A Review on Microfluidic Platforms Applied to Nerve Regeneration
Abstract
1. Introduction
2. Design of Microfluidic Devices
2.1. Materials
2.2. Structures
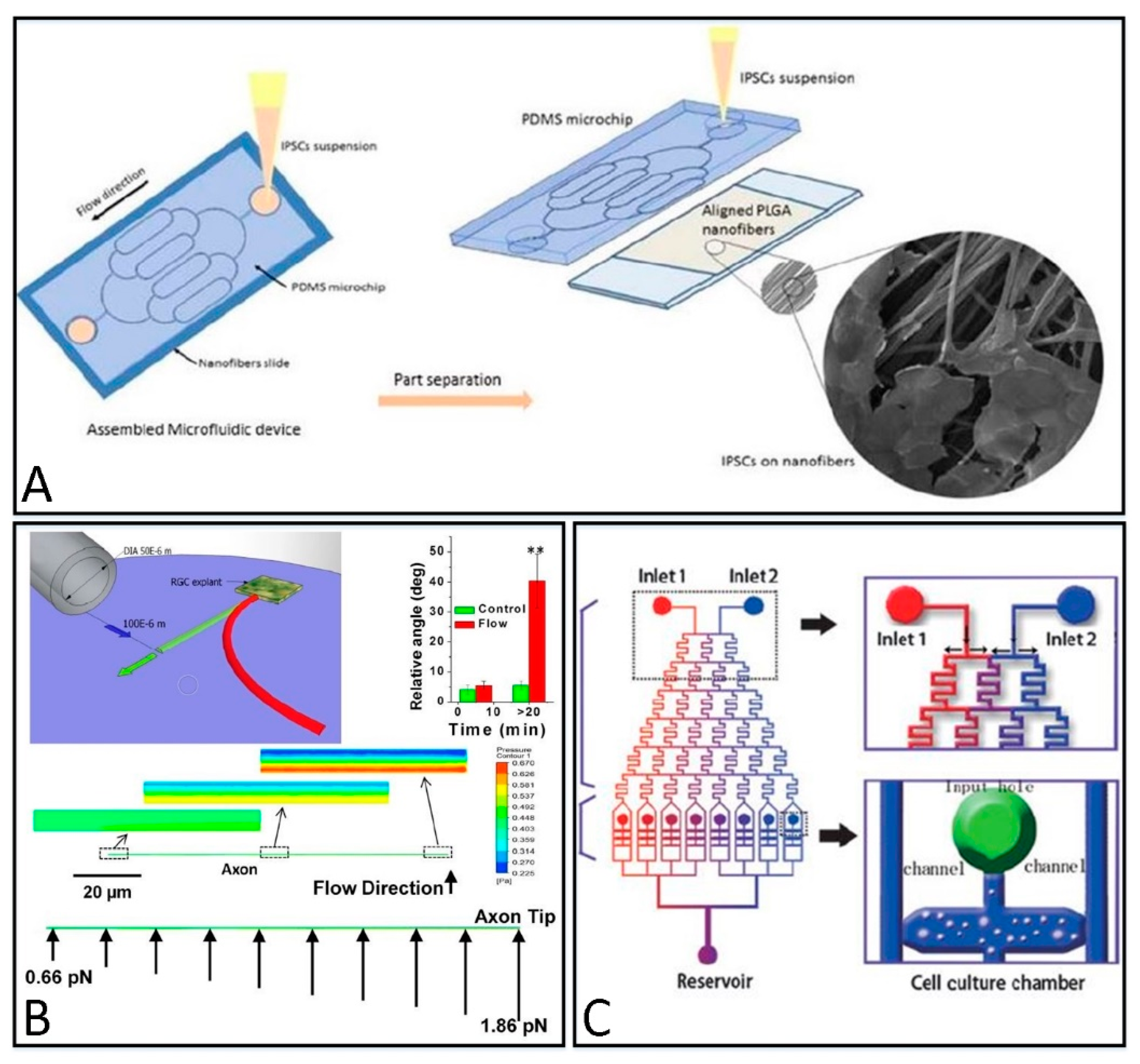
2.2.1. 2D Culture Structure
2.2.2. 3D Culture Structure
3. Microfluidic Platforms Applied to Nerve Repair and Outgrowth
3.1. Nerve Repair
3.1.1. Microfluidic Platforms for Different Repairing Methods
3.1.2. Microfluidic Platforms for Different Injury Models
3.2. Neurite Outgrowth
4. Microfluidic Platforms Applied to Axonal Guidance
4.1. Topographical Guidance
4.2. Mechanical Guidance
4.3. Chemical Gradients Guidance
5. Challenges, Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.spinalcord.com/blog/complete-vs.-incomplete-spinal-cord-injuries (accessed on 22 March 2022).
