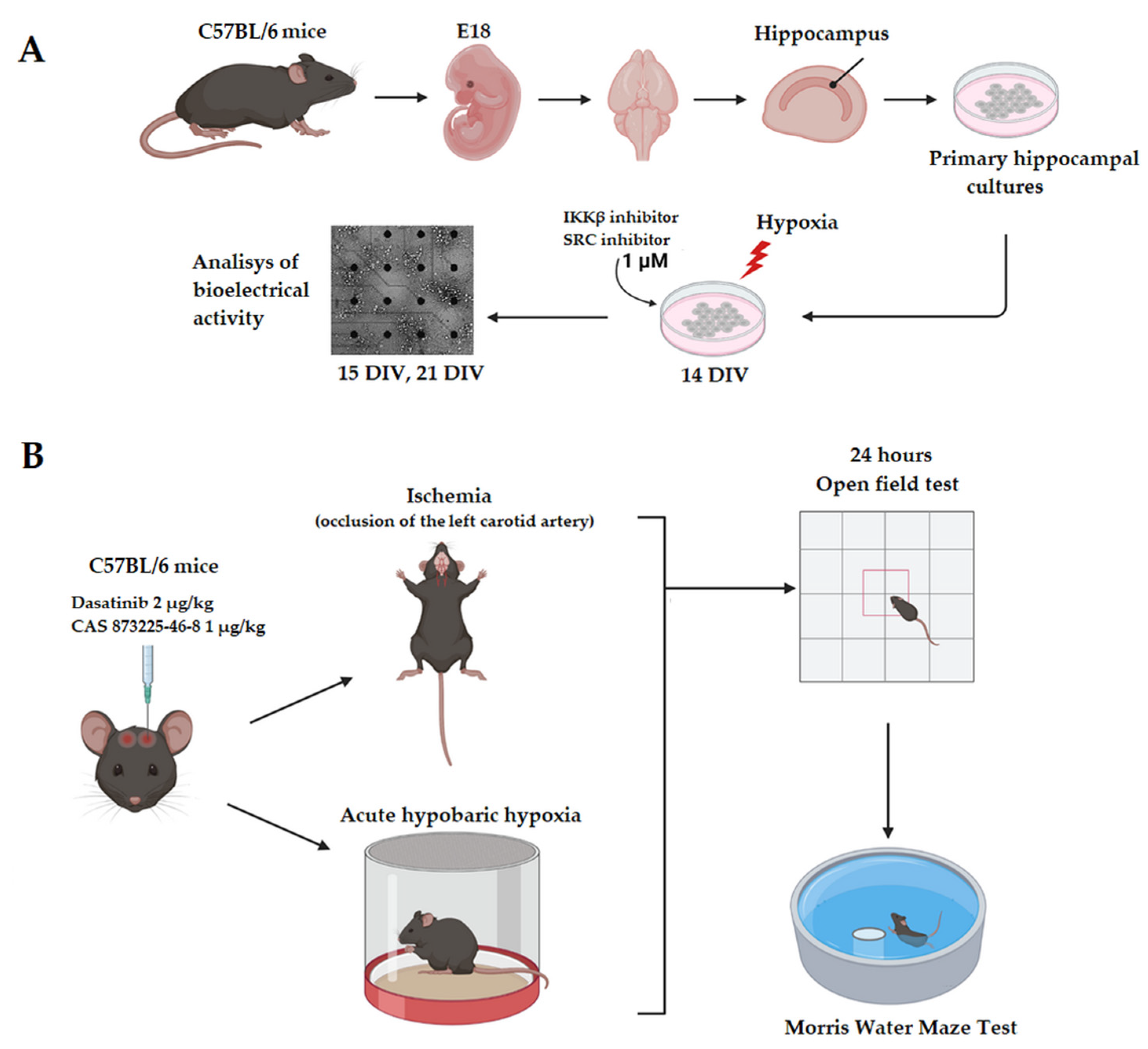
1. Introduction
Cerebral ischemia (ischemic stroke) is a widespread pathology consisting in a decrease in the supply of oxygen and energy to brain tissues, which can lead to acute and chronic dysfunction and death of neurons [
1,
2]. Damage to brain tissues located in the area of the primary ischemic nucleus is most often incurable; however, further development of nerve cell damage initiated by an episode of oxygen deficiency and impaired energy metabolism can last for several days. At this time, there is secondary damage to brain cells in the area of the so-called penumbra, and exactly this period of time makes it possible to implement various therapeutic approaches in order to prevent the development of pathological processes [
3]. Despite the fact that treatments for ischemic stroke have been actively developed in recent decades, there are still no effective therapies that have a high neuroprotective efficacy with minimal side effects [
4,
5].
