Abstract
Cynara cardunculus L. inflorescence infusion has been used for several centuries as curd in traditional cheese making, such as some highly prized Portuguese cheeses. To promote the sustainable use of all C. cardunculus plants, C. cardunculus extract leaves decoction (CL), inflorescence decoction (CI), chlorogenic acid (CA) (a compound in the plant leaves), and rosmarinic acid (RA) (a similar phenolic compound) solutions were tested for antimicrobial activity against bacteria that may appear on the cheese rind. The antimicrobial activity was evaluated by 15 bacterial strains using two different methodologies: solid and liquid. The influence of these extracts and the phenolic compounds on melanin bioproduction by Pseudomonas putida ESACB 191 was also studied. CA and RA (1 mg/mL) showed antimicrobial activity. CL and CA reduced P. putida ESACB 191 growth in the liquid assay and melanin bioproduction by 6.20 Log CFU/mL and 50%, respectively. Cynarin, CA, and its derivates were identified as the main phenolic compounds (52%) of CL, which may justify its inhibitory action on bacterial growth and melanin bioproduction. Thus, future perspectives include the application of CL extracts with antimicrobial activity in edible films and/or coatings to applied in cheese rind to increase the shelf time.
1. Introduction
C. cardunculus L. is a Mediterranean halophyte, commonly designated as cardoon, belonging to the Asteraceae family [1,2]. It comprises three botanical varieties: var. altilis DC, var. scolymus (L.) Fiori, and var. sylvestris (Lamk) Fiori [2]. Traditional applications of C. cardunculus include the use of the blanched leaves and fleshy leaf petioles in soups, stews, and salads [3]. Flowers of C. cardunculus are rich in proteases, namely cardosins A and B, and aqueous extracts of C. cardunculus inflorescence have been used for centuries (particularly in Mediterranean regions) as coagulants in traditional ewes’ milk cheese making, providing distinctive characteristics of texture and flavor in cheeses. In some well-known protected designation of origin (PDO) Portuguese cheeses, such as “Serra da Estrela”, “Castelo Branco”, “Azeitão”, “Évora”, “Niza” and “Serpa”, the C. cardunculus inflorescences, specifically their stigma, are used for their high content of aspartic proteases and high milk-clotting activity [4]. Many studies support the important role of C. cardunculus in human nutrition, due to its high content of nutraceutical and bio-active compounds such as inulin and antioxidant phenolics [2,5]. Furthermore, C. cardunculus extracts have shown hepatoprotective, anti-tumour [6], antibacterial and anti-HIV activity and the ability to inhibit cholesterol biosynthesis [7] and LDL oxidation [3]. The decoction extracts have several phenolic acids [8,9] like chlorogenic acid, cynarine and some flavonoids like luteolin glycosylated [10]. The literature has already described C. cardunculus var. scolymus (L.) leaves extract as having antimicrobial activity [11], however the use of these extracts on Gram-negative bacteria requires high concentrations to have an antimicrobial action [12]. The inflorescence of C. cardunculus L. has been the subject of several studies [13,14]. The literature reports the chemical identification and antimicrobial activities of extracts of C. cardunculus L. obtained from extractions with organic solvents. Nevertheless, in this work, we intend to study the antimicrobial activity of extracts of aqueous nature from both inflorescence and leaves of C. cardunculus L. against bacteria mainly belonging to the genus Pseudomonas spp. isolated from cheese and/or the cheese making environment.
Brownish pigmentation was described as a problem in cheese made from raw milk from sheep or goats [15,16]. It has been reported by cheese producers to cause severe economic losses, since it is difficult to enter in the food market [15,16]. Pseudomonas spp. have been isolated from the rind of brownish cheese in several environments, producing extracellular molecules like enzymes [17] and biopigments [18].
The inflorescence of C. cardunculus L. is already used in the traditional production of cheese as milk coagulant. To contribute to the sustainable use of the C. cardunculus L. plant, the main purposes of this study are the chemical characterization of aqueous leaves extract by mass spectrophotometry, the evaluation of the antimicrobial activity of C. cardunculus L. extracts against bacteria strains isolated in dairy industries, and the evaluation of the influence of C. cardunculus L. extracts in terms of inhibition of brown pigmentation.