- Burns, A.S.; Marino, R.J.; Flanders, A.E.; Flett, H. Clinical diagnosis and prognosis following spinal cord injury. Handb. Clin. Neurol. 2012, 109, 47–62. [Google Scholar]
- Shrirao, A.B.; Kung, F.H.; Omelchenko, A.; Schloss, R.S.; Boustany, N.N.; Zahn, J.D.; Firestein, B.L. Microfluidic platforms for the study of neuronal injury in vitro. Biotechnol. Bioeng. 2018, 115, 815–830. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.W.; Park, J.W.; Byun, J.H.; Vahidi, B.; Rhee, S.W.; Jeon, N.L. Integrated microfluidics platforms for investigating injury and regeneration of CNS axons. Ann. Biomed. Eng. 2012, 40, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Millet, L.J.; Gillette, M.U. Over a century of neuron culture: From the hanging drop to microfluidic devices. Yale J. Biol. Med. 2012, 85, 501. [Google Scholar] [PubMed]
- Park, J.W.; Kim, H.J.; Kang, M.W.; Jeon, N.L. Advances in microfluidics-based experimental methods for neuroscience research. Lab Chip 2013, 13, 509–521. [Google Scholar] [CrossRef]
- Hengst, U.; Deglincerti, A.; Kim, H.J.; Jeon, N.L.; Jaffrey, S.R. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat. Cell Biol. 2009, 11, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Koito, H.; Li, J.; Han, A. Multi-compartment neuron–glia co-culture platform for localized CNS axon–glia interaction study. Lab Chip 2012, 12, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Torrieri, G.; Santos, H.A. The importance of microfluidics for the preparation of nanoparticles as advanced drug delivery systems. Expert Opin. Drug Deliv. 2018, 15, 469–479. [Google Scholar] [CrossRef]
- Harink, B.; Le Gac, S.; Truckenmüller, R.; van Blitterswijk, C.; Habibovic, P. Regeneration-on-a-chip? The perspectives on use of microfluidics in regenerative medicine. Lab Chip 2013, 13, 3512–3528. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.; Han, A.; Li, J. Microfluidic systems for axonal growth and regeneration research. Neural Regen. Res. 2014, 9, 1703. [Google Scholar]
- Taylor, A.M.; Rhee, S.W.; Tu, C.H.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. Microfluidic multicompartment device for neuroscience research. Langmuir 2003, 19, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Rhee, S.W.; Jeon, N.L. Microfluidic chambers for cell migration and neuroscience research. In Microfluidic Techniques; Humana Press: New Jersey, NJ, USA, 2006; pp. 167–177. [Google Scholar]
- Rhee, S.W.; Taylor, A.M.; Tu, C.H.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. Patterned cell culture inside microfluidic devices. Lab Chip 2005, 5, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, B.; Park, J.W.; Kim, H.J.; Jeon, N.L. Microfluidic-based strip assay for testing the effects of various surface-bound inhibitors in spinal cord injury. J. Neurosci. Methods 2008, 170, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bamber, Z.A.; Sun, W.; Menon, R.S.; Wheeler, P.C.; Swain, I.D.; Fong, D.T. Functional Electrical Stimulation of Peroneal Muscles on Balance in Healthy Females. Cyborg Bionic Syst. 2021, 2021, 9801097. [Google Scholar] [CrossRef]
- Wang, L.; Ma, L.; Yang, J.; Wu, J. Human Somatosensory Processing and Artificial Somatosensation. Cyborg Bionic Syst. 2021, 2021, 9843259. [Google Scholar] [CrossRef]
- Akiyama, Y.; Nakayama, A.; Nakano, S.; Amiya, R.; Hirose, J. An Electrical Stimulation Culture System for Daily Maintenance-Free Muscle Tissue Production. Cyborg Bionic Syst. 2021, 2021, 9820505. [Google Scholar] [CrossRef]
- Teotia, P.; Van Hook, M.J.; Fischer, D.; Ahmad, I. Human retinal ganglion cell axon regeneration by recapitulating developmental mechanisms: Effects of recruitment of the mTOR pathway. Development 2019, 146, dev178012. [Google Scholar] [CrossRef]
- Li, L.; Ren, L.; Liu, W.; Wang, J.C.; Wang, Y.; Tu, Q.; Wang, J. Spatiotemporally controlled and multifactor involved assay of neuronal compartment regeneration after chemical injury in an integrated microfluidics. Anal. Chem. 2012, 84, 6444–6453. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, T. 3D Cultures in XonaChips; 2020; Available online: https://xonamicrofluidics.com/wp-content/uploads/2020/04/XonaTechNote_3D-culture-matrigel-in-XonaChips_4-6-20_AMTedits-1.pdf (accessed on 22 March 2022).