Currently, kinase enzymes are considered to be promising molecular targets for drug development under various stress factors, including ischemic damage. The role of kinases in the cell’s lifecycle is exceptionally great and diverse. In fact, every signal pathway occurs via a phosphate group transfer reaction, so kinases provide many “points of contact” for therapeutic modulation in many pathological processes. Dysregulation of kinase functions plays an important role in cardiovascular, neurodegenerative, inflammatory, metabolic, and infectious diseases, etc. [
6,
7].
Previously, our research group screened the effect of more than 100 different neuronal kinases’ inhibitors on the viability of nerve cells in primary hippocampal cultures under physiological conditions and in the modeling of glucose deprivation in vitro [
8]. In vitro, we identified and studied in more detail kinases whose blockade had a neuroprotective effect that was manifested in the effective maintenance of the nerve cell viability under glucose deprivation and hypoxic conditions at the level of intact cultures. However, studies of calcium activity showed that the blockade of these kinases did not allow for the maintenance of the collective functional calcium dynamics [
8,
9].
The present work is a further continuation of this comprehensive study. We focused on a deeper study of the effects that blockade of SRC and IKKβ kinases has on neural network activity, for which we studied spontaneous neural network bioelectric activity using multielectrode arrays and also verified the results obtained in cell cultures regarding the neuroprotective effect of inhibition of these kinases in studies in vivo.
The SRC kinase family is an important subgroup of cytoplasmic tyrosine kinases that are not receptors on the cell membrane. Kinases of this family are widely distributed in the brain [
10]. SRC kinase was the first non-receptor tyrosine kinase that was described. The release of different bioactive molecules, including glutamate, cytokines, thrombin, oxyhemoglobin, and ROS during hypoxia can activate SRC. Activated SRC modulates the release of proapoptogenic molecules from mitochondria into the cytosol, which leads to the activation of caspases 3, 7, and 9, followed by DNA fragmentation and cell death. It can be assumed that SRC inhibition will become an effective treatment for ischemic brain damage in the future [
11].
Another interesting kinase is IKKβ. In ischemia, this kinase can activate the transcription factor NF-kB, which plays an important role in the adaptation of the central nervous system to ischemic damage by regulating the expression of target genes and the activity of other molecular pathways involved in the development of ischemic stroke. Meanwhile, various studies indicate that NF-kB can play both positive and negative roles in the regulation of ischemic resistance [
12].
Currently, there is insufficient data on the neuroprotective effects of SRC and IKKβ kinase inhibition. Moreover, there are no studies on the influence of these blockers on the functioning of the brain’s neural networks.
Our work is devoted to the study of the effect of SRC and IKKβ kinase inhibitors on the preservation of the functional activity of neuronal networks of mouse primary hippocampal cultures during modeling of hypoxia in vitro and on the resistance of mice and the preservation of their cognitive and mnestic functions under acute hypobaric hypoxia and ischemic stroke modeling.
2. Materials and Methods
2.1. Research Object
Primary hippocampal cultures obtained from E18 embryonic brains of C57BL/6 (E18) mice were used for in vitro studies. In vivo research studies were carried out on 63 male C57BL/6 mice. To exclude the influence of the phases of the hormonal cycle and circadian rhythms during the study, all experiments were performed on males at the same time of day.
2.2. Ethics Statement
The maintenance of animals and the conduct of all experiments complied with “Rules of working with laboratory animals” (Russia, 2010) and the “International Guiding Principles (Code of Ethics) for Biomedical Research Involving Animals” (CIOMS and ICLAS, 2012). The ethical principles established by the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 2006) were also followed. The research was approved by the bioethics commission of the Lobachevsky State University of Nizhny Novgorod.
