1. Introduction
Cellulose is the main structural component of lignocellulosic fibers, as it provides strength and stability to the cell walls and the fiber as a whole. Additionally, lignin is a highly cross-linked molecular complex with an amorphous structure and acts as a binding agent between individual fiber cells and between the fibrils forming the cell wall [
1]. The annual world production of natural fibers in 2010 was 28.4 million tons. However, despite the growth in research on lignocellulosic fibers, there is still a large amount of undervalued plant residues, which have great potential to be exploited as part of a circular economy [
2,
3,
4]. There are several advantages that are obtained from natural fibers not only for economics but also their low impact on the environment, as they are biodegradable, abundant and reduce pollutants due to waste disposal.
Due to their fineness, strength and high crystallinity, fibers obtained from specimens of the Bromeliaceae family such as pita or ixtle (
Aechmea magdalenae), canoá (
Neoglaziovia variegata), pineapple leaves (
Ananas comosus),
Bromelia pinguin,
Bromelia karatas and
Bromelia sera have been used for the manufacture of footwear, handicrafts and fishing nets, among others [
5,
6,
7,
8]. In addition to those,
Bromelia hemisphaerica Lam. is a perennial herbaceous plant native to Mexico and Central America, where it is popularly known as timbirichi or timbiriche from the Purépecha word “
timbirish úkata”, which means “bunch of fruits” [
9]. The fruits of
B. hemisphaerica are edible, have a creamy white pulp and are arranged in clusters of 60–80 fruits with black seeds. The fruit is used in traditional Mexican medicine as an antiparasitic, antiscorbutic and anti-inflammatory agent and has pharmacological reports of antifungal activity. It grows in the Balsas River basin that comprises the states of Morelos, Guerrero and Michoacán in Mexico [
10,
11]. The juice of the fruit has been characterized in terms of the production of hemisphaericin-C, which is a polymorphic cysteine endopeptidase that gives the consumer an astringent sensation and has high potential in the beer industry and food technology [
12,
13]. Once the enzymes are obtained, the bagasse of the fruit and the leaves of the plant are discarded. These residues are an alternative source for obtaining natural materials such as fiber or microcrystalline cellulose from Mexican plants like timbiriche.
Plant fibers, also called lignocellulosic fibers, are very complex and are defined by their composition of cellulose, lignin and hemicellulose, as well as their thermal, microstructural, physicochemical and optical properties [
5,
14,
15]. In recent years, the study of obtaining fiber through various sources, mainly those such as a residue or renewable waste, such as those from
Bromelia sp., has been increasing because they have become a resource for the production of composite materials due to their availability and ability to interact with various polymer matrices, and their use reduces dependence on synthetic polymers [
16,
17,
18]. In addition to this, conventional methods of extraction of these components have been detrimental. Researchers have sought to replace or reuse solvents with environmentally friendly extraction techniques such as microwave-assisted organosolv extraction, which has been shown to increase the yield and reduce extraction times [
18]. On the other hand, the functionalization of the byproducts obtained from lignocellulosic components opens up a new possibility of thinking about a circular economy and using not only the cellulose but also the lignin that can be used. In recent studies, it has been shown to be a promising candidate for the replacement of materials of fossil origin and the development of materials, although secondary reactions to condensation have limited its functionalization [
19,
20].
Image Digital Analysis (DIA) is an auxiliary tool that allows the statistical analysis of morphological or surface changes [
21]. This incorporation of computer science has impacted areas such as histology, with digitalization providing sectional images of tissue that can be stored in specific formats for manipulation [
22,
23,
24].
In consideration of the above, when the juice is extracted from B. hemisphaerica, the remains of the plant, such as the bagasse of the fruit and the leaves, are residual byproducts that can be exploited. Therefore, the purpose of this work was to evaluate the effect of microwave-assisted extraction in an organosolv process and to determine the microstructural, chemical and thermal characteristics of the fruit and leaf bagasse of B. hemisphaerica as an alternative to synthetic fiber and the potential for its components, such as lignin and microcrystalline cellulose, to be utilized in the food or materials industries.
2. Materials and Methods
2.1. Material
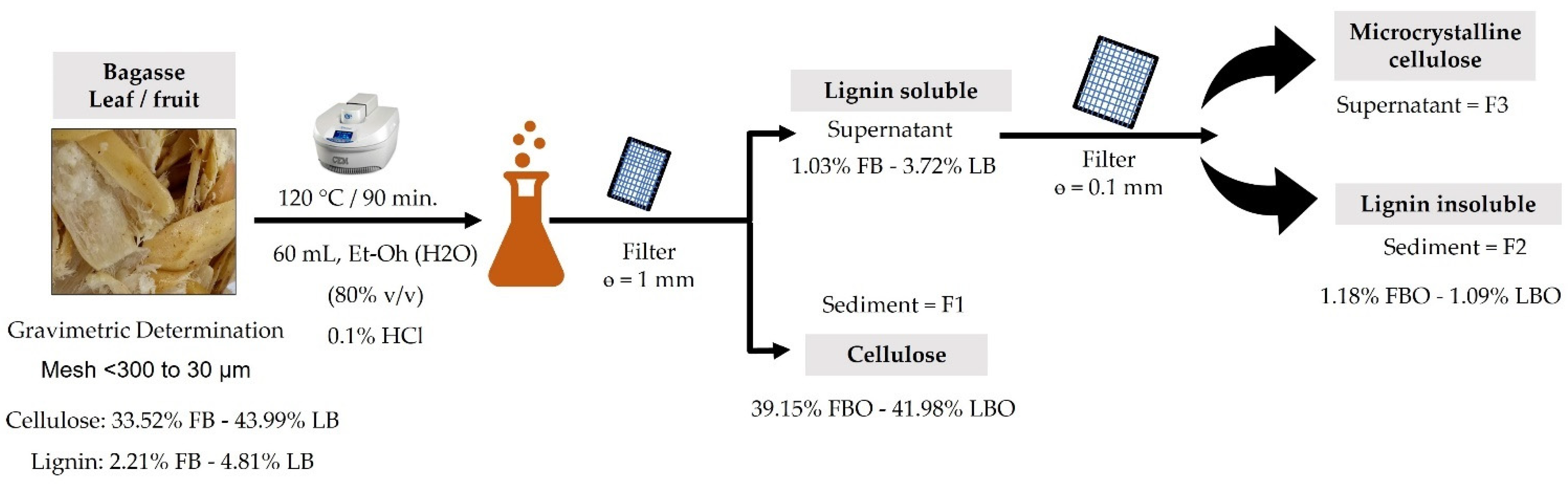