- Yong, Y.; Hughes, C.; Deppmann, C. A microfluidic culture platform to assess axon degeneration. In Axon Degeneration; Humana: New York, NY, USA, 2020; pp. 83–96. [Google Scholar]
- De Vincentiis, S.; Falconieri, A.; Mainardi, M.; Cappello, V.; Scribano, V.; Bizzarri, R.; Raffa, V. Extremely low forces induce extreme axon growth. J. Neurosci. 2020, 40, 4997–5007. [Google Scholar] [CrossRef]
- Gladkov, A.; Pigareva, Y.; Antipova, O.; Kolpakov, V.; Tarabykin, V.; Pimashkin, A. Investigating Mechanisms of Axon Navigation Using Microfluidic Methods. Opera Med. Physiol. 2020, 5, 1–6. [Google Scholar]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.I.; Pagaduan, J.V.; Nordin, G.P.; Woolley, A.T. Single-monomer formulation of polymerized polyethylene glycol diacrylate as a nonadsorptive material for microfluidics. Anal. Chem. 2011, 83, 6418–6425. [Google Scholar] [CrossRef] [PubMed]
- Ogończyk, D.; Węgrzyn, J.; Jankowski, P.; Dąbrowski, B.; Garstecki, P. Bonding of microfluidic devices fabricated in polycarbonate. Lab Chip 2010, 10, 1324–1327. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Chen, G. Fabrication, modification, and application of poly (methyl methacrylate) microfluidic chips. Electrophoresis 2008, 29, 1801–1814. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, S.; Wang, C.; Hu, J.; Li, C.; Zhuang, Z.; Yang, C.J. PMMA/PDMS valves and pumps for disposable microfluidics. Lab Chip 2009, 9, 3088–3094. [Google Scholar] [CrossRef]
- Rogers, C.I.; Oxborrow, J.B.; Anderson, R.R.; Tsai, L.F.; Nordin, G.P.; Woolley, A.T. Microfluidic valves made from polymerized polyethylene glycol diacrylate. Sens. Actuators B Chem. 2014, 191, 438–444. [Google Scholar] [CrossRef]
- Praveen, K.M.; Pious, C.V.; Thomas, S.; Grohens, Y. Non-Thermal Plasma Technology for Polymeric Materials: Applications in Composites, Nanostructured Materials and Biomedical Fields; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–21. [Google Scholar]
- Jiang, G.; Liu, C.; Liu, X.; Zhang, G.; Yang, M.; Liu, F. Construction and properties of hydrophobic association hydrogels with high mechanical strength and reforming capability. Macromol. Mater. Eng. 2009, 294, 815–820. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, C.; Liu, X.; Chen, Q.; Zhang, G.; Yang, M.; Liu, F. Network structure and compositional effects on tensile mechanical properties of hydrophobic association hydrogels with high mechanical strength. Polymer 2010, 51, 1507–1515. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Li, Z.; Gao, G.; Liu, F. Temperature-responsive properties of poly(acrylic acid-co-acrylamide) hydrophobic association hydrogels with high mechanical strength. Macromolecules 2010, 43, 10645–10651. [Google Scholar] [CrossRef]
