Characteristics and Applications of Biochar in Soil–Plant Systems: A Short Review of Benefits and Potential Drawbacks
Abstract
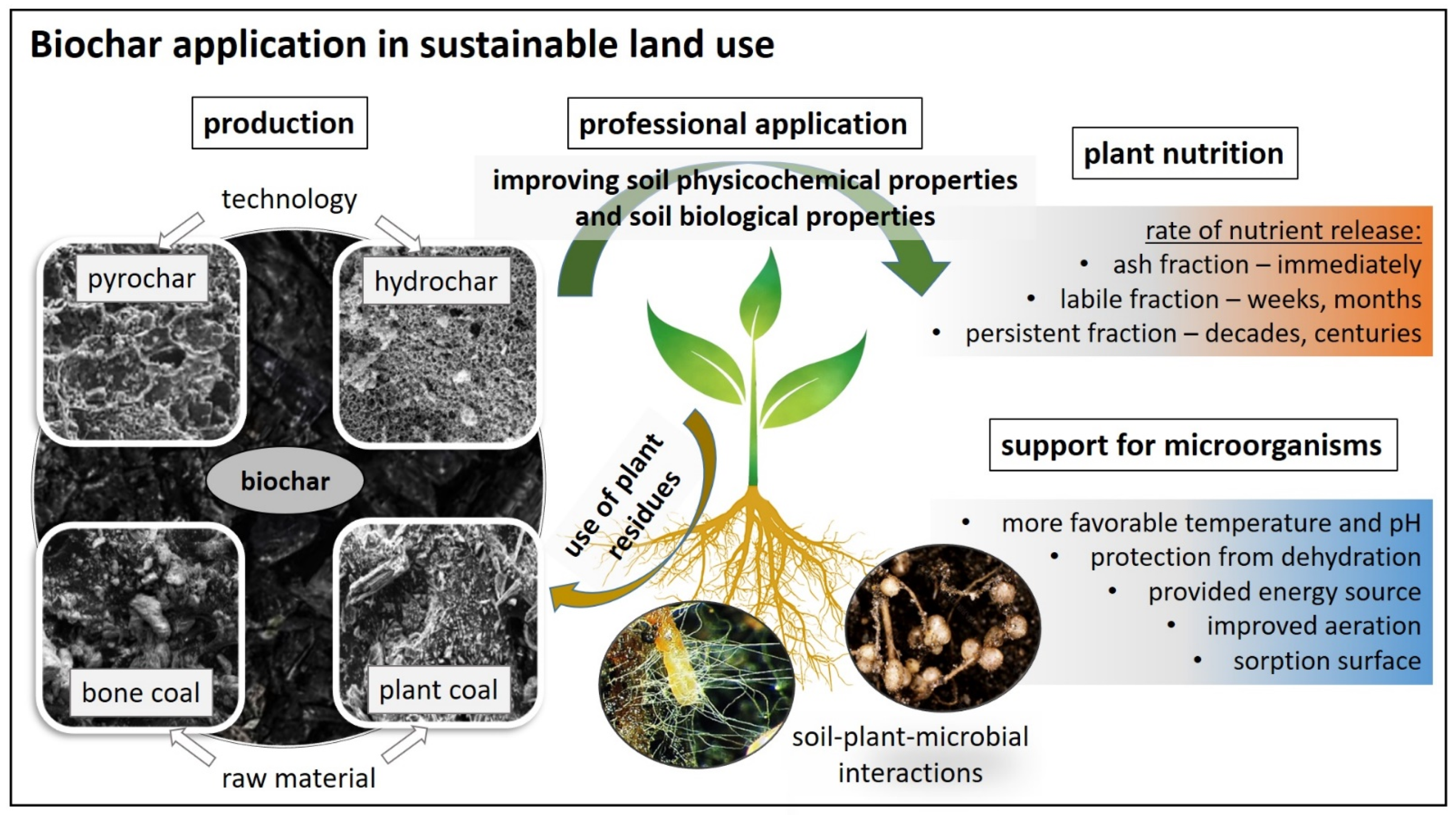
:1. Introduction
2. Approach to the Concept and Production of Biochar
- Biochar produced a relatively low (450–550 °C) pyrolyzing temperature, most often produced with high carbon-content substances according to the literature. It is mainly capable of long-term binding of groundwater and dissolved ions, and usually originating from plant residues, byproducts, and/or animal manures.