2. Materials and Methods
2.1. Plant Material
C. cardunculus L. (n° LISU266732) (Figure 1A) leaves and inflorescences were used. The adult leaves were harvested in March 2018, at Global Positioning System (GPS) coordinates 39°45′37.7″ N 8°27′49.3″ W, Portugal, and the inflorescences (Figure 1B) were harvested in July 2019 (end of flowering). The leaves (ripped roughly by hand) and inflorescence (stigmas) underwent a decoction process and each sample was weighed at a ratio of 10 g to 100 mL of household water and boiled for 20 min. After cooling, the preparation was filtered using Whatman® (Sigma, Germany) qualitative filter paper, Grade 1, and the filtrate was lyophilized.

Figure 1.
Cynara cardunculus (n° LISU266732) morphology of leaves (A) and inflorescence (B).
2.2. C. cardunculus Leaves Extract: Chemical Characterization by LC-HRMS/MS
The chromatographic analysis used to identify the compounds of the C. cardunculus leaves extract was carried out using liquid chromatography-high resolution tandem mass spectrometry (LC-HRMS/MS) with an Elute OLE UHPLC system interface coupled to a quadrupole time-of-flight (QqToF) Impact II mass spectrometer equipped with an electrospray source (ESI) (Bruker DaltoniK GmbH, Bremen, German). The method used was done according to Guedes et al. 2019 [19].
The acquired data were processed by DataAnalysis 4.1 software (Bruker Daltonik GmbH, Bremen, Germany). The identifications were made by considering the suggestions from the DataAnalysis® program version 4.4 from BRUKER and confirmed by using MS/MS analysis (MassFrag® software from Bruker).
2.3. Screening of Antimicrobial Activity
Antimicrobial susceptibility testing was performed according to the EUCAST disk diffusion method [20]. Fifteen strains were tested, of which 11 belong to the microbial culture collection of the Laboratory of Microbiology of Agrarian School of Polytechnic Institute of Castelo Branco, Portugal. The remaining four were Pseudomonas aeruginosa ATCC 27853, Pseudomonas fluorescens ATCC 13525, Staphylococcus aureus ATCC 25923, and Listeria monocytogenes NCTC 11994.
The wild strains came from the dairy factory and their origin is presented in Section 3.2.1.
The agents tested as inhibitor were C. cardunculus leaves decoction extract (CL) and inflorescence decoction extract (CI). Chlorogenic acid (CA) and rosmarinic acid (RA) were used as the standards. In the disk diffusion method, for each white disk (6 mm), 10 µL (0.1 mg/mL) of each antimicrobial agent under study was pipetted. As a negative control, 0.1% chloramphenicol was used, which is a broad-spectrum antibiotic used for growth inhibition control. Sterile distilled water was used as a positive control.
The quantification of Minimum Inhibitory Concentration (MIC) was carried out according to Syed et al., (2016) with some modifications. Using a micropipette, 270 µL of Müeller–Hinton broth (MHB) (OXOID, Basingstoke, Hampshire, England) culture medium was added to column 1 and 150 µL to columns 2 to 9. Column 10 corresponds to the positive control containing only 150 µL of medium and 10 µL of inoculum, while column 11 corresponds to the negative control containing 1350 µL of medium, 15 µL of chloramphenicol (0.1%) and 10 µL of inoculum. In column 12, only 160 µL of culture medium was added. In the first column, 30 µL of extract (10 mg/mL) were added. Eight 1:2 serial dilutions were made using pipetting homogenization between each dilution. After inoculation of 1.5 × 108 UFC/mL (10 μL per well, except for CM), the microplates were placed on a microplate shaker and placed in an incubator for 24 h at 30 °C. After incubation, 30 µL of resazurin solution was added to each well, and the microplates were placed back on the microplate shaker before the previous reading. The addition of resazurin dye acts as a redox indicator, facilitating the reading of the results, where active bacterial cells reduce non-fluorescent resazurin (blue) to fluorescent resorufin (pink) giving a correct quantifiable measure of bacterial metabolic activity. For the determination of the Minimum Bactericidal Concentration (MBC), Petri dishes with Nutrient Agar culture medium (Lyophilchem, Roseto degli Abruzzi (TE), Italy) were divided into squares, and each one of the microorganisms under study were duly identified. From each well of the microplate, 5 µL of its contents were pipetted and placed in the corresponding square. Plates were read 24 h after incubation at 30 °C. The absence of bacterial growth at the lowest concentration of antimicrobial agent corresponds to the minimum bactericidal concentration.