The lignocellulosic fiber was obtained from specimens of B. hemisphaerica Lam. (Bromeliaceae) collected in a fruiting state without noticeable damage in the experimental field “Emiliano Zapata” of the Center for the Development of Biotic Products of the IPN in Mexico (coordinates: 99°5′46.74″ west and 18°49′28.52″ north, 1064 m a.s.l.). The plant material was identified and authenticated by Biol. Gabriel Flores Franco of the HUMO herbarium of the Biodiversity and Conservation Research Center at Universidad Autónoma del Estado de Morelos (Voucher No. 39570). The plants were first carefully cleaned to eliminate organic matter residues, the leaves were removed, and the fruit was separated from the plant. The bagasse was then obtained from the fruit by means of a hydraulic press, applying a pressure of 500 kg × f × cm−2 and dried in a tray dryer at 60 °C for 48 h. Finally, the bagasse fiber was pulverized in a Retsch blade mill with a 0.67-mm mesh (model Grindomix GM 200, Retsch GmbH & Co, Haan, Germany). The particle size obtained was from <300 µm to 30 µm after passing through 30-, 40-, 50- and 100- mesh screens.
2.2. Proximal Determination
The proximate compositions for the lipid (920.039) and protein contents (984.13) were determined according to the AOAC:2000 [
25] standard methods. The nitrogen (N) content was determined by Kjeldahl, and the crude protein was calculated using a conversion factor of 5.7.
2.3. Fiber Chemical Composition
2.3.1. Extraction of Lignocellulosic Components
The extraction of lignocellulosic components was performed following the methodology on the bagasse of ripe fruits by the microwave-assisted organosolv method in an open system [
26]. Here, 3 g of fiber were mixed with 60 mL of hydroalcoholic ethanol (80%
v/
v ethanol in water) and 0.1% hydrochloric acid (
v/
v in water) as a catalyst. They were placed in a 125-mL flat-bottomed round flask at a power of 80 W in a microwave digestion apparatus (CEM, Discover, Model 908005, Matthews, NC, USA). The reaction was carried out in a reflux system for 90 min at a temperature of 120 °C, generated by the equipment as a function of the power and constant stirring. The starting time was considered when the power and temperature reached a steady state. Once the reaction time had elapsed, the suspension was filtered through a metal mesh with a pore size of 1 mm in diameter, where fraction 1 (F1) was obtained. A second filtration of fraction 1 was performed with 0.1-mm pore Walther filter paper and labeled as fraction 2 (F2).
The supernatant of fraction 2 was labeled as fraction 3 (F3) and was used to calculate the yield of the process with Equation (1) from [
27] and as shown in
Figure 1:
where Wf is the final weight of F2 and Wi is the initial weight of the sample of fraction 1 (F1).
2.3.2. Cellulose Quantification
The method of Kurschner and Hoffer in TAPPI:1978 [
28] was used. For this purpose, 0.5 g of fraction 1 was taken on a dry basis (65 °C, 48 h) and mixed in a solution of 96% ethanol and concentrated nitric acid (4:1
v/
v). The mixture was heated at reflux in a water bath for 60 min. This procedure was performed twice before filtering and had the function of separating the polymers that made up the fiber. The residue obtained was washed with hot distilled water for 1 h, followed by washing with a saturated sodium acetate solution and then rinsing with hot distilled water. The residue was dried at 105 °C, cooled in a desiccator and weighed. Equation (2) was used to calculate the percentage of cellulose:
where Wf is the dry weight of the final residue at 105 °C and Wi is the initial weight of the F1 dry sample at 65 °C.
2.3.3. Lignin Quantification
To determine the percentage of insoluble lignin in fraction 2, a modification of the ASTM D-1106-21:2021 standard lignin method was used [
29,
30]. Separately, 30 mg of a dry sample obtained from fraction 2 was placed in a 125-mL Erlenmeyer flask, and 3 mL of 72% (
v/
v) sulfuric acid was added with continuous stirring at 50 °C for 2 h. After this time, the reaction was stopped by reducing the H
2SO
4 content to 4% (
v/
v) and adding distilled water. The flask was sealed with aluminum foil, and to complete the hydrolysis, it was placed in an autoclave at 120 °C and 1.05 bar for 1 h. The solution was filtered with a polyester membrane, and the residue was washed with distilled water and dried in a convection oven at 60 °C before the weight was determined. The percentage of lignin was calculated with Equation (3):
where W
f is the weight of the lignin dry residue at 60 °C and W
i is the weight of the initial dry sample of F2.
2.4. Fourier Transform Infrared Spectroscopy
The functional groups of the leaf and fruit bagasse were determined using an FTIR spectrophotometer (IR Affinity model, Shimadzu, Kyoto, Japan) equipped with attenuated total reflection (ATR). These scanning parameters were used: zinc selenium crystal, % transmittance, number of scans: 100 and a 4-cm−1 resolution. The region used for the analysis was 500–4000 cm−1. The dried and sterilized membranes were mounted on the equipment sample plate. From the transmittance spectra, it was possible to identify interactions between the selected biopolymers.