- Thomas, B.H.; Fryman, J.C.; Liu, K.; Mason, J. Hydrophilic–hydrophobic hydrogels for cartilage replacement. J. Mech. Behav. Biomed. Mater. 2009, 2, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Abdurrahmanoglu, S.; Cilingir, M.; Okay, O. Dodecyl methacrylate as a crosslinker in the preparation of tough polyacrylamide hydrogels. Polymer 2011, 52, 694–699. [Google Scholar] [CrossRef]
- Goy, C.B.; Chaile, R.E.; Madrid, R.E. Microfluidics and hydrogel: A powerful combination. React. Funct. Polym. 2019, 145, 104314. [Google Scholar] [CrossRef]
- Akiyama, Y. Design of temperature-responsive cell culture surfaces for cell sheet engineering. Cyborg Bionic Syst. 2021, 2021, 5738457. [Google Scholar] [CrossRef]
- Park, J.W.; Vahidi, B.; Taylor, A.M.; Rhee, S.W.; Jeon, N.L. Microfluidic culture platform for neuroscience research. Nat. Protoc. 2006, 1, 2128–2136. [Google Scholar] [CrossRef]
- Siddique, R.; Thakor, N. Investigation of nerve injury through microfluidic devices. J. R. Soc. Interface 2014, 11, 20130676. [Google Scholar] [CrossRef]
- Magdesian, M.H.; Sanchez, F.S.; Lopez, M.; Thostrup, P.; Durisic, N.; Belkaid, W.; Colman, D.R. Atomic force microscopy reveals important differences in axonal resistance to injury. Biophys. J. 2012, 103, 405–414. [Google Scholar] [CrossRef]
- Sauer, B.M.; Schmalstieg, W.F.; Howe, C.L. Axons are injured by antigen-specific CD8+ T cells through a MHC class I-and granzyme B-dependent mechanism. Neurobiol. Dis. 2013, 59, 194–205. [Google Scholar] [CrossRef]
- Deleglise, B.; Lassus, B.; Soubeyre, V.; Alleaume-Butaux, A.; Hjorth, J.J.; Vignes, M.; Peyrin, J.M. Synapto-protective drugs evaluation in reconstructed neuronal network. PLoS ONE 2013, 8, e71103. [Google Scholar] [CrossRef]
- Samson, A.J.; Robertson, G.; Zagnoni, M.; Connolly, C.N. Neuronal networks provide rapid neuroprotection against spreading toxicity. Sci. Rep. 2016, 6, 33746. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jeong, S.; Koo, C.; Han, A.; Park, J. A microchip for high-throughput axon growth drug screening. Micromachines 2016, 7, 114. [Google Scholar] [PubMed]
- Park, J.; Kim, S.; Park, S.I.; Choe, Y.; Li, J.; Han, A. A microchip for quantitative analysis of CNS axon growth under localized biomolecular treatments. J. Neurosci. Methods 2014, 221, 166–174. [Google Scholar] [PubMed]