2.4. Inhibition of Growth and Melanin Bioproduction
The study of the influence of C. cardunculus extracts in bacteria growth and in melanin bioproduction was performed according to Ferraz et al. in 2021 [16]. About 2.5 mL of a bacterial suspension (108 CFU/mL) were inoculated in 22.5 mL of MHB (OXOID, Basingstoke, Hampshire, UK) supplemented with 1% L-tyrosine (Acrós Organics, NJ, USA) and 10 mg/mL inhibitory agent.
2.5. Melanin Extraction, Purification, and Quantification
The extraction and purification of melanin were performed according to Ferraz et al., (2021) [16]. To quantify the melanin bioproduction, a reverse-phase high pressure liquid chromatography using a diode array detector (RP-HPLC-DAD) was used.
The HPLC analysis was carried out using an Elite LaChrom® VWR Hitachi liquid chromatograph equipped with a L-2300 and L-2300 Diode array detector (Tokyo, Japan). A column LiChroCART® 100 RP-8 (5 μm) LiChrospher® 250-4 was used. The samples were analyzed by HPLC according to the method used by Guedes et al. in 2019 [19]. A calibration curve was made for melanin quantification. Several concentrations (0.1 to 1.0 mg/mL) of pure melanin, dissolved in methanol, were used to establish a calibration curve: y = 288,641x + 122,937, R² = 0.99.
2.6. Statistical Analysis
All of the assays were done in triplicate and results are presented as the average value ± standard deviation, both calculated using Microsoft® Excel 2016 (Microsoft Office 365, Redmond, Washington, DC, USA). To determine if there were significant differences between samples, an analysis of variance (ANOVA) was performed with α = 0.05, (95% confidence level) using Microsoft® Excel 2016 (Microsoft Office 365, Redmond, Washington, DC, USA).
3. Results and Discussion
3.1. Aqueous Extract from C. cardunculus Leaves: Compound Identification by LC-HRMS/MS
The compounds present in the leaves decoction extract of C. cardunculus (CL) are shown in Table 1.

Table 1.
Characterization of compounds present in leaves decoction extract of C. cardunculus L. by HPLC-DAD-ESI (Negative mode) HRMS.
The results showed that this extract is composed mainly (52%) of chlorogenic acid and their derivatives, cynarin, and caffeoylquinic acids. The compound with a retention time of 5.1 min was identified as chlorogenic acid and showed the highest intensity (303, 408). Fifteen percent of flavonoid compounds are luteolin glycosylated compounds. Several studies [1,13,21] also reported the presence of these compounds in the leaves of C. cardunculus L.
Two hormones were also detected, gibberellin A8 and A28, which are endogenous hormone growth regulators [22]. Gibberellines mediate various plant developmental and growth processes, including seed germination, shoot elongation and flower initiation and development [23]. The high intensity of gibberellin detected in C. cardunculus leaf extract is probably due to the growth stage in which the plant C. cardunculus L. was in.
The chemical composition of C. cardunculus leaf extract is similar to the chemical composition of inflorescence extract already reported in the literature. Cynarin and caffeoylquinic acid were generally the main molecules in the aqueous extracts of inflorescence already studied and were also present in the aqueous C. cardunculus leaf extract studied here [13].
3.2. Influence of C. cardunculus Extracts on Bacterial Growth
3.2.1. Antimicrobial Activity of C. cardunculus Extracts Using Solid Method
In this study, the inhibitor agents used were C. cardunculus leaves decoction extract (CL) and inflorescence decoction extract (CI), and chlorogenic acid (CA) to analyze the effect on several bacteria collected in the dairy factory. For this study, rosmarinic acid was also added due to its similar chemical structure to chlorogenic acid, both caffeic acid derivatives.
The inflorescence aqueous extract is traditionally used in cheese production, so the inflorescence decoction extract was included in this study. Eleven wild strains isolated from the dairy industry and four reference strains from ATCC and NCTC were tested.
The results of the antimicrobial activity of the extracts of C. cardunculus and standard compounds against the fifteen bacterial cultures are shown in Table 2.

Table 2.