2.5. FT Raman Spectroscopy
The Raman spectra of the bagasse samples before and after organosolv delignification (FB, FBO; LB, LBO) were recorded on a DXR Raman Microscope Laser Raman spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.6. X-ray Diffraction Determination
Structural analysis was performed using a diffractometer (Rigaku, Mini-flex 600, The Woodlands, TX, USA) with an X-ray tube with CuKα radiation (ʎ = 1.54 Å) and with line focus at 40 kV and 15 mA. A Soller slit with 0.5-mm Ni filter was placed in the incident beam. In the diffracted optics, a high-speed ultra Dtex detector was used. The scanning was performed from 2° to 60° with a step size of 0.01 and a speed of 3° min
−1. The crystallinity index (I
c) was evaluated using the Buschle-Diller and Zeronian equation (Equation (4)) [
31]:
where
I1 is the intensity at the minimum (2θ = 18.8°), which is the value of the area under the curve corresponding to the amorphous portion in the diffractogram, and
I2 is the intensity associated with the crystalline region of cellulose (2θ = 22.7°), which is the total area of the diffractogram including the curve of the amorphous material [
32]. All analyses were performed at least twice.
2.7. Differential Scanning Calorimetry Determination
A differential scanning calorimeter (DSC) (DuPont 9000, Wilmington, DE, USA) previously calibrated with indium was used for this determination. Here, 2 mg of the sample’s leaf fiber and fruit bagasse (dry basis) was weighed directly into aluminum trays, and deionized water was added with a micro syringe to obtain a solid suspension with a 65–75% (
w/
w) water content before sealing. The trays were allowed to equilibrate for 1 h at room temperature. After this time, the pans were heated at a heating rate of 10 °C min
−1 from 40 to 250 °C. For all measurements, an empty pan was used as a reference [
33].
2.8. Microstructural Characterization
2.8.1. Optical Microscopy
The birefringence of the fibers and cellulose was evaluated by polarized light microscopy with a Nikon Eclipse 80i microscope (Nikon Corp., Tokyo, Japan). Images were captured using the 4× and 10× magnification lenses of the optical microscope and a digital camera (CCD MTI DC330, Oxford Ins., Abingdon, UK).
2.8.2. Scanning Electron Microscopy
An environmental scanning electron microscope (ESEM) (Carl Zeiss EVO LS10, Jena, Germany) was used to evaluate the morphological characteristics of the fibers. Sections of 5 × 5 cm were taken from the upper and lower sides of the leaves (basal, middle and distal) and from the bagasse of the fruit (mature and immature). The samples were placed in aluminum stubs with carbon conductive tape. A manometric pressure of 30 Pa of water vapor, an electron beam with a voltage of 20 kV and a backscattered electron detector (NTS BSD) were used. The samples were analyzed before and after microwave-assisted organosolv extraction. All images were stored in TIFF format with a resolution of 2048 × 1536 pixels [
34].
2.8.3. Confocal Scanning Laser Microscopy
For analysis of the fiber components (cellulose, lignin and pectin), a Confocal Scanning Laser Microscope (CLSM) LSM 800 microscope with ZEN 2.6 Blue Edition software was used (Carl Zeiss, Jena, Germany). The fiber samples were mounted on a glass coverslip with a nominal thickness of 1.5 (0.16–0.19 mm). Calcofluor White M2R was used to stain the cellulose, and the lignin was identified with autofluorescence on the lignocellulosic material using the lambda function of MCBL. The laser wavelength used was 405 nm (blue color) at 3% excitation. Focal slices of the sample were made with a pinhole aperture of 0.5 airy units (AU). All the micrographs were captured at 200X using a Plan-Neofluar 20X/0.5 objective, and all images were stored in TIFF format with a resolution of 1024 × 1024 pixels. As with electron microscopy, to compare the effect of the chemical digestion method (microwave-assisted organosolv), the samples were analyzed before and after chemical digestion [
35].
2.9. Image Texture Analysis
All images obtained from the electron microscopy (ESEM) were converted to grayscale images. Subsequently, the algorithms of the gray level co-occurrence matrix (GLCM) and shifting differential box counting (SDBC) were applied to obtain texture parameters from the grayscale images [
36]. All image processing was performed using the Image J v.1.50d software (National Institutes of health, Bathesda, MD, USA). The texture parameters were extracted from the images obtained from microscopy techniques (energy (or second angular moment), contrast and entropy) with the GLCM textura plugin included in Image J. The values of the different parameters obtained were averaged with their respective standard deviations (average standard deviation) obtained from the equations for the texture parameters extracted from the grayscale SEM images [
27]. The statistical values were obtained using one-way analysis of variance (ANOVA). Significant differences between the means were compared using the post hoc Tukey methodology (
p < 0.05) followed by the multiple comparison test of Duncan (
p < 0.01) to evaluate the differences between the samples in the image texture analysis. The graphs and equations of the results were generated using SPSS Statistics 26 (IBM Corporation, Armonk, NY, USA).
2.10. Statistical Analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) to evaluate the response variables. The descriptive statistics were calculated using SPSS Statistics 26 software (IBM Corporation, Armonk, NY, USA). All experiments were performed in triplicate, and the results were expressed as the mean ± standard deviation (SD).
3. Results and Discussion
3.1. Chemical Compositions of the Fibers
The cellulose content of the leaf bagasse was significantly higher than that of the fruit bagasse (
Table 1), which is related to a lignocellulosic matrix of the primary cell wall of the leaf tissue cells with more organized and rigid structures. They are mostly composed of cellulose, which is responsible for the crystalline fraction of the fiber [
37,
38].
The lignin content was significantly higher in the leaves compared with the fruit bagasse. Lignin is a component of the cell wall in all vascular plants and has an important role in the control and transport of water through the cell wall, which is an important property in xerophytic plants such as
B. hemisphaerica due to its thermoplastic properties that confer support [
33].