- Oda, H.; Kihara, K.; Morimoto, Y.; Takeuchi, S. Cell-based biohybrid sensor device for chemical source direction estimation. Cyborg Bionic Syst. 2021, 2021, 8907148. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; Dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef]
- Li, X.; Valadez, A.V.; Zuo, P.; Nie, Z. Microfluidic 3D cell culture: Potential application for tissue-based bioassays. Bioanalysis 2012, 4, 1509–1525. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Zhao, S.P.; Ma, Y.; Lou, Q.; Zhu, H.; Yang, B.; Fang, Q. Three-dimensional cell culture and drug testing in a microfluidic sidewall-attached droplet array. Anal. Chem. 2017, 89, 10153–10157. [Google Scholar]
- Tang, Y.; Qiu, Q.F.; Zhang, F.L.; Xie, M.; Huang, W.H. Quantifying orientational regeneration of injured neurons by natural product concentration gradients in a 3D microfluidic device. Lab Chip 2018, 18, 971–978. [Google Scholar]
- Romano, N.H.; Lampe, K.J.; Xu, H.; Ferreira, M.M.; Heilshorn, S.C. Microfluidic gradients reveal enhanced neurite outgrowth but impaired guidance within 3D matrices with high integrin ligand densities. Small 2015, 11, 722–730. [Google Scholar]
- Choi, J.; Kim, S.; Jung, J.; Lim, Y.; Kang, K.; Park, S.; Kang, S. Wnt5a-mediating neurogenesis of human adipose tissue-derived stem cells in a 3D microfluidic cell culture system. Biomaterials 2011, 32, 7013–7022. [Google Scholar] [CrossRef] [PubMed]
- Kunze, A.; Bertsch, A.; Giugliano, M.; Renaud, P. Microfluidic hydrogel layers with multiple gradients to stimulate and perfuse three-dimensional neuronal cell cultures. Procedia Chem. 2009, 1, 369–372. [Google Scholar] [CrossRef]
- Kunze, A.; Giugliano, M.; Valero, A.; Renaud, P. Micropatterning neural cell cultures in 3D with a multi-layered scaffold. Biomaterials 2011, 32, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Kunze, A.; Valero, A.; Zosso, D.; Renaud, P. Synergistic NGF/B27 gradients position synapses heterogeneously in 3D micropatterned neural cultures. PLoS ONE 2011, 6, e26187. [Google Scholar] [CrossRef] [PubMed]
- Nasser, T.I.N.; Spencer, G.E.; Outgrowth, N. Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128012383. [Google Scholar]
- Cui, J.; Wang, H.P.; Shi, Q.; Sun, T. Pulsed Microfluid Force-Based On-Chip Modular Fabrication for Liver Lobule-Like 3D Cellular Models. Cyborg Bionic Syst. 2021, 2021, 9871396. [Google Scholar] [CrossRef]
- Louis, F.; Piantino, M.; Liu, H.; Kang, D.H.; Sowa, Y.; Kitano, S.; Matsusaki, M. Bioprinted vascularized mature adipose tissue with collagen microfibers for soft tissue regeneration. Cyborg Bionic Syst. 2021, 2021, 1412542. [Google Scholar] [CrossRef]