2.3. Cultivation of Primary Cultures
Primary hippocampal cultures were obtained from embryos (day 18 of gestation) of the C57BL/6 mouse line. Euthanasia of pregnant mice was carried out by dislocation of the cervical vertebrae. Surgical scissors and forceps were used to extract the embryos; next, hippocampi were extracted and placed in phosphate buffered saline (PBS) (Thermo Fisher, Waltham, MA, USA). It was followed by enzymatic dissociation using 0.25% trypsin solution (Thermo Fisher, USA) for 20 min in a CO2 incubator. After centrifugation, hippocampal cells were plated in glass-bottomed dishes (SPL Life Sciences, Naechon-Myeon, Korea).
Cell cultures were cultured using Neurobasal medium (Thermo Fisher, Waltham, MA, USA) supplemented with fetal calf serum (Biosera, Nuaille, France), bioactive supplement B27 (Thermo Fisher, Waltham, MA, USA), and L-glutamine (Thermo Fisher, Waltham, MA, USA) in a CO2 incubator at 35.50 °C. The culture medium was replaced every two days.
2.4. Modeling of Hypoxia In Vitro
On the 14th DIV the acute normobaric hypoxia was simulated for hippocampal cultures (
Figure 1A). For this purpose, the cultural Neurobasal medium was replaced with a medium with a low oxygen content (0.37 mL/L) [
13]. Cell cultures were incubated in a hypoxic medium for 10 min, then the medium was replaced again with a standard Neurobasal medium.
2.5. Modeling of Acute Hypobaric Hypoxia In Vivo
Acute hypobaric hypoxia (AHH) modeling in vivo was carried out using a hermetic pressure chamber in which a mouse was placed. A pressure of 220–240 mm Hg was created in the chamber, which corresponds to an altitude of 10,000 m above sea level. Hypoxia was simulated no more than 10 min before the second agonal inspiration of the animal. The interval from the moment the mouse rose to the height until agonal breath, the animal stopped breathing, or after 10 min, was considered the survival time.
2.6. Modeling of Ischemic Brain Injury In Vivo
Modeling of ischemic brain damage in mice was carried out by unilateral left carotid artery occlusion under inhalation anesthesia with isoflurone (Karizoo, Spain). The left carotid artery was tied with a non-absorbable ligature suture (MedMart, Russia). After the sutured wound was treated with Streptocid bacteriostatic powder (Meligen, Russia). After all surgical interventions, which took 10–15 min, each mouse was placed in a separate cage with free 24-h access to food and water.
2.7. Pharmacological Inhibition of Intracellular Kinases
The SRC and IKKβ kinase inhibitors used for in vitro studies were selected from a library of inhibitors assembled by the Drug Discovery group at the Ontario Institute for Cancer Research (OICR) and kindly provided to us by Dr. Rima Al-awar. These compounds were used as 100% solutions in DMSO. In vitro treatment of the kinase inhibitors was performed on the 14th day of cultivation of primary nerve cell culture at a concentration of 1 μM 20 min before the modeling of ischemia factors, during and after the replacement of the culture medium with a normoxic one (
Figure 1A).
In vivo experiments used commercial blockers similar to those used in in vitro experiments: Dasatinib (SRC kinase blocker, Sigma-Aldrich, Taufkirchen, Germany) and CAS 873225-46-8 (Ikk kinase blocker, Sigma-Aldrich, Taufkirchen, Germany). These blockers were administered intraventricularly to C57BL/6 mice at a dose of 2 μg/kg for Dasatinib and 1 μg/kg for CAS 873225-46-8 (
Figure 1B).
2.8. Analysis of the Spontaneous Bioelectrical Activity of Primary Neuronal Cultures
The multielectrode system USB-MEA120-2-InVBC-System-E-Standard (Multichannel Systems, Reutlingen, Germany) was used to investigate the dynamics of spontaneous bioelectrical activity of neuro-glial networks via non-invasive long-term recording of extracellular potentials. For this, according to previously developed protocols [
13], murine brain hippocampal cells with a density of 9000 cells/cm
2 were cultured for 14 days on a MEA60 multielectrode array (Multichannel Systems, Germany). Registration of spontaneous bioelectrical activity was carried out immediately before hypoxia modeling, during hypoxia, immediately after the return to the normoxic medium, and within seven days of exposure to the stressor. The obtained electrophysiological data were analyzed in the MATLAB software environment using the original set of MEAMAN algorithms (certificate of state registration of the computer program No. 2012611190) [
14,
15].