As for the lipid content, it was significantly higher in the fiber of the leaves with respect to the fruit bagasse, possibly due to the fact that steroids are found in the membranes that are responsible for providing stability. In addition, most plants of the genus
Bromelia have developed photoprotection mechanisms to prevent excess light energy and changes in water availability, such as having low frequency of stomata, spongy parenchyma and impermeable cuticles due to a coating of the leaves by waxy materials [
24,
39].
The protein content was significantly higher in the leaf bagasse compared with the bagasse of the fruit. When plants respond to water deficit stress at the cellular and molecular level, one of the responses is the modification of gene expression. To adapt to stress, the plants activate a large set of genes, which leads to the accumulation of proteins specifically associated with the cell plant organ stress acting in the regulation of other genes, which translate into signals in response to water stress [
40].
Prior to organosolv extraction, the cellulose and lignin contents of the fruit bagasse fiber (FB) and leaf (LB) were quantified. In the case of the fruit, a content of 33.52% cellulose and 2.21% lignin was obtained, and for the leaves, a content of 43.99% cellulose and 4.81% lignin was obtained. Subsequently, in order to delignify the fibrous material, a microwave-assisted organosolv treatment was carried out at 120 °C for 90 min. Then, the solution was filtered through a mesh with a pore size of 1 mm in diameter, and a sediment was obtained that was named F1. Due to the weight of the cellulose, this macromolecule was found in this fraction, and so it was quantified, obtaining a value of 39.15% for the case of the leaves. The cellulose content increased significantly due to the effect of the organosolv method, which degraded the lignin that fulfills the function of covering the cellulose, increasing the content of this component.
In the case of the LB, the cellulose content after organosolv was 41.98%, a value significantly lower than the initial cellulose content. This behavior is explained by the predominantly more crystalline nature of the leaf fiber compared with the fruit fiber. This is the reason why the fiber when receiving an organosolv treatment not only degraded the lignocellulosic fraction that covered the fiber but also affected the cellulose fraction.
On the other hand, the soluble lignin fraction was found in the supernatant of this filtrate because its density is lower than that of cellulose. The F1 fraction was filtered again with a 0.1-mm pore size mesh, obtaining two fractions. In the F2 fraction (sediment), where the insoluble fraction of the lignin was found because it had a higher density compared with the F3 fraction (supernatant) [
41,
42], regarding the quantification of lignin after organosolv, the value obtained for the FBO and LBO fractions were 1.18% and 1.09%, respectively. In both cases, the content of lignocellulosic material was significantly reduced due to its degradation by organosolv.
Finally, in the supernatant F3, both for the fruit and leaf bagasse, micrometric-sized particles known as microcrystalline cellulose were obtained.
3.2. Fourier Transform Infrared Spectroscopy
The FTIR spectra of the fiber samples analyzed in this work were compared to the cellulose, hemicellulose and lignin standards. The FTIR spectra showed similar behavior, with most of the peaks located on the same wave number range. The peaks of 3660–2800 cm
−1 were present in all the samples because they are characteristic of the stretching vibration of OH and CH bonds in polysaccharides. The shape of the spectra was like those seen for other types of plant fibers and published in previous papers [
43,
44,
45]. The 3450 cm
−1 band observed in the spectra corresponded to the hydroxyl groups. A peak wave number of 2900 cm
−1 was also identified, which is attributed to the asymmetric stretching of CH and CH
2 [
44,
46]. Both bands are characteristic of organic materials, exhibiting high peak intensities as seen in the spectra of
Figure 2.
The bands at 1730 and 1625 cm
−1 correspond to the acetyl groups and C–O bonds characteristic of the hemicellulose. These bands were affected by the presence of pectin, whose peaks are found at 1735, 1680–1600 and 1260 cm
−1 [
47,
48]. They showed a different behavior which is associated with a chemical modification of their fractions of hemicellulose and pectin. In the spectrum of samples before organosolv (FB and LB), they showed peaks in the range of 1750–1250 cm
−1 also observed in the standards of hemicellulose and lignin. The presence of lignin was observed in the band of 1595 cm
−1 due to aromatic vibrations in the C=C in the plane aromatic vibrations [
47]. The cellulose is characterized by the bands of 1170–1150, 1050 and 1030 cm
−1 [
44].
With the spectra, it was possible to observe the differences between the FB and LB, the presence of hemicellulose and pectin in the fruit bagasse and the larger number of characteristic vibrations of the lignin and cellulose crystalline and the amorphous material in the leaf bagasse. Otherwise, only minor differences were observed after organosolv, which were associated with a chemical modification of the fibers of the material associated with hemicellulose and lignin. In the spectra of the fibers before and after organosolv, the typical bands assigned to cellulose were observed in the region of 1630–900 cm
−1, where the peaks located at 1633 cm
−1 correspond to the vibration of water molecules absorbed in the cellulose [
37]. The band around 1420–1430 cm
−1 is associated with the amount of the crystal structure of the cellulose, while the band at 897 cm
−1 is assigned to the amorphous region of the cellulose [
14].
3.3. FT Raman Espectra Spectroscopy
The Raman spectra in different ranges of fruit and leaf bagasse (
Figure 3), with respect to xylem cellulose, were from 2800 to 3030 cm
−1 (
Figure 3A), and the lignin phloem promoted by the delignification process by organosolv was from 1540 to 1700 cm
−1 (
Figure 3B). These signals were previously reported to different vascular cell types (e.g., xylem and phloem) in woody and herbaceous plants by the Raman spectroscopy technique [
49] and were used to interpret the xylem and phloem molecular components in the cell plants.
Plant cell walls are highly complex structures due to the presence of saccharides, and during organosolv delignification, these undergo changes. In the first range (
Figure 3A) of the Raman spectra, it was not possible to visualize significant changes due to the delignification process. For the fruit and leaf bagasse xylem and phloem residuals, Raman analysis was carried out in combination with the DRX methods for use as a predictive model for estimating the cellulose crystallinity for cellulose and amorphous polysaccharides as lignin’s presence [
50]. The complex multicomponent lignocellulose samples were described with the functional groups for the FTIR spectroscopy technique.