Antimicrobial activity of C. cardunculus extracts measured by the inhibition zone (mm) of: CL—Leaves decoction, CI—Inflorescence decoction, and standard compounds, CA—chlorogenic and RA—rosmarinic acids; C− is the negative control (0.001 mg/mL chloramphenicol), C+ is the positive control (distilled and sterilized water). The antimicrobial agents were tested at a concentration of 1 mg/mL. NI—No Inhibition. Different superscript letters in the same line correspond to statistically different values (p ≤ 0.05).
CL and CI showed no inhibitory activity on all strains tested. All strains, except Staphylococcus aureus ATCC 25923, were inhibited by CA and RA. In this case, it was verified that the standard compounds were better inhibitors then C. cardunculus extracts. CA and RA showed the same antimicrobial activity as the negative control, for a confidence level of 95%. For example, Pseudomonas putida ESACB 191 showed similar antimicrobial activity for CA and a decrease of approximately 1 mm for RA compared to the negative control (p ≤ 0.05).
C. cardunculus inflorescence and leaves extracts were described as having antibacterial activity [13,24,25]. However, these studies used C. cardunculus extracts extracted with organic solvents such as ethanol and methanol among others. The extracts tested were extracted with water, so they are complete extracts where several phenolic and flavonoid compounds are present, as well as mucilage, making the proportion of active compounds lower than the extracts used in other studies. This is why the results showed antimicrobial activity by CA, whereas the main compound of CL (Table 1) and CL showed no antimicrobial activity.
Considering the results obtained, the study of MIC and MBC using chlorogenic acid and rosmarinic acid was carried out. All strains showed the same MIC/MBC of 1 mg/mL, except Pseudomonas putida ESACB 27 and Pseudomonas fluorescens ESACB 67, which showed lower MIC/MBC values of about 0.5 mg/mL. Matejczyk et al., 2018 also showed RA and caffeic acid MIC values in Gram+ bacteria of 0.5 mg/mL and 0.25 mg/mL in Gram− bacteria [26]. CA and RA are phenolic acids that are esters of caffeic acid, so they showed a similar MIC value. For all the strains tested, the MBC value corresponded to the MIC, showing that these extracts do not possess an inhibitory but a bactericidal activity. It is described that several classes of polyphenols such as phenolic acids like chlorogenic acid, flavonoids and tannins serve as a defense mechanism against microorganisms [21,25].
3.2.2. Antimicrobial Activity of C. cardunculus Extracts Using Liquid Method
P. putida ESACB 191 showed the same response as Alcaligenes faecalis ESACB 7 and P. fluorescens ESACB 67 against the antimicrobial action of CA and RA. However, this strain was described by Ferraz et al. in 2021 [16] as producing melanin and was therefore selected for further testing.
To understand whether the inhibition of P. putida ESACB 191 by phenolic acids, detected in the disk diffusion method described above, is influenced by physicochemical properties such as the penetration of antimicrobial agents into the disk, colony forming units (CFU) were used to measure cell viability in the presence of: chlorogenic acid (CA), rosmarinic acid (RA), CA:RA mixture (50:50), C. cardunculus leaves extract (CL), C. cardunculus inflorescence extract (CI) and CL:CI mixture (50:50). To compare the antimicrobial activity, the same concentration of antimicrobial agents (1 mg/mL) was used in both methods. The results of the influence of the samples on the growth of P. putida ESACB 191 in a liquid assay are shown in Figure 2.
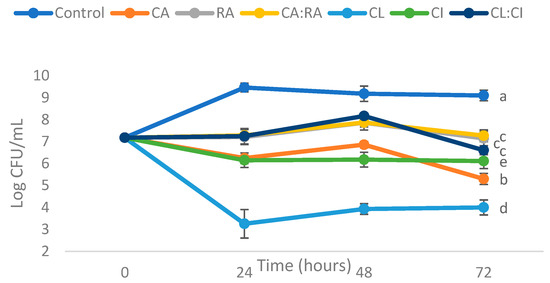
Figure 2.
P. putida ESACB 191 growth curves by action of CA—Chlorogenic acid; RA—Rosmarinic acid; CA:RA—50% Chlorogenic acid + 50% Rosmarinic acid, and C. cardunculus extracts: CL—Leaves decoction; CI—Inflorescence decoction; CL:CI—50% leaves decoction + 50% inflorescence decoction. The concentration 1 mg/mL was used for all samples tested in Müller–Hinton Broth with 1% L-tyrosine.