2.9. Open Field Test
The general locomotor and orienting-exploratory activity and anxiety of the experimental animals were tested in the “Open Field”. The test was performed one day after the modeling of AHH or ischemia in the IR Actimeter (OpenField LE800S; Panlab, Harvard Apparatus, Barcelona, Spain). The infrared activity monitor IR Actimeter includes a two-dimensional 45 cm × 45 cm square frame and an infrared beam system for detecting the animal’s movements. All parameters were recorded and analyzed using ActiTrack software (PanLab, Harvard Apparatus, Barcelona, Spain). The following behavioral reactions of mice were analyzed: an orienting-exploratory activity (the number of squares crossed around the arena perimeter, the number of upright postures); emotional state (the residence time in the arena center); passive reaction fear (frequency of grooming acts and the number of boluses).
2.10. Morris Water Maze Test
For the test in the Morris maze, a round pool with a diameter of 90 cm was filled with warm opaque water (26 °C) and a moving platform 10 cm in diameter was placed in a certain area in the pool 1–2 cm below the water surface. Animals were trained for five days after modeling the damaging factor. The training cycle consisted of three attempts lasting 60 s each. Putting the animal into the pool on different sides trained them to find the platform by a visual landmark. If the animal did not find the platform until the end of the testing time, it was placed on the platform forcibly for 30 s. Further, the reconsolidation of the long-term memory of the animal was assessed 24 h after the last training session. This parameter was assessed by testing animals in a pool without a platform for 1 min. The duration of the animal’s stay in the area where the platform was previously located to the total testing time (delayed coefficient of retention) was estimated with Smart Tracking (Panlab Harvard Apparatus, Barcelona, Spain).
2.11. Morphology Studies
To study the morphology of the brain, histological studies were carried out. In experimental animals, the brain was isolated and placed for a day in a 10% formalin solution. Further, the brain was fixed at room temperature for cryoprotection for 24–48 h in sucrose solutions (15% and 30%). Brain slices with a thickness of 10 µm were prepared using a freezing sliding cryostat Leica CM1520 (Leica, Wetzlar, Germany). The prepared sections were stained with hematoxylin-eosin according to the standard technique (PanReac AppliChem, Darmstadt, Germany) [
16]. To fix the stained sections, the slides were incubated in alcohols and xylenes with an increase in their concentrations. At the end, the samples were filled with a mounting medium (Consul-Mount, Fisher Scientific, Göteborg, Sweden) and examined using a Zeiss Primo Star microscope (Zeiss, Jena, Germany) with an Axio CamMRc camera (Zeiss, Jena, Germany).
To immunohistochemically study the polyclonal chicken anti-GFAP (marker of astrocytes) primary antibody (Abcam, Cambridge, UK, 1:500 dilution) and polyclonal goat anti-MAP2 (marker of differentiated neurons) primary antibody (Abcam, Cambridge, UK, 1:500 dilution) were used. Goat Anti-Rabbit IgG H&L Alexa Fluor®® 488 (ThermoFisher, USA, 1:1000) and goat antibodies anti-Chicken IgY (H + L) Alexa Fluor®® 647 (ThermoFisher, USA, 1:1000) were used as secondary antibodies. The stained samples were observed using a Zeiss LSM 800 fluorescence confocal microscope (Carl Zeiss, Oberkochen, Germany).
When performing histological and immunocytochemical studies, sections of the sensorimotor cortex were prepared from at least five animals from each experimental group. Five to seven sections were obtained from each animal. At least ten visual fields were analyzed on each section.
2.12. MRI
To visualize the effect of inhibition of SRC and IKKβ kinases, a study was carried out on a high-field magnetic resonance tomograph (Agilent Technologies DD2-400 9 T (400 MHz), Agilent Technologies, Santa Clara, California, USA, UK) with an M2M (H1) volumetric coil. The animals were under general anesthesia: Zoletil (Virbac, Carros, France) was administered intraperitoneally and Xila (Interchemie werken “De Adelaar” B.V., Waalre, The Netherlands) intramuscularly. The animals were placed vertically inside the tomograph. The study took place in T1 and T2 modes of the weighted image. Tomographic images were obtained using the VnmrJ program, which were then analyzed using the “Fiji is just ImageJ” program.