The Raman spectroscopy technique is complementary for the interpretation of the changes suffered by the Bromelia hemisphaerica fruit and leaf bagasse after having been subjected to the microwave-assisted organosolv delignification process. Resulting from the microwave-assisted organosolv process, a bagasse with a structure of greater crystallinity in the case of the fruit and with a visually significant reduction in lignin with the final component absent were found.
3.4. Microstructural Characterization
3.4.1. Optical Microscopy
Polarized light microscopy is a simple method to characterize the changes that some materials undergo, such as the conservation of an ordered state or crystallinity.
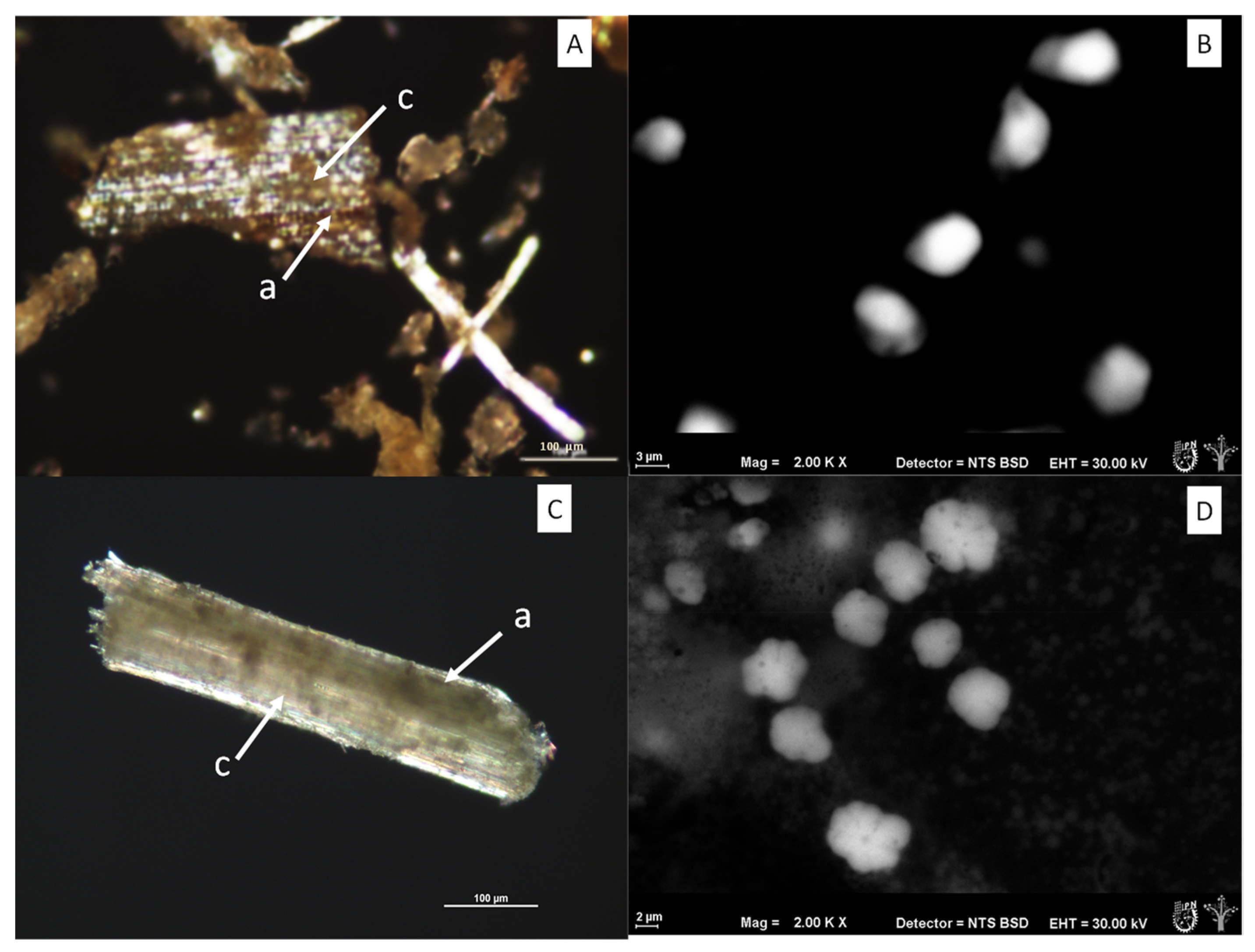
Figure 4 shows the polarized light micrographs of the fruit bagasse fibrils (A,B) and leaf (C,D), as well as the ESEM micrographs of the microcrystalline cellulose obtained from its processing, in which sizes to the order of 2–10 µm were observed.
The particle size decreased marginally without the use of chemical methods. In
Figure 4B,D, spherical and ellipsoidal morphologies without agglomerations can be observed, while polarized light micrographs show agglomerations and birefringence characteristic of the ordered state of incompletely hydrolyzed cellulose crystals. This technique is very useful for monitoring the loss or gain of crystallinity.
The presence of amorphous and crystalline cellulose can be seen in both bagasse by means of the polarized light technique in
Figure 4, as well as the presence of phloem with amorphous characteristics and xylem tissue mainly with crystalline structures [
49]. It is possible the decrease in the crystallinity index in the leaf bagasse after the organosolv process allowed for obtaining crystalline microcellulose (CMC) of a homogeneous size (
Figure 4B,D) and a greater quantity in the supernatant of the extraction system compared with that obtained from the fruit bagasse.
3.4.2. Scanning Electron Microscopy
The texture parameters were studied, such as the second moment (ASM), contrast, correlation, inverse difference moment (IDM), entropy and fractal dimension through Shilling Differencial Box Counting (SDBC) to the gray level (
Table 2).