- Biochar produced from animal bones at high temperatures (600–650 °C or higher) with a high calcium phosphate content, along with apatite minerals with significantly lower carbon contents.
3. Physicochemical Properties of Soils Affected by Biochar
4. Biological Properties of Soils Affected by Biochar
5. Soil Productivity Effects of Biochar
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.; De Neve, S. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Ragályi, P.; Bernhardt, B.; Rékási, M.; Draskovits, E.; Molnár, S.; Molnár, M.; Uzinger, N. Effect of biochar and microbial inoculant on the element compostion and element yield of maize on acidic and on calcareous sandy soils. Agrokémia Talajt. 2019, 68, 115–137. [Google Scholar] [CrossRef]
- Pabar, S.A.; Mónok, D.; Kotroczó, Z.; Biró, B. Soil microbial parameters and synergies between bean growth and microbial inoculums as a dependence of five soils with different characteristics. Hung. Agric. Eng. 2020, 37, 27–33. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Kocsis, T.; Biró, B.; Ulmer, Á.; Szántó, M.; Kotroczó, Z. Time-lapse effect of ancient plant coal biochar on some soil agrochemical parameters and soil characteristics. Environ. Sci. Pollut. Res. 2018, 25, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion-a review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar]
- Fuchs, M.R.; Garcia-Perez, M.; Small, P.; Flora, G. Campfire lessons: Breaking down the combustion process to understand biochar production and characterization. Biochar J. 2014. Available online: www.biochar-journal.org/en/ct/44 (accessed on 1 April 2022).
- Conte, P.; Nestle, N. Water dynamics in different biochar fractions. Magn. Reson. Chem. 2015, 53, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Mohammad-Khah, A.; Ansari, R. Activated charcoal: Preparation, characterization and applications: A review article. Int. J. ChemTech Res. 2009, 1, 859–864. [Google Scholar]
- Kleber, M.; Hockaday, W.; Nico, P.S. Characteristics of biochar: Macro-molecular properties. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 143–170. [Google Scholar]
- Kocsis, T.; Pabar, S.A.; Ferschl, B.; Kotroczó, Z.; Mohácsi-Farkas, C.; Biró, B. Biotic and abiotic risks of soil biochar treatment for food safety and human health. Acta Univ. Sapientiae Aliment. 2020, 13, 69–84. [Google Scholar] [CrossRef]
- Lawal, A.A.; Hassan, M.A.; Zakaria, M.R.; Yusoff, M.Z.M.; Norrrahim, M.N.F.; Mokhtar, M.N.; Shirai, Y. Effect of oil palm biomass cellulosic content on nanopore structure and adsorption capacity of biochar. Bioresour. Technol. 2021, 332, 125070. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Banik, C.; Laird, D.A. Quantification and characterization of chemically-and thermally-labile and recalcitrant biochar fractions. Chemosphere 2018, 194, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, B.; Liu, G.; Abbas, Q.; Wang, R.; Ali, M.U.; Ullah, H.; Zhou, C. Systematic investigation on combustion characteristics and emission-reduction mechanism of potentially toxic elements in biomass-and biochar-coal co-combustion systems. Appl. Energy 2017, 208, 142–157. [Google Scholar] [CrossRef]
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar effects on crop yields with and without fertilizer: A meta-analysis of field studies using separate controls. Soil Use Manag. 2020, 36, 2–18. [Google Scholar] [CrossRef]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of biochar addition on the humic substances of composting manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef]
- Mao, J.D.; Johnson, R.L.; Lehmann, J.; Olk, D.C.; Neves, E.G.; Thompson, M.L.; Schmidt-Rohr, K. Abundant and stable char residues in soils: Implications for soil fertility and carbon sequestration. Environ. Sci. Technol. 2012, 46, 9571–9576. [Google Scholar] [CrossRef]
- Grossman, J.M.; O’Neill, B.E.; Tsai, S.M.; Liang, B.; Neves, E.; Lehmann, J.; Thies, J.E. Amazonian anthrosols support similar microbial communities that differ distinctly from those extant in adjacent, unmodified soils of the same mineralogy. Microb. Ecol. 2010, 60, 192–205. [Google Scholar] [CrossRef]
- International Energy Agency. Annual Report—IEA Bioenergy. Task 34 Pyrolysis of Biomass. 2006. Available online: http://www.globalbioenergy.org/uploads/media/0707_IEA_-_Bioenergy_annual_report.pdf (accessed on 1 April 2022).
