentelicum |
| M15 | Side 2 | Calcite, quartz * | 500 μm | HO | Mosaic, polygonal | Straight | 2.00 | −1.51 | Carrara |
| M16 | Side 2 | Calcite, quartz * | 350 μm | HO | Mosaic, polygonal | Straight | 2.10 | −1.51 | Carrara |
| M17 | Side 2 | Calcite, mica *, quartz * | 500 μm | HO | Mosaic, polygonal | Straight | 2.15 | −1.67 | Carrara |
| M18 | Side 6 | Calcite, mica *, quartz * | 500 μm | HO | Mosaic, polygonal | Straight | 2.35 | −1.16 | Carrara |
| M19 | Side 6 | Calcite, mica *, dolomite *, quartz * | 500 μm | HO | Mosaic, polygonal | Straight | 2.11 | −1.76 | Carrara |
| M20 | Side 4 | Calcite, Kfeld *, quartz * | 1.5 mm | HE | Mortar | Straight/curved | 3.07 | −1.97 | Hymettus |
| M21 | Side 1 | Calcite, dolomite, mica *, quartz * | 2 mm | HE | Mortar, with deformed crystals | Curved/sutured | 2.33 | −3.15 | Hymettus |
| M22 | Side 6 | Calcite, quartz * | 500 μm | HO | Mosaic, polygonal | Straight | 2.07 | −2.22 | Carrara |
| M23 | Side 4 | Calcite, quartz * | 500 μm | HO | Mosaic, polygonal | Straight | 2.00 | −2.08 | Carrara |
| M24 | Side 4 | Calcite, dolomite *, quartz * | 500 μm | HO | Mosaic, polygonal | Straight | 2.21 | −2.02 | Carrara |
| M25 | Side 9 | Calcite, dolomite *, quartz * | 2 mm | HE | Mortar, bimodal distribution | Curved/sutured | 4.16 | −1.60 | Thassos/Proconneso |
| M26 | Side 6 | Calcite, dolomite *, mica * | 600 μm | HE | Lineated | Curved/embayed | 2.61 | −7.23 | Pentelicum |
| M27 | Side 3 | Calcite, mica * | 700 μm | HE | Lineated | Curved/embayed | 2.54 | −7.03 | Pentelicum |
| Mortar Samples | Position | Depth | Quartz | Calcite | Plagioclase | K Feldspar | Other |
|---|---|---|---|---|---|---|---|
| S1 | Attic, side 10 | 10–15 cm | +++ | + | + | - | Muscovite (*), chlorite (*) |
| S2 | Attic, side 10 | 9–14 cm | +++ | ++ | + | + | Muscovite (+), chlorite (*) |
| S3 | Attic, side 4–5 | 9–25 cm | +++ | + | + | + | Muscovite (*), chlorite (*) |
| 25–30 cm | +++ | + | + | + | Muscovite (*), chlorite (*) | ||
| S4 | Attic, side 4 | 4–20 cm | +++ | + | + | - | Muscovite (*), chlorite (*) |
| 20–35 cm | +++ | ++ | ++ | * | Muscovite (*), chlorite (*), portlandite (*) | ||
| S5 | Women’s gallery, side 1 | 7–12 cm | +++ | ++ | + | + | Muscovite (+), chlorite (*) |
| 12–26 cm | +++ | ++ | + | + | Muscovite (*), chlorite (*) | ||
| 26–38 cm | +++ | ++ | ++ | + | Muscovite (*), chlorite (*) | ||
| S6 | Basements, side 9–10 | 0–10 cm | ++ | +++ | + | - | Muscovite (*) |
| 10–32 cm | +++ | ++ | * | - | Muscovite (*) | ||
| S7 | Basements, side 9–10 | 0–11 cm | +++ | ++ | * | * | Muscovite (*), chlorite (*), vaterite (*) |
| 11–32 cm | +++ | + | + | - | Muscovite (*), chlorite (*), gypsum (*) | ||
| S8 | Basements, side 8 | 0–12 cm | +++ | ++ | ++ | + | Muscovite (+), chlorite (*) |
| 12–38 cm | +++ | + | + | * | Muscovite (*), chlorite (*) | ||
| S9 | Basements, side 9 | 0–11 cm | +++ | + | * | - | Muscovite (*) |
| 11–21 cm | +++ | + | + | + | Muscovite (*), chlorite (*) | ||
| 21–38 cm | +++ | ++ | + | - | Muscovite (*), chlorite (*) | ||
| S10 | Basements, side 9–10 | 0–15 cm | +++ | ++ | * | - | Muscovite (*) |
| 15–38 cm | +++ | ++ | * | * | Muscovite (*) | ||
| S11 | Basements, side 9 | 0–5 cm | +++ | ++ | + | + | Muscovite (*) |
| S12 | Basements, central part | 0–4 cm | +++ | ++ | + | + | Muscovite (*), vaterite (+) |
| S13 | Basements, central part | 0–5 cm | +++ | + | + | - | Muscovite (*), chlorite (*), gypsum (*), vaterite (+) |
| S14 | Basements, central part | 0–4 cm | +++ | ++ | + | * | Muscovite (*), chlorite (*) |
| S15 | Basements, central part | 0–2 cm | +++ | ++ | + | * | Muscovite (*), chlorite (*) |
| S16 | Basements, side 7 | 4–12 cm | +++ | + | * | - | Muscovite (*) |
| S17 | Basements, side 6 | 0–2 cm | +++ | + | + | + | Muscovite (*), chlorite (*) |
| Mortar Samples | Depth | Binder | Aggregate | B/A | Macroporosity |
|---|---|---|---|---|---|