Frontal sections of the brain 1 mm in thickness were obtained using the following sequences: MGEMS (multi-gradient echo-multi-slice) with the following parameters: TR = 1000 ms, TE = 1.49 ms, number of echo signals—6, field of view—20 × 20 mm; diffusion-weighted pulsed SEMS (spin echo multislice) with the following parameters: TR = 1200 ms, TE = 2 ms, FOV—20 × 20 mm. The image matrix was set to 256 × 256. The total scanning time per animal was 34 min 08 s.
2.13. Statistical Analysis
Statistical analysis was performed using GraphPad Prism (v.9.0) program. Quantitative results are presented as a mean ± standard mean error (SEM) or a median together with the second and third quartiles of normal distribution. One-way ANOVA was followed by Bonferroni post-hoc test for multiple comparisons, which was used to analyze the data in the case of a normal distribution (normal distribution was assessed using the Shapiro-Wilk test). A nonparametric Mann–Whitney U test was used for data distribution other than normal. At least three independent biological replicates were used for all experiments. The difference between the groups was considered significant if the p-value was less than 0.05 (p < 0.05).
3. Results
We have previously shown that the modeling of hypoxia leads to a significant (
p ˂ 0.05 ANOVA) decrease in the viability of primary hippocampal nerve cell cultures by 35% compared to intact ones. The application of both IKKβ and SRC kinase blockers maintained cell viability at the level of intact cultures (1.25 and 1.22 times higher than in the Hypoxia group, respectively) [
9].
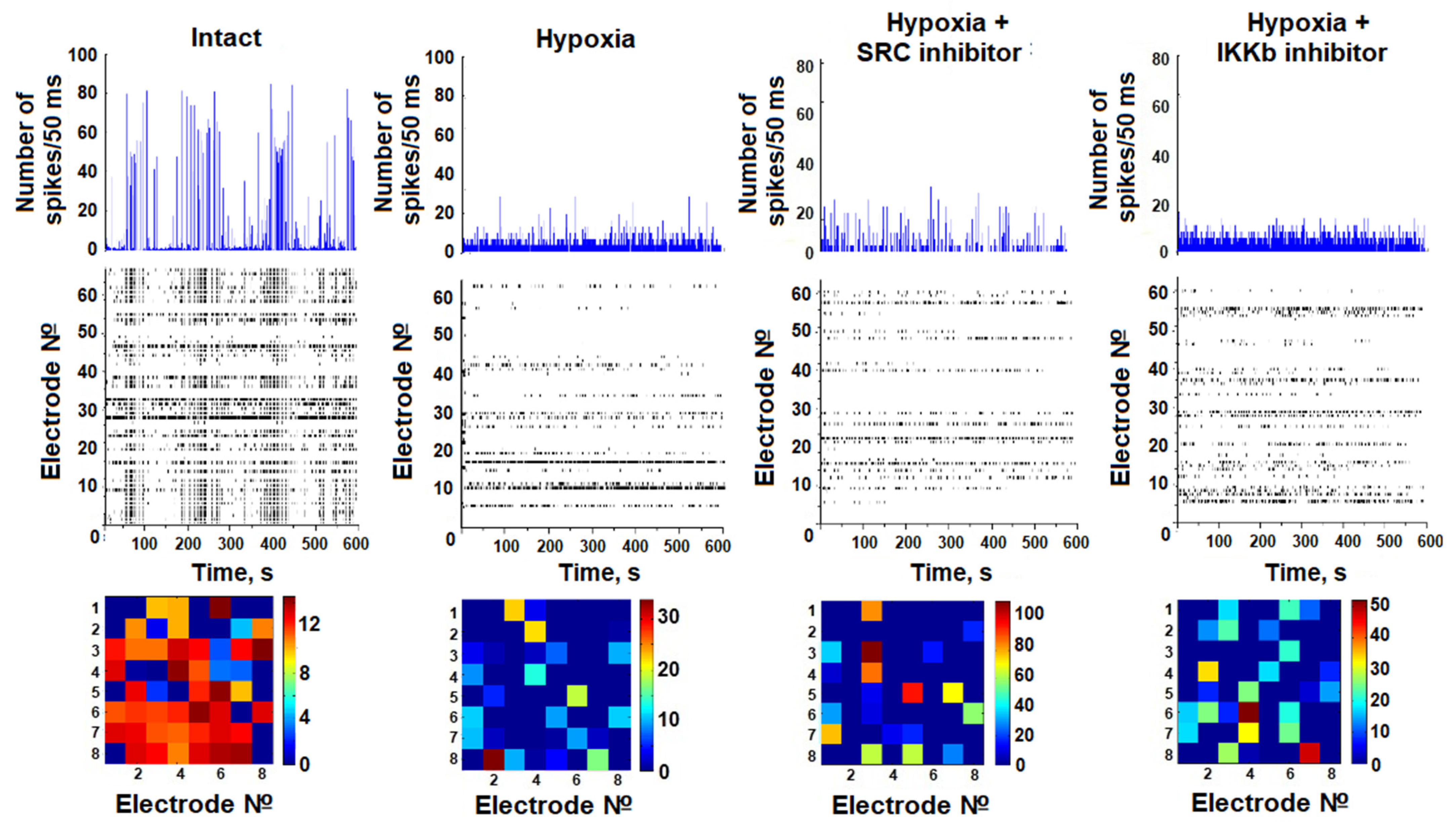
In the present work, we studied the neural network burst bioelectrical activity of dissociated hippocampal nerve cell cultures during hypoxia modeling against the background of blockade of IKKβ and SRC kinases. A baseline level of activity of the cultures was recorded at 14 DIV, after which they were randomly divided into experimental groups. The effect of hypoxia on bioelectrical activity was carried out on days one and seven after the modeling of hypoxia. On day 1 after the modeling of hypoxia, no significant differences between the groups were found.