- Sala-Jarque, J.; Mesquida-Veny, F.; Badiola-Mateos, M.; Samitier, J.; Hervera, A.; Del Río, J.A. Neuromuscular activity induces paracrine signaling and triggers axonal regrowth after injury in microfluidic Lab-On-Chip devices. Cells 2020, 9, 302. [Google Scholar] [CrossRef]
- Hosmane, S.; Fournier, A.; Wright, R.; Rajbhandari, L.; Siddique, R.; Yang, I.H.; Thakor, N. Valve-based microfluidic compression platform: Single axon injury and regrowth. Lab Chip 2011, 11, 3888–3895. [Google Scholar] [CrossRef]
- Tong, Z.; Segura-Feliu, M.; Seira, O.; Homs-Corbera, A.; Del Río, J.A.; Samitier, J. A microfluidic neuronal platform for neuron axotomy and controlled regenerative studies. RSC Adv. 2015, 5, 73457–73466. [Google Scholar] [CrossRef]
- Mika, J.K.; Schwarz, K.; Wanzenboeck, H.D.; Scholze, P.; Bertagnolli, E. Investigation of neurotrophic factor concentrations with a novel in vitro concept for peripheral nerve regeneration. J. Neurosci. Res. 2015, 93, 1631–1640. [Google Scholar] [CrossRef]
- Morrison, B., III; Cater, H.L.; Benham, C.D.; Sundstrom, L.E. An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. J. Neurosci. Methods 2006, 150, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Pfister, B.J.; Weihs, T.P.; Betenbaugh, M.; Bao, G. An in vitro uniaxial stretch model for axonal injury. Ann. Biomed. Eng. 2003, 31, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Dollé, J.P.; Morrison, B., III; Schloss, R.S.; Yarmush, M.L. An organotypic uniaxial strain model using microfluidics. Lab Chip 2013, 13, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.C.; Dickson, T.C.; King, A.E.; Breadmore, M.C.; Guijt, R.M. Microfluidic culture platform for studying neuronal response to mild to very mild axonal stretch injury. Biomicrofluidics 2014, 8, 044110. [Google Scholar] [CrossRef]
- Bang, S.; Lee, S.; Hwang, K.S.; Kim, J.; Choi, N.; Kim, H.N. Three-Dimensional Axotomy and Regeneration on Open-Access Microfluidic Platform. IEEE Trans. NanoBiosci. 2021. [CrossRef] [PubMed]
- Taylor, A.M.; Berchtold, N.C.; Perreau, V.M.; Tu, C.H.; Jeon, N.L.; Cotman, C.W. Axonal mRNA in uninjured and regenerating cortical mammalian axons. J. Neurosci. 2009, 29, 4697–4707. [Google Scholar] [CrossRef]
- Zhang, J.N.; Michel, U.; Lenz, C.; Friedel, C.C.; Köster, S.; d’Hedouville, Z.; Koch, J.C. Calpain-mediated cleavage of collapsin response mediator protein-2 drives acute axonal degeneration. Sci. Rep. 2016, 6, 37050. [Google Scholar] [CrossRef] [PubMed]