| S1 | S1 (10–15) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Numerous lumps | Composition: quartz (monocrystalline), feldspar, rock fragments (quartzite, sandstone, calcarenite, micritic limestone) and few cocciopesto grains Homogeneous grain size distribution Grain size: 100 μm–700 μm Shape: sub rounded | 1/3–1/4 | Medium amount due to pores of irregular shape |
| S2 | S2 (9–14) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Numerous lumps | Composition: quartz (monocrystalline), feldspar, calcite, rock fragments (quartzite, sandstone, calcarenite, micritic limestone) Homogeneous grain size distribution Grain size 100 μm–>1 mm Shape: sub angular/sub rounded | 1/3–1/4 | Medium amount due to pores of irregular shape |
| S3 | S3 (9–25) cm | Natural hydraulic lime and homogeneous structure and micritic texture. Some lumps | Composition: quartz (monocrystalline), feldspar, calcite, quartzite, few carbonates rock fragments and cocciopesto Homogeneous grain size distribution Mean grain size 150–200 μm, few mm grains Shape: sub angular | 1/3 | Medium amount due to pores elongated and sub rounded shape |
| S3 (25–30) cm | Natural hydraulic lime and homogeneous structure and micritic texture. Numerous lumps | Composition: quartz (monocrystalline), feldspar, rock fragments (quartzite, calcarenite, micritic limestone, flint) and few cocciopesto grains Homogeneous grain size distribution Grain size 100 μm–>1 mm Shape: sub angular | 1/3 | Medium amount due to pores of sub rounded shape | |
| S4 | S4 (4–20) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Slight recrystallized binder Some lumps | Composition: quartz (monocrystalline), feldspar, rock fragments (quartzite, sandstone, calcarenite, micritic limestone) and few cocciopesto grains Homogeneous grain size distribution Grain size: 100 μm–700 μm Shape: sub angular | 1/3 | Medium amount due to microcracks and pores of sub rounded shape |
| S4 (20–35) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder Some lumps | Composition: quartz (monocrystalline), feldspar, rock fragments (quartzite, sandstone, calcarenite, micritic limestone) and few cocciopesto grains Homogeneous grain size distribution Grain size 100 μm–1 mm Shape: sub angular/sub rounded | 1/2–1/3 | Medium/high amount due to pores of irregular and sub rounded shape | |
| S5 | S5 (7–12) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Numerous lumps | Composition: quartz (monocrystalline), feldspar, rock fragments (quartzite, sandstone, micritic limestone) Heterogeneous grain size distribution Grain size 100 μm–600 μm Shape: sub angular/sub rounded | 1/3 | Medium amount due to microcracks and pores of irregular shape |
| S5 (12–26) cm | Natural hydraulic lime and heterogeneous structure and micritic texture. Recrystallized binder. Numerous lumps | Composition: quartz (monocrystalline), feldspar, several fragments of carbonate rocks and quartzite, sandstone rocks Homogeneous grain size distribution Grain size 100 μm–600 μm Shape: sub angular/ sub rounded | 1/2–1/3 | Medium amount due to pores of irregular and sub rounded shape | |
| S5 (26–38) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Numerous lumps | Composition: quartz (monocrystalline), feldspar, rock fragments (quartzite, calcarenite, micritic limestone, marble) and few cocciopesto grains Homogeneous grain size distribution Grain size 100 μm–600 μm Shape: sub angular/sub rounded | 1/2–1/3 | Medium amount due to microcracks and pores of sub rounded and irregular shape | |
| S6 | S6 (0–10) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, abundant fragments of carbonate rocks Homogeneous grain size distribution Grain size 100 μm–1 mm Shape: sub angular/sub rounded | 1/3–1/4 | High amount due to pores of irregular |