- Rehrah, D.; Reddy, M.R.; Novak, J.M.; Bansode, R.R.; Schimmel, K.A.; Yu, J.; Ahmedna, M. Production and characterization of biochars from agricultural by-products for use in soil quality enhancement. J. Anal. Appl. Pyrolysis 2014, 108, 301–309. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Mahmoud, M.E.; Ahmed, S.B.; Huff, M.D.; Lee, J.W.; Kumar, S. Biochar from woody biomass for removing metal contaminants and carbon sequestration. J. Ind. Eng. Chem. 2015, 22, 103–109. [Google Scholar] [CrossRef]
- Côrtes, L.N.; Druzian, S.P.; Streit, A.F.M.; Godinho, M.; Perondi, D.; Collazzo, G.C.; Dotto, G.L. Biochars from animal wastes as alternative materials to treat colored effluents containing basic red 9. J. Environ. Chem. Eng. 2019, 7, 103446. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Production and characterization of bio-chars from biomass via pyrolysis. Energy Sources Part A 2006, 28, 413–422. [Google Scholar] [CrossRef]
- Gaunt, J.L.; Lehmann, J. Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ. Sci. Technol. 2008, 42, 4152–4158. [Google Scholar] [CrossRef]
- Omara, P.; Aula, L.; Oyebiyi, F.B.; Eickhoff, E.M.; Carpenter, J.; Raun, W.R. Biochar application in combination with inorganic nitrogen improves maize grain yield, nitrogen uptake, and use efficiency in temperate soils. Agronomy 2020, 10, 1241. [Google Scholar] [CrossRef]
- Werner, S.; Akoto-Danso, E.K.; Manka’abusi, D.; Steiner, C.; Haering, V.; Nyarko, G.; Marschner, B. Nutrient balances with wastewater irrigation and biochar application in urban agriculture of Northern Ghana. Nutr. Cycl. Agroecosyst. 2019, 115, 249–262. [Google Scholar] [CrossRef]
- Verheijen, F.G.; Zhuravel, A.; Silva, F.C.; Amaro, A.; Ben-Hur, M.; Keizer, J.J. The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs sandy loam soil in a column experiment. Geoderma 2019, 347, 194–202. [Google Scholar] [CrossRef]
- Hussain, R.; Garg, A.; Ravi, K. Soil-biochar-plant interaction: Differences from the perspective of engineered and agricultural soils. Bull. Eng. Geol. Environ. 2020, 79, 4461–4481. [Google Scholar] [CrossRef]
- Rawal, A.; Joseph, S.D.; Hook, J.M.; Chia, C.H.; Munroe, P.R.; Donne, S.; Webber, J.B.W. Mineral–biochar composites: Molecular structure and porosity. Environ. Sci. Technol. 2016, 50, 7706–7714. [Google Scholar] [CrossRef] [Green Version]
- Libisch, B.; French, H.K.; Hartnik, T.; Anton, A.; Biró, B. Laboratory scale evaluation of selected remediation techniques for propylene-glycol based aircraft deicing fluids. Environ. Technol 2012, 33, 717–724. [Google Scholar] [CrossRef]
- Fekete, I.; Lajtha, K.; Kotroczó, Z.; Várbíró, G.; Varga, C.; Tóth, J.A.; Berki, I. Long-term effects of climate change on carbon storage and tree species composition in a dry deciduous forest. Glob. Chang. Biol. 2017, 23, 3154–3168. [Google Scholar] [CrossRef] [PubMed]
- Feigl, V.; Anton, A.; Uzigner, N.; Gruiz, K. Red mud as a chemical stabilizer for soil contaminated with toxic metals. Water Air Soil Pollut. 2012, 223, 1237–1247. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and quantification of biochar alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Jones, D.L.; Hill, P.; Bastami, M.S.; Tu, C.L. Influence of biochar produced from different pyrolysis temperature on nutrient retention and leaching. Arch. Agron. Soil Sci. 2018, 64, 850–859. [Google Scholar] [CrossRef]
- Xiao, L.; Meng, F. Evaluating the effect of biochar on salt leaching and nutrient retention of Yellow River Delta soil. Soil Use Manag. 2020, 36, 740–750. [Google Scholar] [CrossRef]