In the intact culture, spontaneous burst bioelectrical activity of neural networks was observed on day 21 of development. The average number of small network bursts was 36.33 ± 4.39 bursts/10 min, and the average number of spikes per burst was 90.33 ± 11.63. It has been shown that hypoxic damage leads to irreversible damage of bioelectrical network activity by day 7 after hypoxia modeling. On day 7 of the posthypoxic period, the number of small network bursts of impulses significantly decreased (15.33 ± 2.75 bursts/10 min). Moreover, the number of spikes in bursts decreased dramatically to 11.58 ± 0.62 (
Table 1).
Inhibition of SRC kinase in hypoxic damage supported the partial maintenance of neural network activity at a functional level. Despite the fact that the level of spontaneous bioelectrical activity was reduced relative to intact cultures, on day 7 of the posthypoxic period, the number of spikes forming a burst in the Hypoxia + IKKβ inhibitor group and Hypoxia + SRC inhibitor group was significantly higher than in the Hypoxia group (36.16 ± 6.05 and 28.71 ± 4.18 respectively). Blockade of the IKKβ kinase also partially maintained bioelectrical activity during hypoxia modeling (7th posthypoxic day number spikes in burst 36.16 ± 6.05).
The analysis of activation patterns shows an increase in the time of impulse propagation between the electrodes in the posthypoxic period. Blockade of IKKβ and SRC kinases does not lead to maintenance of the structure of network activity in the late posthypoxic period (
Figure 2).
At the next stage of the work, we studied the effect of SRC and IKKβ kinase blockers on the resistance of animals to hypoxic and ischemic damage in vivo. The effect of intraventricular application of appropriate blockers on the animals’ tolerance to acute hypobaric hypoxia was investigated (
Table 2).
The survival rate of animals in the Control group (with intraventricular administration of PBS) was 33.3%. Both SRC and IKKβ kinase blockade had no effect on survival. It is important to note that inhibition of SRC significantly increased animals’ survival time at altitude compared to the Control (Control—3.77 ± 0.81 min; SRC inhibitor—6.68 ± 0.84 min). Moreover, the blockade of this kinase significantly increased the share of highly resistant animals (Control—8.3%, SRC inhibitor—50%) (
Figure 3).