- Lezana, J.P.; Dagan, S.Y.; Robinson, A.; Goldstein, R.S.; Fainzilber, M.; Bronfman, F.C.; Bronfman, M. Axonal PPAR γ promotes neuronal regeneration after injury. Dev. Neurobiol. 2016, 76, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Karthikeyan, K.; Chirvi, S.; Davé, D.P. Neuro-optical microfluidic platform to study injury and regeneration of single axons. Lab Chip 2009, 9, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Hellman, A.N.; Vahidi, B.; Kim, H.J.; Mismar, W.; Steward, O.; Jeon, N.L.; Venugopalan, V. Examination of axonal injury and regeneration in micropatterned neuronal culture using pulsed laser microbeam dissection. Lab Chip 2010, 10, 2083–2092. [Google Scholar] [CrossRef]
- Gokce, S.K.; Hegarty, E.M.; Mondal, S.; Zhao, P.; Ghorashian, N.; Hilliard, M.A.; Ben-Yakar, A. A multi-trap microfluidic chip enabling longitudinal studies of nerve regeneration in Caenorhabditis elegans. Sci. Rep. 2017, 7, 9837. [Google Scholar] [CrossRef] [PubMed]
- Hyung, S.; Lee, S.R.; Kim, Y.J.; Bang, S.; Tahk, D.; Park, J.C.; Jeon, N.L. Optogenetic neuronal stimulation promotes axon outgrowth and myelination of motor neurons in a three-dimensional motor neuron–Schwann cell coculture model on a microfluidic biochip. Biotechnol. Bioeng. 2019, 116, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Hesari, Z.; Soleimani, M.; Atyabi, F.; Sharifdini, M.; Nadri, S.; Warkiani, M.E.; Dinarvand, R. A hybrid microfluidic system for regulation of neural differentiation in induced pluripotent stem cells. J. Biomed. Mater. Res. Part A 2016, 104, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Folch, A. Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates. Exp. Cell Res. 2005, 311, 307–316. [Google Scholar] [CrossRef]
- Ristola, M.; Fedele, C.; Hagman, S.; Sukki, L.; Kapucu, F.E.; Mzezewa, R.; Narkilahti, S. Directional Growth of Human Neuronal Axons in a Microfluidic Device with Nanotopography on Azobenzene-Based Material. Adv. Mater. Interfaces 2021, 8, 2100048. [Google Scholar] [CrossRef]
- Gu, L.; Black, B.; Ordonez, S.; Mondal, A.; Jain, A.; Mohanty, S. Microfluidic control of axonal guidance. Sci. Rep. 2014, 4, 6457. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Hogue, I.B.; Cung, K.; Purohit, P.K.; McAlpine, M.C. Tension-induced neurite growth in microfluidic channels. Lab Chip 2013, 13, 3735–3740. [Google Scholar] [CrossRef]
- Wang, C.J.; Li, X.; Lin, B.; Shim, S.; Ming, G.L.; Levchenko, A. A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound ECM molecules and diffusible guidance cues. Lab Chip 2008, 8, 227–237. [Google Scholar] [CrossRef]
- Yin, B.S.; Li, M.; Liu, B.M.; Wang, S.Y.; Zhang, W.G. An integrated microfluidic device for screening the effective concentration of locally applied tacrolimus for peripheral nerve regeneration. Exp. Ther. Med. 2015, 9, 154–158. [Google Scholar] [CrossRef][Green Version]