- Bachmann, H.J.; Bucheli, T.D.; Dieguez-Alonso, A.; Fabbri, D.; Knicker, H.; Schmidt, H.P.; Zehetner, F. Toward the standardization of biochar analysis: The COST action TD1107 interlaboratory comparison. J. Agric. Food Chem. 2016, 64, 513–527. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Tian, Y.; Zhang, H.; Cheng, Y.; Zhang, J. A soil management strategy for ameliorating soil acidification and reducing nitrification in tea plantations. Eur. J. Soil Biol. 2018, 88, 36–40. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Lehmann, J. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Yang, F.; Wang, C.; Sun, H. A comprehensive review of biochar-derived dissolved matters in biochar application: Production, characteristics, and potential environmental effects and mechanisms. J. Environ. Chem. Eng. 2021, 9, 105258. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Oesterle, P.; Wurzer, C.; Mašek, O.; Jansson, S. Removal of contaminants of emerging concern from multicomponent systems using carbon dioxide activated biochar from lignocellulosic feedstocks. Bioresour. Technol. 2021, 340, 125561. [Google Scholar] [CrossRef] [PubMed]
- Jatav, H.S.; Rajput, V.D.; Minkina, T.; Singh, S.K.; Chejara, S.; Gorovtsov, A.; Kalinitchenko, V.P. Sustainable approach and safe use of biochar and its possible consequences. Sustainability 2021, 13, 10362. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Nguyen, B.; Lehmann, J.; Hockaday, W.C.; Joseph, S.; Masiello, C.A. Temperature sensitivity of black carbon decomposition and oxidation. Env. Sci. Technol. 2010, 44, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Magrini-Bair, K.A.; Czernik, S.; Pilath, H.M.; Evans, R.J.; Maness, P.C.; Leventhal, J. Biomass derived, carbon sequestration, designed fertilizers. Ann. Environ. Sci. 2009, 3, 217–225. [Google Scholar]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W.; Duangchan, A.; Darunsontaya, T. The effects of pyrolysis conditions on the chemical and physical properties of rice husk biochar. Int. J. Mater. Sci. 2013, 3, 97–103. [Google Scholar]
- Forján, R.; Rodríguez-Vila, A.; Cerqueira, B.; Covelo, E.F. Comparison of the effects of compost versus compost and biochar on the recovery of a mine soil by improving the nutrient content. J. Geochem. Explor. 2017, 183, 46–57. [Google Scholar] [CrossRef]
- Yargicoglu, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef]
- Zhang, H.; Voroney, R.P.; Price, G.W. Effects of temperature and processing conditions on biochar chemical properties and their influence on soil Cand N transformations. Soil Biol. Biochem. 2015, 83, 19–28. [Google Scholar] [CrossRef]
- Baigorri, R.; San Francisco, S.; Urrutia, Ó.; García-Mina, J.M. Biochar-Ca and biochar-Al/-Fe-mediated phosphate exchange capacity are main drivers of the different biochar effects on plants in acidic and alkaline soils. Agronomy 2020, 10, 968. [Google Scholar] [CrossRef]
- Laird, D.; Rogovska, N. Biochar effects on nutrient leaching. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 553–574. [Google Scholar]
- Zhang, J.; Wang, Q. Sustainable mechanisms of biochar derived from brewers’ spent grain and sewage sludge for ammonia–nitrogen capture. J. Clean. Prod. 2016, 112, 3927–3934. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Novak, J. Biochar, Soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Moezzi, A.; Chorom, M.; Enayatizamir, N. Application of biochar changed the status of nutrients and biological activity in a calcareous soil. J. Soil Sci. Plant Nutr. 2020, 20, 450–459. [Google Scholar] [CrossRef]
- Foster, E.J.; Baas, P.; Wallenstein, M.D.; Cotrufo, M.F. Precision biochar and inoculum applications shift bacterial community structure and increase specific nutrient availability and maize yield. Appl. Soil Ecol. 2020, 151, 103541. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, X.; Qu, Z.; Jia, Y.; Hu, M.; Li, C. Effects of biochar application and irrigation methods on soil temperature in farmland. Water 2019, 11, 499. [Google Scholar] [CrossRef] [Green Version]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Short-term effects of biochar on soil properties and wheat yield formation with meat bone meal and inorganic fertiliser on a boreal loamy sand. Agric. Ecosyst. Environ. 2014, 191, 108–116. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Parkin, T.B. Effect of biochar on soil greenhouse gas emissions at the laboratory and field scales. Soil Syst. 2019, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Barracosa, P.; Cardoso, I.; Marques, F.; Pinto, A.; Oliveira, J.; Trindade, H.; Pereira, J.L. Effect of biochar on emission of greenhouse gases and productivity of cardoon crop (Cynara cardunculus L.). J. Soil Sci. Plant Nutr. 2020, 20, 1524–1531. [Google Scholar] [CrossRef]