| S6 (10–32) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, abundant fragments of carbonate rocks Homogeneous grain size distribution Grain size 100 μm–1 mm Shape: sub angular/sub rounded | 1/3–1/4 | High amount due to pores of irregular shape | |
| S7 | S7 (0–11) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, rock fragments (micritic limestone, flint, quartzite, calcarenite) Homogeneous grain size distribution Grain size 100 μm–1 mm Shape: sub angular/sub rounded | 1/3–1/4 | High amount due to pores of irregular shape |
| S7 (11–32) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Some lumps. | Composition: quartz (monocrystalline), feldspar, abundant fragments of carbonate rocks, fragments of sandstone Homogeneous grain size distribution Grain size 100 μm–1 mm Shape: sub angular/sub rounded | 1/3–1/4 | High amount due to pores of irregular shape | |
| S8 | S8 (0–12) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, quartzite, few fragments of carbonate rocks and flint Homogeneous grain size distribution Grain size 100 μm–1 mm Shape: sub angular/sub rounded | 1/4 | Medium/ high amount due to pores of irregular shape |
| S8 (12–38) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Numerous lumps | Composition: quartz (monocrystalline), feldspar, quartzite, few fragments of carbonate rocks Homogeneous grain size distribution Grain size 100 μm–1 mm Shape: sub angular/sub rounded | 1/3–1/4 | Low amount due to pores of irregular shape | |
| S9 | S9 (0–11) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, mica, quartzite, few fragments of carbonate rocks Homogeneous grain size distribution Grain size 100 μm–500 μm Shape: sub angular/sub rounded | 1/3–1/4 | High amount due to pores of irregular shape |
| S9 (11–21) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, mica, quartzite, few fragments of carbonate rocks and cocciopesto Homogeneous grain size distribution Grain size 100 μm–700 μm Shape: sub angular/sub rounded | 1/3–1/4 | High amount due to pores of irregular shape | |
| S9 (21–38) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, mica, quartzite, few fragments of carbonate rocks (with fossil, calcarenite) Homogeneous grain size distribution Grain size 100 μm–500 μm Shape: sub angular/sub rounded | 1/3 | High amount due to pores of irregular shape | |
| S10 | S10 (0–15) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, mica, quartzite, large and abundant fragments of carbonate rocks (with fossil, calcarenite) Heterogeneous grain size distribution Grain size 100 μm–700 μm Shape: sub angular | 1/3 | High amount due to pores of irregular shape |
| S10 (15–38) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Few lumps | Composition: quartz (monocrystalline), feldspar, mica, quartzite, calcite, abundant fragments of carbonate rocks (with fossil, calcarenite) and fragments of cocciopesto Heterogeneous grain size distribution Grain size 100 μm–700 μm Shape: sub angular | 1/3 | High amount due to pores of irregular shape | |
| S11 | S11 (0–21) cm | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Few lumps | Composition: quartz (monocrystalline), feldspar, quartzite, flint, calcite, abundant fragments of carbonate rocks (with fossil, calcarenite) and fragments of cocciopesto Homogeneous grain size distribution Grain size 100 μm–800 μm Shape: sub angular | 1/3–1/4 | High amount due to pores of irregular shape |
| S12 | Lime hydraulicized with crushed ceramics and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Absent lumps | Composition: cocciopesto, calcite, carbonate fragments and some fragments of sandstone, abundant fragments of carbonate rocks Heterogeneous grain size distribution (bimodal) Grain size 50 μm–1 mm Shape: sub angular/sub rounded | 1/2–1/3 | Medium amount due to pores of irregular shape | |