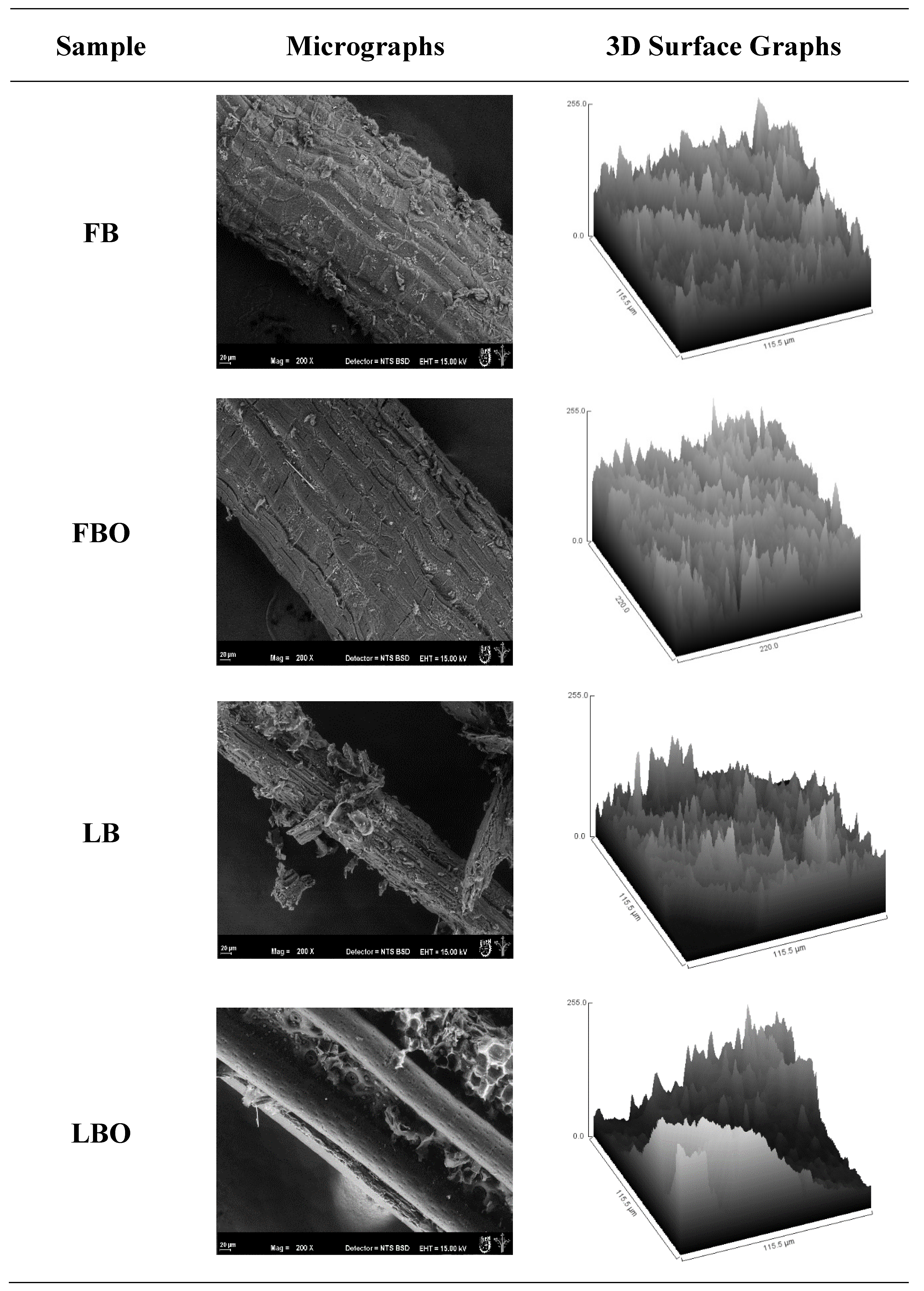
Figure 5 shows the microstructure of the lignocellulosic material surface from the fruit and leaves of
B. hemisphaerica Lam. with an environmental scanning electron microscope (ESEM). In the FB sample, lignocellulosic material from the bagasse of the fruit prior to the organosolv process was observed. The amorphous material is associated with hemicellulose and pectin present in the tissues of the xylem and phloem of the parenchyma.
Additionally, the presence of packages of calcium oxalate crystals in the form of raffidia has already been reported in several plants of the Bromeliaceae family [
51], whose function is to neutralize oxalic acid, which is reintroduced into the metabolism of the plant. Otherwise, after the organosolv process in the fruit bagasse (FBO), all the lignocellulosic material from the bagasse of the fruit disappeared. The presence of amorphous material related to the hydrolysis of thermolabile components such as pectin and hemicellulose disappeared while the fibrillar structure remained intact.
These changes suffered in the microstructure of the fruit after the process were reflected in the image entropy, where the FBO had a higher image entropy (8.9) than the LBO (8.17) (
Table 2). The morphometric changes are in
Figure 5, where 3D charts represent the images obtained from SEM. It was reported that for the 3D figures, the areas with white tones indicate pores or empty cavities in the internal structure of the system and darker tones are related to more compact structures [
52].
The FB sample had a larger surface of black tones, which indicates a more compact structure [
53] that could be related to the presence of all the elements, such as hemicellulose and pectin, which are part of the FB before the organosolv process and indicate a more organized structure. However, the FBO sample changed color in the 3D graph from black to white after the organosolv process and after losing components like pectin and hemicellulose by hydrolysis, which generated a more heterogeneous structure that was reflected in the high entropy of the image (8.9) and the change in color of the 3D graph (darker to clearer).