- Graber, E.R.; Frenkel, O.; Jaiswal, A.K.; Elad, Y. How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag. 2014, 5, 169–183. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Boateng, A.A.; Han, Y.; Douds Jr, D.D. Inactivation of E. coli O157: H7 in cultivable soil by fast and slow pyrolysis-generated biochar. Foodborne Pathog. Dis. 2014, 11, 215–223. [Google Scholar] [PubMed]
- Kwon, T.; Shibata, H.; Kepfer-Rojas, S.; Schmidt, I.K.; Larsen, K.S.; Beier, C.; Gualmini, M. Effects of climate and atmospheric nitrogen deposition on early to mid-term stage litter decomposition across biomes. Front. For. Glob. Chang. 2021, 90, 1–18. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher-Jenull, J.; Ceccherini, M.T.; Pietramellara, G.; Renella, G.; Schloter, M. Beyond microbial diversity for predicting soil functions: A mini review. Pedosphere 2020, 30, 5–17. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Tóth, J.A.; Nagy, P.T.; Krakomperger, Z.; Veres, Z.; Kotroczó, Z.; Kincses, S.; Fekete, I.; Papp, M.; Mészáros, I.; Viktor, O. The effects of climate change on element content and soil pH (Síkfőkút DIRT project, Northern Hungary). In The Carpathians: Integrating Nature and Society towards Sustainability. Environmental Science and Engineering; Kozak, J., Ostapowicz, K., Bytnerowicz, A., Wyżga, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 77–88. [Google Scholar]
- Ennis, C.J.; Evans, A.G.; Islam, M.; Ralebitso-Senior, T.K.; Senior, E. Biochar: Carbon sequestration, land remediation, and impacts on soil microbiology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2311–2364. [Google Scholar] [CrossRef] [Green Version]
- Paz-Ferreiro, J.; Méndez, A.; Gascó, G.; Guo, M.; He, Z.; Uchimiya, S.M. Application of biochar for soil biological improvement. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 145–174. [Google Scholar]
- Zavalloni, C.; Alberti, G.; Biasiol, S.; Delle Vedove, G.; Fornasier, F.; Liu, J.; Peressotti, A. Microbial mineralization of biochar and wheat straw mixture in soil: A short-term study. Appl. Soil Ecol. 2011, 50, 45–51. [Google Scholar] [CrossRef]
- Smirnova, E.V.; Giniyatullin, K.G.; Okunev, R.; Valeeva, A.A.; Guseva, I.A. The effect of pre-incubation duration of soil-biochar model mixtures on the results of determination the intensity of substrate-induced respiration (methodological aspects of study). Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1360–1366. [Google Scholar]
- Bandara, T.; Franks, A.; Xu, J.; Chathurika, J.B.A.J.; Tang, C. Biochar aging alters the bioavailability of cadmium and microbial activity in acid contaminated soils. J. Hazard. Mater. 2021, 420, 126666. [Google Scholar] [CrossRef]
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Datta, R. Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef] [Green Version]
- Videgain-Marco, M.; Marco-Montori, P.; Martí-Dalmau, C.; Jaizme-Vega, M.D.C.; Manyà-Cervelló, J.J.; García-Ramos, F.J. The Effects of Biochar on Indigenous Arbuscular Mycorrhizae Fungi from Agroenvironments. Plants 2021, 10, 950. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Shafi, M.I.; Beamont, E.; Anawar, H.M. Poultry litter biochar increases mycorrhizal colonisation, soil fertility and cucumber yield in a fertigation system on sandy soil. Agriculture 2020, 10, 480. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Soil microbial responses to biochars varying in particle size, surface and pore properties. Pedosphere 2015, 25, 770–780. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, J.; Liu, X.; Yan, Q.; Hu, Y. Short-term impact of fire-deposited charcoal on soil microbial community abundance and composition in a subtropical plantation in China. Geoderma 2020, 359, 113992. [Google Scholar] [CrossRef]