Figure 2 shows that all samples reduce the growth of P. putida ESACB 191 compared to the control at a 95% confidence level, within the first 24 h of incubation time. CL showed the greatest reduction in bacterial growth at 24 h, followed by CI and CA.
RA and the mixtures CA:RA and CL:CI did not show significant differences between them, however they were the ones that reduced the growth of P. putida ESACB 191 less compared with the control, for a confidence level of 95%.
Table 3 shows the reduction of the tested compounds in P. putida ESACB 191 growths at 24, 48 and 72 h of incubation. None of the tested compounds showed differences between incubation time periods, but they showed differences between them.

Table 3.
Influence of C. cardunculus decoction extracts and standard compounds in P. putida ESACB 191 growth reduction (Log CFU/mL), over the incubation time (24, 48 and 72 h). Different superscript letters in the same line, correspond to statistically different values (p ≤ 0.05).
CL showed the most significant decrease in bacterial growth, for a 95% confidence level, which corresponds to a mean decrease of 60% in the growth of P. putida ESACB 191. CI decreased bacterial growth by about 33% on average.
CA also showed a mean decrease of 33%, however it was statistically different from CI at a 95% confidence level. RA, CA:RA and CL:CI did not show significant differences between them in decreasing the growth of P. putida ESACB 191 over time, however they were the ones that had less influence on the decrease of bacterial growth.
Contrary to the results obtained in the antimicrobial activity test in solid medium (disk diffusion method), in the liquid test, the CL and CI extracts were the ones that showed the greatest influence in reducing the growth of P. putida ESACB 191. These results suggest that the disk usage decreases the diffusion capacity of the extracts in the culture medium. The discrepancies between the methods can be explained by the fact that the aqueous extracts tested are mixtures of compounds, some of them with relatively high molecular weight compounds, as in the case of CL 533 m/z. Due to their molecular weight and probably to their poor hydrophilicity, they do not dissolve easily into the agar medium. The compounds present in the extracts have limited migration into the agar, erroneously leading to their non activity as bacteria growth inhibitors [27,28,29].
CL has chlorogenic acid and its derivatives as the major component of its chemical constitution (Table 1) and presented an antimicrobial action that resulted in a 66% decrease in the growth of P. putida ESACB 191. This decrease results not only from the presence of chlorogenic acid that showed less antimicrobial activity than CL, but from the synergistic effect of all compounds present in this extract. Chlorogenic acid, cynarin, luteolin and caffeoylquinic acids in general were all responsible for the antibacterial activity detected in the work of other investigators [21,30].
3.3. Influence of C. cardunculus Extracts in Melanin Bioproduction
The P. putida ESACB 191 strain is responsible for the brownish color observed in the rind of cheeses, which has been identified as melanin [16].
Therefore, the study of the influence of RA, CA, CA: RA, CL, CI, and CL: CI, on melanin bioproduction was tested for P. putida ESACB 191 strain (Figure 3).
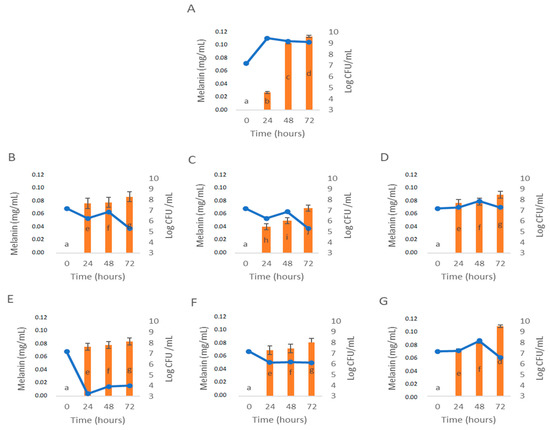
Figure 3.
Influence of C. cardunculus extracts and phenolic acids in melanin bioproduction by P. putida ESACB 191. (A). Control; (B). Rosmarinic acid; (C). Chlorogenic acid; (D). 50% Chlorogenic acid + 50% Rosmarinic acid; (E). Leaf decoction extract; (F). Inflorescence decoction extract; (G). 50% Leaves decoction + 50% Inflorescence decoction. The concentration 1 mg/mL was used for all samples tested in Müller–Hinton Broth with 1% of L-tyrosine. Lines represent P. putida ESACB 191 growth curve (Log CFU/mL) and bars represent melanin bioproduction (mg/mL).