| S13 | Natural hydraulic lime and homogeneous structure and microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, rock fragments (micritic limestone, quartzite) and few fragments of cocciopesto Heterogeneous grain size distribution Grain size 100 μm–800 μm Shape: sub angular | 1/2 | High amount due to pores of irregular shape | |
| S14 | Natural hydraulic lime and homogeneous structure and micritic texture. Some lumps | Composition: quartz (monocrystalline), feldspar, sandstone few fragments of carbonate and rare fragments of cocciopesto Homogeneous grain size distribution Grain size 100 μm–500 μm Shape: sub angular | 1/1–1/2 | Medium amount due to pores of irregular shape | |
| S15 | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, few fragments of carbonate rocks and cocciopesto Homogeneous grain size distribution Grain size 100 μm–500 μm Shape: sub angular/sub rounded | 1/3 | High amount due to pores of irregular shape | |
| S16 | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Recrystallized binder. Some lumps | Composition: quartz (monocrystalline), feldspar, fragments of sandstone, few fragments of carbonate rocks and cocciopesto Homogeneous grain size distribution Grain size 100 μm–500 μm Shape: sub angular | 1/3–1/4 | Medium amount due to pores of irregular shape | |
| S17 | Natural hydraulic lime and heterogeneous structure and micritic/microsparitic texture. Some lumps | Composition: quartz (monocrystalline), feldspar, few fragments of carbonate rocks and cocciopesto Homogeneous grain size distribution Grain size 100 μm–800 μm Shape: sub angular | 1/3 | High amount due to pores of irregular shape |
| Brick Samples | Groundmass | Framework | Macroporosity | XRD Data |
|---|---|---|---|---|
| 1A | Low birefringence | Composition: quartz, micas, plagioclase and k feldspar Well sorted Grain size 150–300 μm Shape: sub rounded/sub angular | Low, presence inside the pores of recrystallization of calcite | Quartz +++ Calcite * Plagioclase + K feldspar * Muscovite * |
| 1B | Low birefringence | Composition: quartz, micas and plagioclase Well sorted Grain size 150–300 μm Shape: sub angular | Low, presence inside the pores of recrystallization of calcite | Quartz +++ Calcite * Plagioclase + Diopside * |
| 1C | Low birefringence | Composition: quartz, plagioclase, carbonate rock fragments Medium sorted Grain size 150–500 μm Shape: sub angular | Medium | Quartz +++ Calcite + Plagioclase + |
| 1D | Low birefringence | Composition: quartz, micas, plagioclase, carbonate rock fragment Grain size distribution (bimodal) 150–200 μm 1–2 mm Shape: sub rounded/sub angular | Medium | Quartz +++ Calcite * Plagioclase + Muscovite * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calandra, S.; Cantisani, E.; Vettori, S.; Ricci, M.; Agostini, B.; Garzonio, C.A. The San Giovanni Baptistery in Florence (Italy): Assessment of the State of Conservation of Surfaces and Characterization of Stone Materials. Appl. Sci. 2022, 12, 4050. https://doi.org/10.3390/app12084050
Calandra S, Cantisani E, Vettori S, Ricci M, Agostini B, Garzonio CA. The San Giovanni Baptistery in Florence (Italy): Assessment of the State of Conservation of Surfaces and Characterization of Stone Materials. Applied Sciences. 2022; 12(8):4050. https://doi.org/10.3390/app12084050
Chicago/Turabian StyleCalandra, Sara, Emma Cantisani, Silvia Vettori, Marilena Ricci, Beatrice Agostini, and Carlo Alberto Garzonio. 2022. "The San Giovanni Baptistery in Florence (Italy): Assessment of the State of Conservation of Surfaces and Characterization of Stone Materials" Applied Sciences 12, no. 8: 4050. https://doi.org/10.3390/app12084050
APA StyleCalandra, S., Cantisani, E., Vettori, S., Ricci, M., Agostini, B., & Garzonio, C. A. (2022). The San Giovanni Baptistery in Florence (Italy): Assessment of the State of Conservation of Surfaces and Characterization of Stone Materials. Applied Sciences, 12(8), 4050. https://doi.org/10.3390/app12084050