- Makoto, K.; Tamai, Y.; Kim, Y.S.; Koike, T. Buried charcoal layer and ectomycorrhizae cooperatively promote the growth of Larix gmelinii seedlings. Plant Soil 2010, 327, 143–152. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Blackwell, P.; Abbott, L.K.; Storer, P. Direct and residual effect of biochar application on mycorrhizal colonization, growth and nutrition of wheat. Austral. J. Soil Res. 2010, 48, 546–554. [Google Scholar] [CrossRef]
- Dangi, S.; Gao, S.; Duan, Y.; Wang, D. Soil microbial community structure affected by biochar and fertilizer sources. Appl. Soil Ecol. 2020, 150, 103452. [Google Scholar] [CrossRef]
- Füzy, A.; Biró, B.; Tóth, T. Effect of saline soil parameters on endomycorrhizal colonisation of dominant halophytes in four Hungarian sites. Span. J. Agric. Res. 2010, 1, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Horel, Á.; Potyó, I.; Szili-Kovács, T.; Molnár, S. Potential nitrogen fixation changes under different land uses as influenced by seasons and biochar amendments. Arab. J. Geosci. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rékási, M.; Szili-Kovács, T.; Takács, T.; Bernhardt, B.; Puspán, I.; Kovács, R.; Uzinger, N. Improving the fertility of sandy soils in the temperate region by combined biochar and microbial inoculant treatments. Arch. Agron. Soil Sci. 2019, 65, 44–57. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Qamar, R.; Ali, M.; Rehman, A.; Farooq, M.; Zamir, S.I.; Hussain, M. Improvement in Soil Characteristics of Sandy Loam Soil and Grain Quality of Spring Maize by Using Phosphorus Solublizing Bacteria. Sustainability 2019, 11, 7049. [Google Scholar] [CrossRef] [Green Version]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Rizwan, M.; ur Rehman, M.Z.; Ali, S.; Abbas, T.; Maqbool, A.; Bashir, A. Biochar is a potential source of silicon fertilizer: An overview. Biochar Biomass Waste 2019, 12, 225–238. [Google Scholar]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Grafe, M.; Kurth, J.K.; Panten, K.; Raj, A.D.; Baum, C.; Zimmer, D.; Schulz, S. Effects of different innovative bone char-based P fertilizers on bacteria catalyzing P turnover in agricultural soils. Agric. Ecosyst. Environ. 2021, 314, 107419. [Google Scholar] [CrossRef]
- Nigri, E.M.; Santos, A.L.; Mesquita, P.L.; Viana, P.R.; Rocha, S.D. Simultaneous removal of strontium and refractory organic compounds from electrodialysis effluents by modified bovine bone char. Desalin. Water Treat. 2019, 145, 189–201. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Lehmann, J.; Bauerle, T.; Vanek, S.; Hestrin, R.; Nigussie, A. Phosphorus availability from bone char in a P-fixing soil influenced by root-mycorrhizae-biochar interactions. Plant Soil 2016, 408, 95–105. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, S.; Zhang, X.; Chen, J.; He, X.; Zhang, Q. Effects of pyrolysis temperature on soil-plant-microbe responses to Solidago canadensis L.-derived biochar in coastal saline-alkali soil. Sci. Total Environ. 2020, 731, 138938. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Komkiene, J.; Baltrenaite, E. Biochar as adsorbent for removal of heavy metal ions [Cadmium (II), Copper (II), Lead (II), Zinc (II)] from aqueous phase. Int. J. Environ. Sci. Technol. 2016, 13, 471–482. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, A.H.; Usman, A.R.; Al-Omran, A.; Ok, Y.S.; Ahmad, M.; Al-Wabel, M.I. Carbon mineralization and nutrient availability in calcareous sandy soils amended with woody waste biochar. Chemosphere 2015, 138, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rogovska, N.; Laird, D.A.; Rathke, S.J.; Karlen, D.L. Biochar impact on Midwestern Mollisols and maize nutrient availability. Geoderma 2014, 230, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Varma, A.; Tripathi, S.; Prasad, R. (Eds.) Plant Microbe Symbiosis; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Khatabi, B.; Gharechahi, J.; Ghaffari, M.R.; Liu, D.; Haynes, P.A.; McKay, M.J.; Salekdeh, G.H. Plant–microbe symbiosis: What has proteomics taught us? Proteomics 2019, 19, 1800105. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Biró, B.; Köves-Péchy, K.; Vörös, I.; Takács, T.; Eggenberger, P.; Strasser, R.J. Interrelations between Azospirillum and Rhizobium nitrogen-fixers and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in sterile, AM-free or normal soil conditions. Appl. Soil Ecol. 2000, 15, 159–168. [Google Scholar] [CrossRef]
- Ogawa, M.; Okimori, Y. Pioneering works in biochar research, Japan. Soil Res. 2010, 48, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Yu, M.; Lu, X.; Tang, C.; Liu, X.; Brookes, P.C.; Xu, J. Combined application of biochar and nitrogen fertilizer benefits nitrogen retention in the rhizosphere of soybean by increasing microbial biomass but not altering microbial community structure. Sci. Total Environ. 2018, 640, 1221–1230. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; de Quadros, P.D.; Davis-Richardson, A.; Camargo, F.A.; Clark, I.M.; Triplett, E.W. Soil pH determines microbial diversity and composition in the park grass experiment. Microb. Ecol. 2015, 69, 395–406. [Google Scholar] [CrossRef]
- Shamseldin, A.; Werner, D. High salt and high pH tolerance of new isolated Rhizobium etli strains from Egyptian soils. Curr. Microbiol. 2005, 50, 11–16. [Google Scholar] [CrossRef]
- McHenry, M.P. Soil organic carbon, biochar, and applicable research results for increasing farm productivity under Australian agricultural conditions. Commun. Soil Sci. Plant Anal. 2011, 42, 1187–1199. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]

| Site | Soil | Fertile Layer (cm) | Age (year) | Clay (%) | pH | Carbon (mg g−1) | Nitrogen (mg g−1) | C:N Ratio |
|---|---|---|---|---|---|---|---|---|
| Hatahara | Terra Preta | 43–69 | 600–1000 | 27.0 | 6.4 | 22.0 | 1.0 | 22 |
| Control | 0–10 | 600–1000 | 35.9 | 4.6 | 21.8 | 1.6 | 14 | |
| Lago Grande | Terra Preta | 0–16 | 900–1100 | 22.6 | 5.9 | 31.5 | 1.8 | 18 |
| Control | 0–8 | 900–1100 | 26.7 | 4.2 | 17.5 | 1.3 | 13 | |
| Acutuba | Terra Preta | 48–83 | 2000–3000 | 10.4 | 5.6 | 15.7 | 1.0 | 16 |
| Control | 0–30 | 2000–3000 | 8.5 | 4.7 | 15.4 | 0.8 | 19 | |
| Dona Stella | Terra Preta | 190–210 | 6700–8700 | 0.3 | 5.0 | 16.5 | 1.1 | 15 |
| Control | 0–12 | 6700–8700 | 0.3 | 3.9 | 10.2 | 0.4 | 26 |
| Method of Pyrolysis | Liquid (Bio-Oil, Bio-Fuel) | Solid (Biochar) | Gas (Synthesis Gas, Bio-Gas) |
|---|---|---|---|
| Moderate temperature (~500 °C), short hot vapor residence time (<2 s) | 75% (25% water) | 12% | 13% |
| Moderate temperature (<500 °C), moderate hot vapor residence time (10–20 s) | 50% (50% water) | 20% | 30% |
| Slow temperature (~400 °C), very long solid residence time | 30% (70% water) | 35% | 35% |
| High temperature (>800 °C), long vapor residence time | 5% tar | 10% | 85% |
| Raw Material | Production (°C) | pH | C g kg−1 | N g kg−1 | C:N Ratio | P g kg−1 | K g kg−1 | Literature |
|---|---|---|---|---|---|---|---|---|
| Green waste | 450 | 6.2 | 680 | 1.7 | 400 | 0.2 | 1 | Chan et al., 2007 [45] |
| Poultry litter | 450 | 9.9 | 380 | 20 | 19 | 25 | 22 | Chan et al., 2007 [45] |
| Residue (Zea mays) | 350 | 675 | 9.3 | 73 | 10.4 | Nguyen and Lehmann, 2009 [46] | ||
| Residue (Zea mays) | 600 | 790 | 9.2 | 86 | 6.7 | Nguyen and Lehmann, 2009 [46] | ||
| Peanut shell (Arachis hypogaea) | 400 | 499 | 11 | 45 | 0.6 | 6.2 | Margini-Bair et al., 2009 [47] | |
| Rice husk | 370–550 | 8.5 | 470 | 5.9 | 80 | 1.03 | 7.9 | Prakongkep et al., 2014 [48] |