Figure 3A corresponds to the control test that had the optimal conditions for P. putida ESACB 191 to produce melanin as described in the previous study [16]. It was verified, at a 95% confidence level, that there are significant differences in the bioproduction of melanin, except for the CL:CI mixture (Figure 3G) which showed similar melanin bioproduction with the Control (Figure 3A) at 72 h. RA (Figure 3B), CA:RA (Figure 3D), CL (Figure 3E) and CI (Figure 3F) extracts, although they showed differences in the bioproduction of melanin by P. putida ESACB 191 compared to the control (Figure 3A), did not differ from each other for a 95% confidence level.
However, the influence of CA (Figure 3C) on melanin bioproduction resulted in significant differences at 24, 48 and 72 h when compared to the control (Figure 3A). Namely, at 48 h there was a drop in the bioproduction of melanin by P. putida ESACB 191 of 50%, for a confidence level of 95%. This tyrosinase inhibitory action by chlorogenic acid agrees with the literature where it is described that chlorogenic acid affects the production of melanin through the inhibition of tyrosinase when converted into metabolites in cells [31,32].
The growth curves present in the results of Figure 3 help us understand the bioproduction of melanin as a function of the cell viability of P. putida ESACB 191. Thus, it is possible to verify that in the presence of the CL extract there is a break in bacterial growth, but it does not affect the bioproduction of melanin. This means that P. putida ESACB 191 secreted tyrosinase into the culture medium during the initial growth of the bacteria [33,34,35,36]. However, tyrosinase activities are induced in the stationary phase, where they are involved in extracellular melanin production [36]. This explains the reason for melanin production by P. putida ESACB 191 with less than 6.20 Log CFU/mL of viable cells in the presence of CL extract.
The effect of all samples tested on melanin bioproduction is presented in Figure 4.
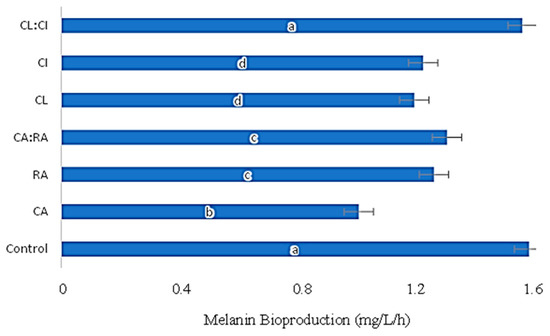
Figure 4.
P. putida ESACB 191 melanin bioproduction (mg/L/h) by action of several C. cardunculus extracts: CL:CI—50% Leaf decoction + 50% Inflorescence decoction. CI—Inflorescence decoction. CL—Leaves decoction. CA:RA—50% Chlorogenic acid + 50% Rosmarinic acid. RA—Rosmarinic acid. CA—Chlorogenic acid. The concentration 1 mg/mL was used for all samples tested in Müller–Hinton Broth with 1% of L-tyrosine. The a-d letters correspond to statistically different values (p ≤ 0.05).
CA was the phenolic compound with the greatest influence on melanin bioproduction by P. putida ESACB 191, resulting in a significant decrease of 43% in yield (mg/L/h) compared to control, for a confidence level of 95% (Figure 4).
The extracts, both from the leaves and from the inflorescence, influence the melanin production in the same measure, 25%, but the mixture of these extracts caused a smaller decrease than the individual extracts. A similar effect was seen with phenolic acids, as they had a better effect on reducing melanin production than when used as a mixture.
The CL and CI extracts, on the other hand, did not show significant differences in bioproduction between them. However, CL showed a greater reduction (33%) in melanin bioproduction compared to the control (Figure 4).
Chlorogenic acid showed the greatest reduction in melanin bioproduction, followed by CL extract. As shown in Table 1, CL extract is mainly composed of chlorogenic acid and its derivatives, which justifies its inhibitory action on the growth of P. putida ESACB 191 and its melanin bioproduction.