Regarding the leaf bagasse,
Figure 5 (LB) shows the lignocellulosic material from the leaf bagasse prior to the organosolv process, where with the associated amorphous material, unlike the fruit, the leaves had a higher entropy (8.42) prior to the organosolv process, which indicates the presence of amorphous material associated with the presence of leaf trichomes in the epithelium of the leaves. Otherwise, when the leaves passed through the organosolv process, the presence of amorphous material disappeared (
Figure 5 (LBO)), and only the lignocellulosic material remained, which is shown in this type of element in a more homogeneous microstructure and in a lower entropy (8.17), which must be corroborated by CLSM. The SDBC parameter confirmed the changes that occurred in the leaf after processing (2.65–2.27) and a change in roughness, where it decreased, because it was related to the complexity and higher ordering of the texture [
53,
54]. The ASM is an energy parameter and quantifies the homogeneity in the image, which is the opposite of entropy [
54]. The LBO showed a higher value (6.32), and
Figure 5 shows a texture more uniform and less rough.
As for contrast, the results showed similar values and less change in the fruit (328.74–351.26), unlike the leaf (180.15–115.68). This indicates that there was a great influence from the organosolv treatment on the lignocellulosic material of the leaf, so there could have been a greater loss of hemicellulose and lignin, leaving the cellulose free. This may be beneficial, since the method would allow a higher fraction of microcrystalline cellulose to be obtained in the future, expanding its possible use as a composite. All the texture parameters showed a significant difference in the leaf treatment in the Tukey post hoc test, followed by a Duncan test (
p < 0.01). Unlike the fruit, in which the values were similar, it was indicated that there was greater heterogeneity in the composition of lignocellulosic components in the leaf than in the fruit, as shown by the lower IDM values (1.33–1.78) that related to the heterogeneity of the image [
55].
3.4.3. Laser Scanning Confocal Microscopy (CLSM)
Figure 6 shows tridimensional micrographs obtained with the Z-stack mode of operation of the FB, FBO, LB and hemispherical LBO fibrous material samples. In
Figure 6 (FB), the fruit bagasse fiber was observed before the organosolv process, where the presence of cellulose is indicated in blue and lignin in green.
It was observed that the organosolv process exposed the cellulose, which presented a greater intensity in the blue channel (31.12), corresponding to cellulose in the FBO sample, which means that there was a greater content of the component. Values that are related to the results are presented in
Section 3.1, where the FBO sample showed 39.15 ± 1.33% cellulose, representing approximately a 20% greater value than the FB sample (33.52 ± 0.43% and blue channel intensity of 23.32). Although the FBO sample presented a higher intensity in the green channel (29.27), the FB sample presented a greater content of lignin (2.21 ± 0.05%), which represents approximately a 46% greater value than the FBO sample (1.18 ± 0.11%).
These are related because, despite the fact that the FBO sample presented more intensity at certain points, throughout the entire sample, there was greater homogeneity in the blue color (
Figure 6 (FB)), represented by the fact that the FB sample had the lignin in more of the surface area compared with the organosolv processed sample (
Figure 6 (FBO)), indicating that this method reduced the presence of amorphous material related to the hydrolysis of thermolabile components such as pectin and hemicellulose, while the fibrillar structure remained intact.
On the other hand, after performing the organosolv process and capturing the 3D micrograph, it can be observed that the presence of amorphous material was reduced (cellulose and lignin) in the leaf samples (LBO). In relation to the lignin content, the LBO samples showed a lower content (1.09 ± 0.02%) in relation with the LB sample (4.81 ± 0.14%), which was related to the intensity of the green channel, where the LBO sample showed 10% less intensity (18.22) than the LB sample (20.33). Nevertheless, for the cellulose content, the samples (LB and LBO) presented the same behavior as the FB and FBO samples for lignin, where the higher intensity in the blue channel was presented by the LBO sample (39.63); however, the content was almost 5% less than that of the LB sample.
CLSM allowed us to corroborate the presence, distribution and interaction of cellulose and lignin before and after the organosolv process. These results are important because these components are found together naturally in the walls of plant cells. It was also verified that the organosolv process partially disorganizes the lignocellulosic matrix that constitutes the cell pairs in the tissues and that the presence of cellulose (blue color) and lignin (green color) is observed more clearly after performing the extraction process because the amorphous material disappears [