| Wood (Quercus ilex) | 400 | 9.9 | 676 | 5.3 | 128 | 3.2 | Forján et al., 2014 [49] | |
| Pine wood (Pinus spp.) | ~480 | 8.4 | 532 | 3.7 | 143 | 9.4 | Yargicoglu et al., 2015 [50] | |
| Wood (Quercus spp.) | 400 | 6.9 | 427 | 3.3 | 130 | 0.6 | 3.8 | Zhang et al., 2015 [51] |
| Wood (Quercus spp.) | 600 | 9.5 | 455 | 4.1 | 111 | 0.6 | 4.4 | Zhang et al., 2015 [51] |
| Mechanism | Rhizobium | Bacteria | Mycorrhiza | Filamentous Fungi |
|---|---|---|---|---|
| Protecting surface | 0 | + | + | + |
| Improved hydration | + | + | + | + |
| N availability | − | + | 0 | + |
| P, Ca, Mg, K availability | + | + | − | − |
| Micronutrient availability | + | + | − | + |
| pH increase | + | + | 0 | 0 |
| pH decrease | − | − | 0 | 0 |
| Sorption of microorganisms | − | + | + | + |
| Biofilm formation | + | + | 0 | 0 |
| Sorption of dissolved organic matter as an energy source for microorganisms | 0 | + | 0 | + |
| Soil Characteristics | Advantages of BC | Disadvantages of BC | Suggestions to Treat |
|---|---|---|---|
| Soil physical conditions | |||
| Texture, porosity | - BC can be used to improve soil quality [21,86] | - Soil type is crucial in positive effect [29,30,31,32] - Site-specific application needed [10,60] | - Previous selection and study needed to avoid improper use |
| Surface area, plant-nutrition | - Adsorption and fixing of elements or leachable materials (e.g., nitrate) [37] - Protects soil biota [33,34] | - Plant nutrition might be limited (e.g., in drought conditions) [38,39] | - Consider the stressed environmental condition (watering, soil inoculation) |
| Aeration, better oxygenation | - Supports aerobic processes, protects soil biota [2,3] | - Potential of reduced SOM and humus content [42] | - Use soil-dependent treatments, add organic materials |
| Soil chemistry | |||
| pH | - Near-neutral conditions, better for the soil life [100] | - Some nutritive elements become less available [36] | - Consider current soil characteristics and act accordingly |
| SOM, humus, carbon | - Sequesters carbon [4,5] - Mitigates climate change [58,59] | - Indirect effect on soil biota might reduce SOM [63,64] | - Proper use might be required |
| Toxic materials, heavy metals | - Improved decontamination [16,81,89] - Heavy metal adsorption and fixing [99,100] | - Potential accumulation of toxic compounds [5] | - Inoculation by adapted microbes might improve remediation |
| Soil biology | |||
| Survival of soil biota | - Large surfaces can provide niche [65,66,67] - Drought protection and improved stress tolerance [70,84,88] | - Dependence on microbial physiological groups [98] - Limitation in microbial distribution [12] | - Focus on soil-borne plant pathogens might be helpful |
| Activity of soil biota | - Enhanced plant nutrition [94,96,97] | - Competition with plants for nutrients [43] | - Proper C:N ratio to avoid penthozan effect - Optimization for specific soil–plant systems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocsis, T.; Ringer, M.; Biró, B. Characteristics and Applications of Biochar in Soil–Plant Systems: A Short Review of Benefits and Potential Drawbacks. Appl. Sci. 2022, 12, 4051. https://doi.org/10.3390/app12084051
Kocsis T, Ringer M, Biró B. Characteristics and Applications of Biochar in Soil–Plant Systems: A Short Review of Benefits and Potential Drawbacks. Applied Sciences. 2022; 12(8):4051. https://doi.org/10.3390/app12084051
Chicago/Turabian StyleKocsis, Tamás, Marianna Ringer, and Borbála Biró. 2022. "Characteristics and Applications of Biochar in Soil–Plant Systems: A Short Review of Benefits and Potential Drawbacks" Applied Sciences 12, no. 8: 4051. https://doi.org/10.3390/app12084051
APA StyleKocsis, T., Ringer, M., & Biró, B. (2022). Characteristics and Applications of Biochar in Soil–Plant Systems: A Short Review of Benefits and Potential Drawbacks. Applied Sciences, 12(8), 4051. https://doi.org/10.3390/app12084051