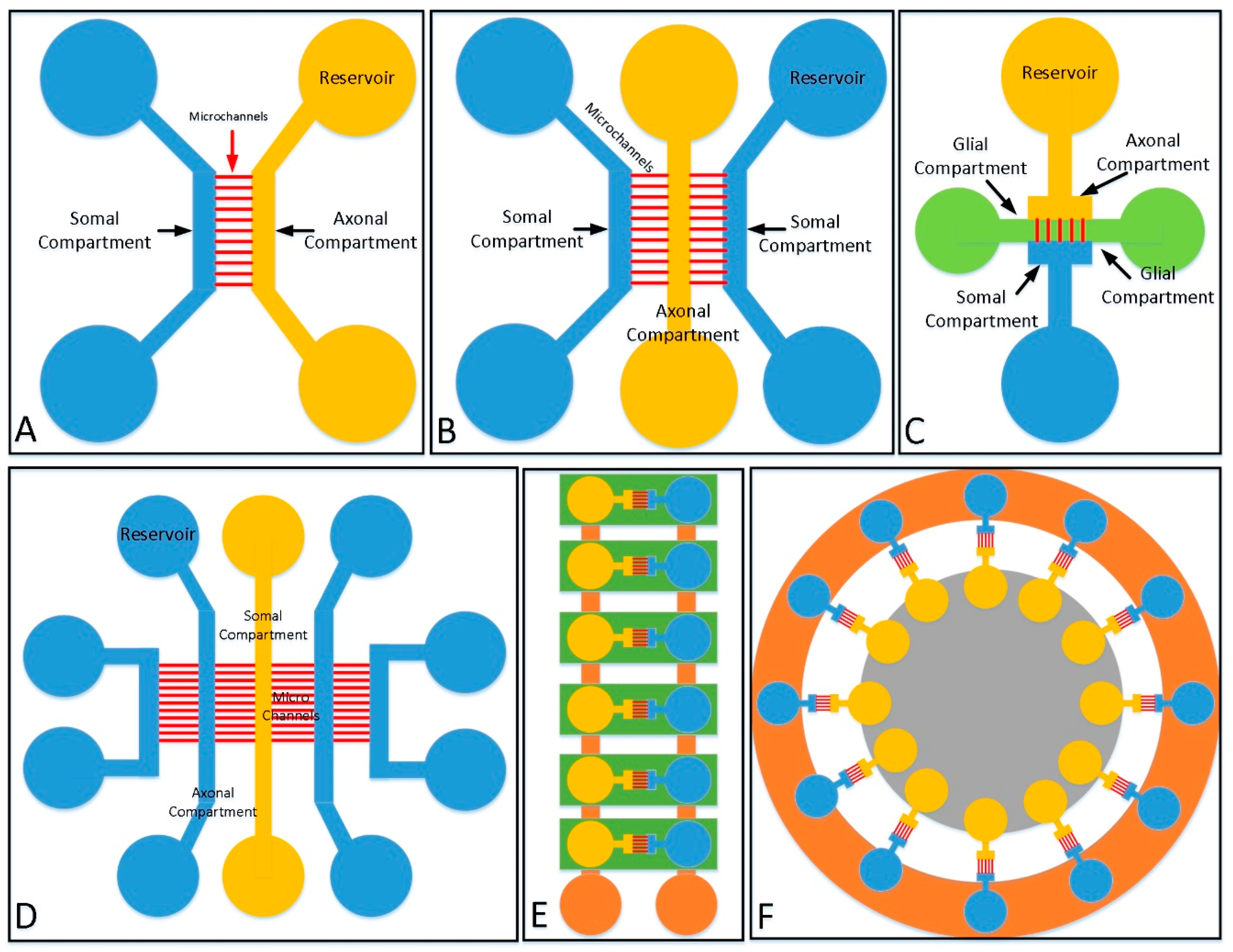
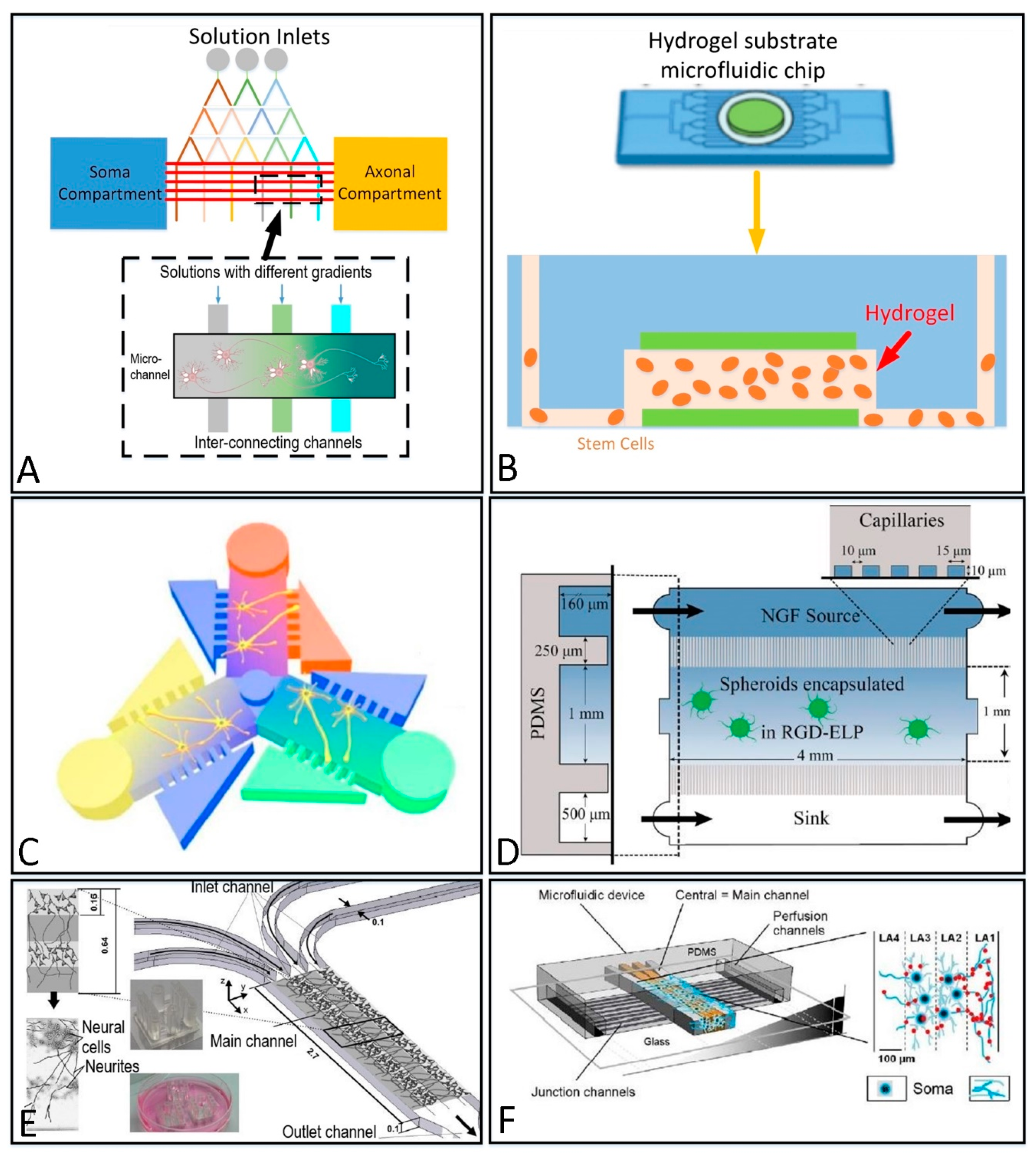
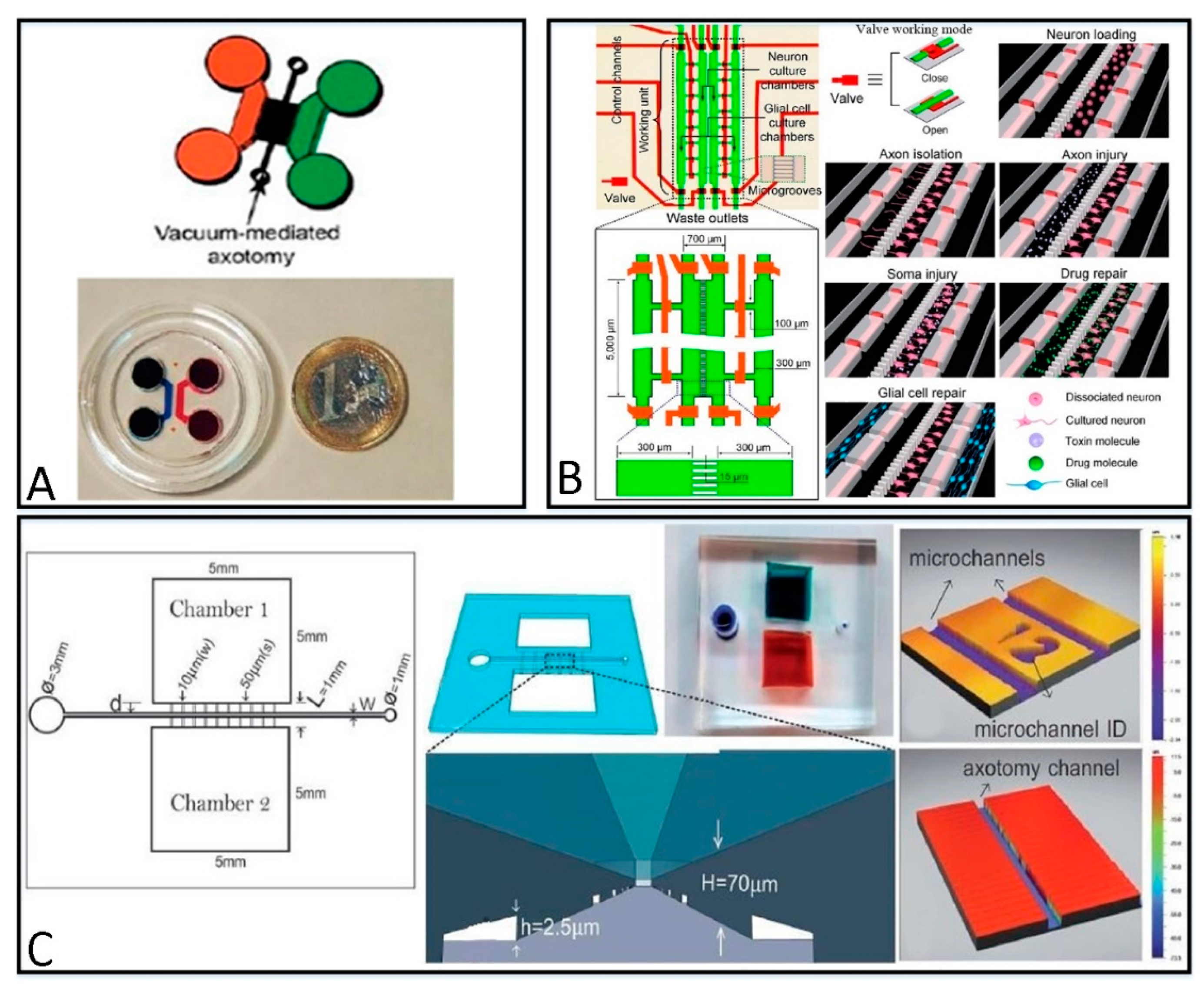
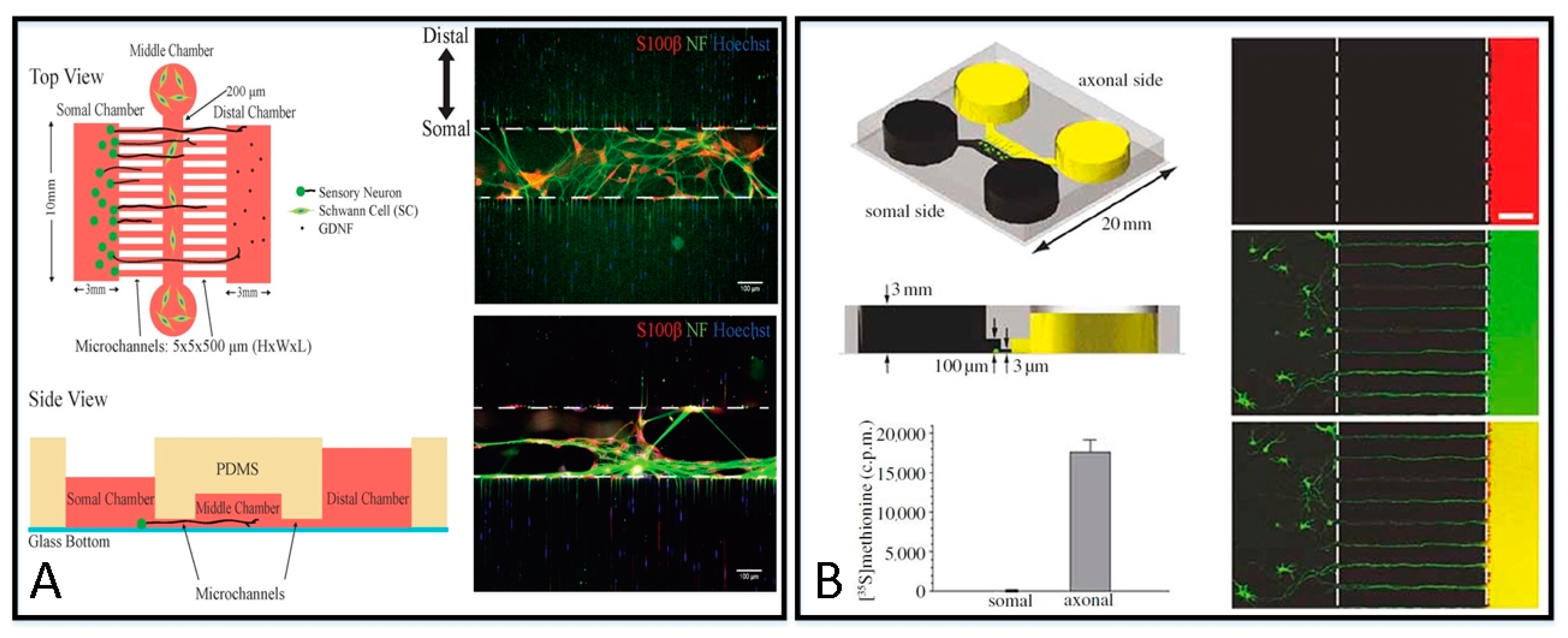
| Fabrication Material | Categories | Themo-Stability | Surface Stability | Optical Transparency | Gas Permeability | Biocompatibility | Hydrophilicity | Cost |
|---|---|---|---|---|---|---|---|---|
| Silicon | Inorganic | High | High | No | No | ✘ | Hydrophilic | High |
| Glass | Inorganic | High | High | High | No | ✘ | Hydrophilic | High |
| Ceramic | Inorganic | High | High | No | No | ✘ | Hydrophilic | Low |
| Thermoplastic | polymeric | High | Medium | High | Low | ✓ | Hydrophobic | Low |
| PDMS | polymeric | Medium | Low | High | High | ✓ | Hydrophobic | Low |
| Hydrogel | Hydrogel | Low | N/A | Low | No | ✓ | Hydrophobic/ Hydrophilic | Low |
| Methods | Advantages | Disadvantages | |
|---|---|---|---|
| Structure of Classical methods |
| ||
| Structure in Figure 1 |
|
| |
| Structure in Figure 2A,B,D |
|
| |
| Structure in Figure 2C |
|
| |
| Research | Repairing Method | Injury Mechanism |
|---|---|---|
| Dollé, J. P. [69] | NGF | Physical injury (compression) |
| Yap, Y. C. [70] | NGF | Physical injury (compression) |
| Bang, S. [71] | Drug + NGF | Physical injury (puncture) |
| Taylor, A. M. [72] | Nan | Vacuum-assisted injury |
| Zhang, J. N. [73] | NGF | Vacuum-assisted injury |
| Lezana, J. P. [74] | Drug + NGF | Vacuum-assisted injury |
| Kim, Y. T. [75] | Drug | Laser injury |
| Hellman, A. N. [76] | Drug | Laser injury |
| Gokce, S. K. [77] | Drug + neurodevelopmental genes | Laser injury |
| Wang. J. [21] | NGF | Biochemical injury |
| Samson, A. J. [46] | Drug + NGF | Biochemical injury |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, C.; Liu, X.; Tang, R.; He, J.; Arai, T. A Review on Microfluidic Platforms Applied to Nerve Regeneration. Appl. Sci. 2022, 12, 3534. https://doi.org/10.3390/app12073534
Dai C, Liu X, Tang R, He J, Arai T. A Review on Microfluidic Platforms Applied to Nerve Regeneration. Applied Sciences. 2022; 12(7):3534. https://doi.org/10.3390/app12073534
Chicago/Turabian StyleDai, Chuankai, Xiaoming Liu, Rongyu Tang, Jiping He, and Tatsuo Arai. 2022. "A Review on Microfluidic Platforms Applied to Nerve Regeneration" Applied Sciences 12, no. 7: 3534. https://doi.org/10.3390/app12073534
APA StyleDai, C., Liu, X., Tang, R., He, J., & Arai, T. (2022). A Review on Microfluidic Platforms Applied to Nerve Regeneration. Applied Sciences, 12(7), 3534. https://doi.org/10.3390/app12073534