28,
56].
3.5. Structural Analysis Using X-ray Diffraction (XRD)
The cellulose contained in the fiber has a crystalline zone oriented on a nano and microscale. Therefore, each fiber has a characteristic degree of crystallinity, where the percentage of crystallinity is proportional to the mechanical properties of a material, including lignocellulosic materials. In
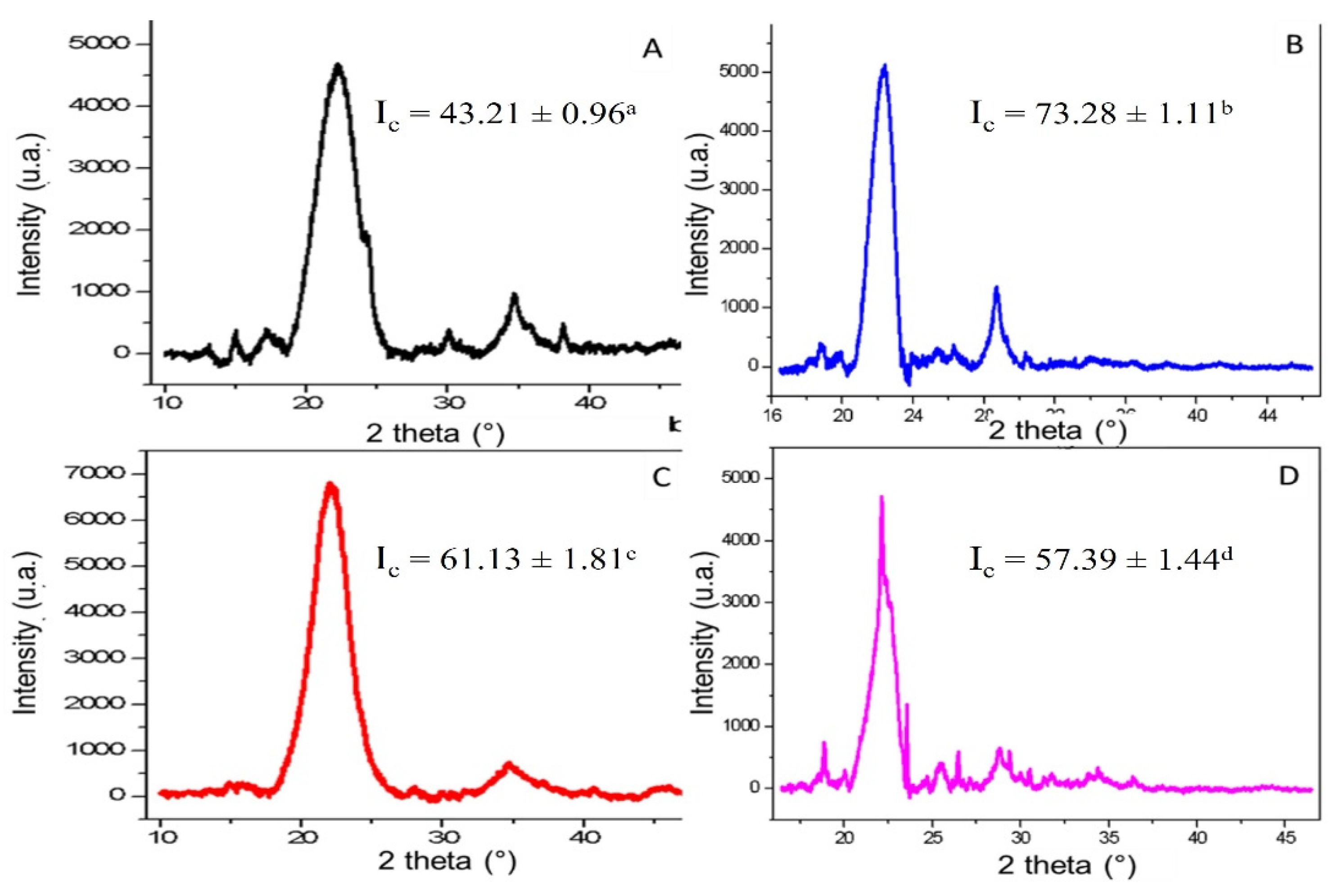
Figure 7, the characteristic diffraction patterns of the plant fibers are shown, and the crystallinity index was calculated using Equation (3), obtaining the following significantly different values.
The bagasse fiber of the fruit (
Figure 7A) had a value of 43.21 ± 0.96% compared with the same fiber after the organosolv method (
Figure 7B), with a value of 73.28 ± 1.11%, showing greater crystallinity in response to the removal of amorphous material from the hemicellulose and lignin fraction as cellulose-coating macromolecules. On the other hand, for the case of the fiber of the leaves (
Figure 7C), it had an I
c of 61.13 ± 1.81%, a value higher than that presented by the fiber in
Figure 7A but less than that in
Figure 7B. The index of crystallinity was higher in the fiber of the leaves than the fruit (before the organosolv process), which is congruent with a higher content of cellulose and proteins in its fiber because several of the components mentioned serve as structural support materials in the fiber.
The X-ray diffraction spectra revealed a highly ordered solid structure after organosolv treatment (Ic between 57% and 73% for the leaves and fruit). Here, the change in the crystalline behavior for both sections of fibers (fruit and leaf) showed an increased crystallinity index in the fruit bagasse, which remained almost unchanged after organosolv treatment for the leaf, which would indicate a minor loss of minimal amorphous material in the leaf or a low content of hemicellulose or lignin. This would represent less processing in the leaves of the plant, unlike the fruit which, although the crystallinity increased, the loss of amorphous material increased.
Figure 7D shows the leaf fiber after the organosolv process, with an I
c of 57.39 ± 1.44%. Unlike the behavior presented with the leaf fiber, that value of the I
c was slightly lower compared with the one in
Figure 7C due to its lower lignin content as a cellulose coating macromolecule in the leaf with respect to the fruit, which could cause the organosolv method to affect the cellulose structure [
15,
26,
28]. The crystallinities of other plants of the Bromeliaceae family used by the resistance of their fibers, such as
Bromelia sp.,
Ananas comosus var. Comosus and
Aechmea magdalenae (leaf fibers), are 58.6, 50.0 and 55.0%, respectively [
14], indicating that the fiber of
B. hemisphaerica leaves is a candidate for use as a biomaterial and that microcrystalline cellulose can be obtained for use as an excipient or future uses as a carrier for colorants and flavors in the food industry.
3.6. Thermal Analysis Using Differential Scanning Calorimetry (DSC)
The DSC data of the samples are shown in
Table 3 and illustrated in
Figure 8, where 8A corresponds to the fruit bagasse fiber, 8B the fruit bagasse fiber after organosolv treatment, 8C the leaf bagasse fiber and 8D the leaf bagasse fiber after organosolv treatment. In all kinetics, the first event attributed to moisture loss from the samples was between 50 and 60 °C, and the second event occurred between 130 and 150 °C. This behavior was possibly associated with the fusion of the degraded sugars that remained in the solution after hydrolysis. Finally, the third event was between 210 and 230 °C and was related to cellulose degradation [
5].
Making a comparison between the fiber of the bagasse of the fruit before and after the organosolv treatment (
Figure 8A,B, respectively), an increase in the To, Tp and ΔH after organosolv treatment was observed because when losing the amorphous material composed of hemicellulose and lignin, a higher temperature is required to begin to degrade the crystalline material and to finish disorganizing the fiber (Tp). The ΔH value also increased, because more energy per unit mass is required to disorganize the crystalline material.
Finally, the Tc was reduced because the fiber no longer had an amorphous material protection or coating. In the case of the leaf fiber before and after organosolv treatment (
Figure 8C,D, respectively), its thermal properties were affected, and an increase in To and Tp was observed while the values of ΔH and Tc were reduced.