4. Conclusions
This study allowed us to identify the chemical composition of a decoction extract of C. cardunculus leaves and to evaluate the antimicrobial activity of several extracts of C. cardunculus as well as their influence on melanin bioproduction. It was found that CL is mainly composed of chlorogenic acid and its derivatives. The antimicrobial activity in solid medium and in liquid medium was evaluated for 15 bacterial strains using CA, RA, CL, CI, CA:RA, CL:CI as antimicrobial agents. RA showed an MBC of 1 mg/mL in 80% of the strains. In liquid medium, CL showed the greatest reduction in the growth of P. putida ESACB 191, around 6.20 Log CFU/mL, which corresponded to 66% of bacteria growth on average. The influence of these extracts and phenolic acids on the bioproduction of melanin was also studied. Chlorogenic acid showed 50% reduction in melanin bioproduction by P. putida ESACB 191 in 48 h.
Chlorogenic acid is one of the main components of phenolic acid derivatives of C. cardunculus leaves extract (CL), which may justify the inhibitory action on bacterial growth and melanin bioproduction found with the plant extract. Thus, in the future, the application of CL extracts with antimicrobial activity in edible films and/or coatings to cheese rind to increase the shelf time can be studied.
Author Contributions
A.R.F.: Investigation, method development C.M.B.S.P.: Microbiology supervision, microbiology resources, funding acquisition, paper reviewing M.L.S.: Work conceptualization, formal analysis; funding acquisition; project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Fundação para a Ciência e Tecnologia (FCT) with financial support to IPCB-CERNAS (UID/AMB/00681/2020) Research Unit Grant from FCT; to BioISI Research Unit through the contract UIDB/04046/2020 from FCT; to CESAM (UID/AMB/50017/2019), through FCT/MEC National funds, and the co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020. This work is recipient of a project (PTDC/Bia-BQM/28355/2017) grant and Ana Rita Ferraz acknowledges a PhD fellowship (SFRH/BD/135692/2018) from FCT (Portugal).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Manuela Goulão for the help provided in the Laboratory of Microbiology (IPCB/ESA) and Rita Alexandra Guedes for analyzing the extract of C. cardunculus by LC-HRMS/MS.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Veríssimo, P.; Esteves, C.; Faro, C.; Pires, E. The vegetable rennet of Cynara cardunculus L. contains two proteinases with chymosin and pepsin-like specificities. Biotechnol. Lett. 1995, 17, 621–626. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostic, M.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Seasonal variation in bioactive properties and phenolic composition of cardoon (Cynara cardunculus var. altilis) bracts. Food Chem. 2021, 336, 127744. [Google Scholar] [CrossRef]
- Mileo, A.M.; Di Venere, D.; Linsalata, V.; Fraioli, R.; Miccadei, S. Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA-MB231. J. Cell. Physiol. 2012, 227, 3301–3309. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Serralheiro, M.L. Studies on the molecular mechanism of cholesterol reduction by Fraxinus angustifolia, Peumus boldus, Cynara cardunculus and Pterospartum tridentatum infusions. J. Med. Plants Res. 2014, 8, 9–17. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Henriques, M.; Ferreira, I.C.F.R. Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterisation. Food Chem. 2015, 167, 131–137. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Cleto, P.; Madeira, P.J.A.; Florêncio, M.H.; Frazatilde, F.N.; Serralheiro, M.L.M. Antioxidant and anti-acetylcholinesterase activity of commercially available medicinal infusions after in vitro gastrointestinal digestion. J. Med. Plants Res. 2013, 7, 1370–1378. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic Compounds from the Leaf Extract of Artichoke (Cynara scolymus L.) and Their Antimicrobial Activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef] [PubMed]
- Scavo, A.; Pandino, G.; Restuccia, C.; Parafati, L.; Cirvilleri, G.; Mauromicale, G. Antimicrobial activity of cultivated cardoon (Cynara cardunculus L. var. altilis DC.) leaf extracts against bacterial species of agricultural and food interest. Ind. Crop. Prod. 2019, 129, 206–211. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alves, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostić, M.; Soković, M.S.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Phenolic Composition and Biological Properties of Cynara cardunculus L. var. altilis Petioles: Influence of the Maturity Stage. Antioxidants 2021, 10, 1907. [Google Scholar] [CrossRef]
- Carreira, A.; Paloma, L.; Loureiro, V. Pigment producing yeasts involved in the brown surface discoloration of ewes’ cheese. Int. J. Food Microbiol. 1998, 41, 223–230. [Google Scholar] [CrossRef]
- Ferraz, A.R.; Pacheco, R.; Vaz, P.D.; Pintado, C.S.; Ascensão, L.; Serralheiro, M.L. Melanin: Production from Cheese Bacteria, Chemical Characterization, and Biological Activities. Int. J. Environ. Res. Public Health 2021, 18, 10562. [Google Scholar] [CrossRef]
- Arslan, S.; Eyi, A.; Özdemir, F. Spoilage potentials and antimicrobial resistance of Pseudomonas spp. isolated from cheeses. J. Dairy Sci. 2011, 94, 5851–5856. [Google Scholar] [CrossRef]
- Abdulkadir, N.; Usman, N.A.H.M.; Gani, H.M.M.M. Bacterial Pigments and its Significance. MOJ Bioequivalence Bioavailab. 2017, 4, 285–288. [Google Scholar] [CrossRef]
- Guedes, L.; Reis, P.B.P.S.; Machuqueiro, M.; Ressaissi, A.; Pacheco, R.; Serralheiro, M.L. Bioactivities of Centaurium erythraea (Gentianaceae) Decoctions: Antioxidant Activity, Enzyme Inhibition and Docking Studies. Molecules 2019, 24, 3795. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing–EUCAST, Antimicrobial susceptibility testing EUCAST disk diffusion method version 8.0 January. Eur. Soc. Clin. Microbiol. Infect. Deseases 2020, 1–21.
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. Comptes Rendus Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Toner, P.; Nelson, D.; Rao, J.R.; Ennis, M.; Moore, J.E.; Schock, B. Antimicrobial properties of phytohormone (gibberellins) against phytopathogens and clinical pathogens. Access Microbiol. 2021, 3, 000278. [Google Scholar] [CrossRef]
- Fratianni, F.; Pepe, R.; Nazzaro, F. Polyphenol Composition, Antioxidant, Antimicrobial and Quorum Quenching Activity of the “Carciofo di Montoro” (Cynara cardunculus var. scolymus) Global Artichoke of the Campania Region, Southern Italy. Food Nutr. Sci. 2014, 5, 2053–2062. [Google Scholar] [CrossRef][Green Version]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef]
- Osés, S.M.; Pascual-Maté, A.; de la Fuente, D.; de Pablo, A.; Fernández-Muiño, M.A.; Sancho, M.T. Comparison of methods to determine antibacterial activity of honeys against Staphylococcus aureus. NJAS-Wagening. J. Life Sci. 2016, 78, 29–33. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk diffusion susceptibility test protocol author information. Am. Soc. Microbiol. 2012, 15, 1–13. [Google Scholar]
- Flanagan, J.N.; Steck, T.R. The Relationship Between Agar Thickness and Antimicrobial Susceptibility Testing. Indian J. Microbiol. 2017, 57, 503–506. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Molecules Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- Oh, H.-N.; Park, D.-H.; Park, J.-Y.; Song, S.-Y.; Lee, S.-H.; Yoon, G.; Moon, H.-S.; Oh, D.-S.; Rhee, S.-H.; Im, E.-O.; et al. Molecules communication tyrosinase inhibition antioxidant effect and cytotoxicity studies of the extracts of Cudrania tricuspidata fruit standardized in chlorogenic acid. Molecules 2019, 24, 3266. [Google Scholar] [CrossRef]
- Al Khatib, M.; Harir, M.; Costa, J.; Baratto, M.C.; Schiavo, I.; Trabalzini, L.; Pollini, S.; Rossolini, G.M.; Basosi, R.; Pogni, R. Spectroscopic characterization of natural melanin from a Streptomyces cyaneofuscatus strain and somparison with melanin enzymatically synthesized by tyrosinase and laccase. Molecules 2018, 23, 1916. [Google Scholar] [CrossRef]
- Plonka, P.M.; Grabacka, M. Melanin synthesis in microorganisms-biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.M.R.; Ribera, J. Microbial production of melanin and its various applications. World J. Microbiol. Biotechnol. 2020, 36, 1–9. [Google Scholar] [CrossRef]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).