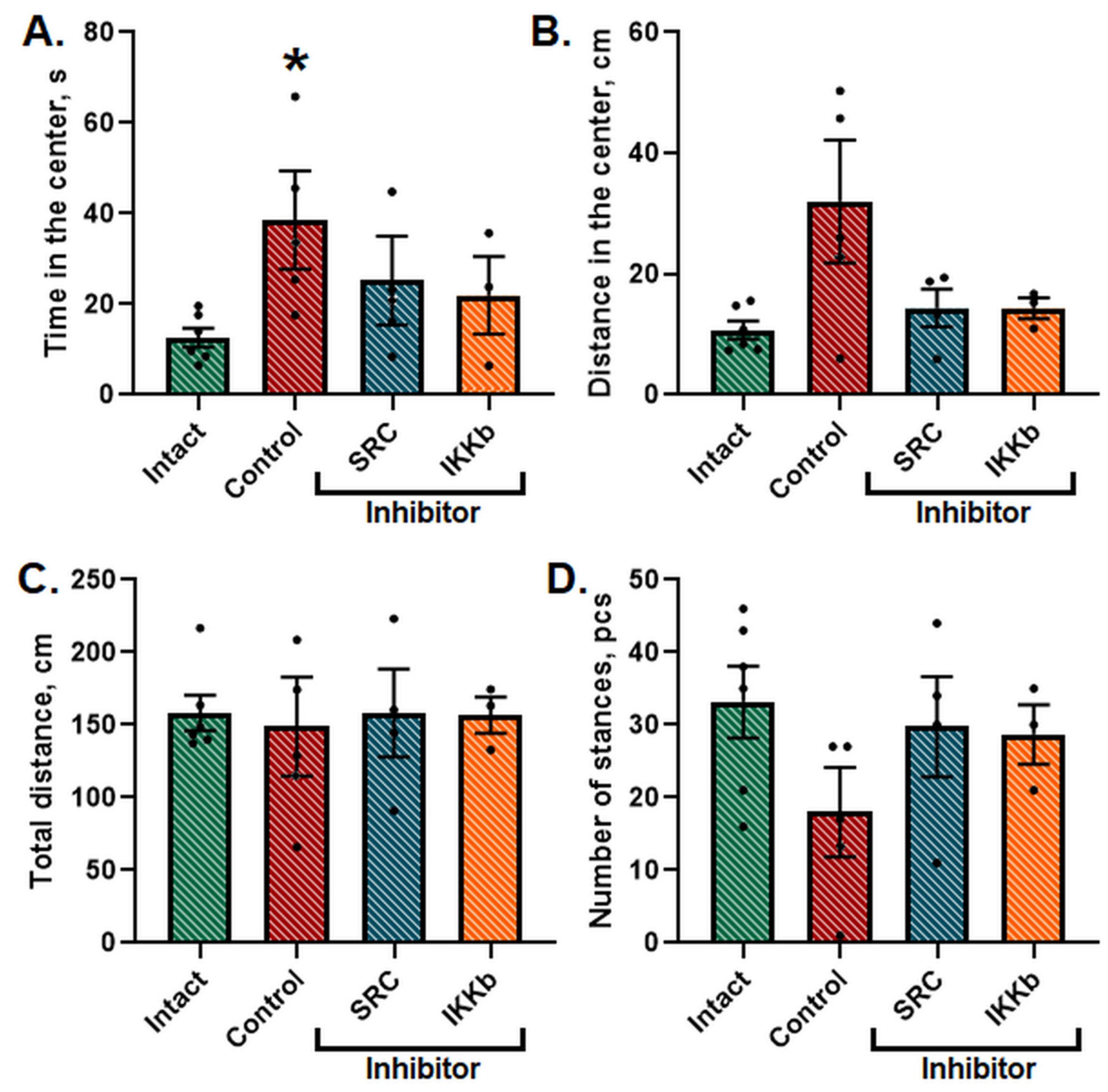
Twenty-four h after the AHH modeling, the locomotor and orientation-exploratory activity of the animals was tested in the IR Actimeter. It was shown that the general level of locomotor and orientation-exploratory activity in animals did not change; however, the animals spent significantly more time in the central area of the field (
Figure 4).
It indicates a change in the natural level of anxiety of the animals since the normal reaction for mice, as for crepuscular burrowing rodents, is to leave brightly lit areas in a center of open space. The application of the studied inhibitors normalized the time and distance spent in the center of the arena to the level of the Intact group.
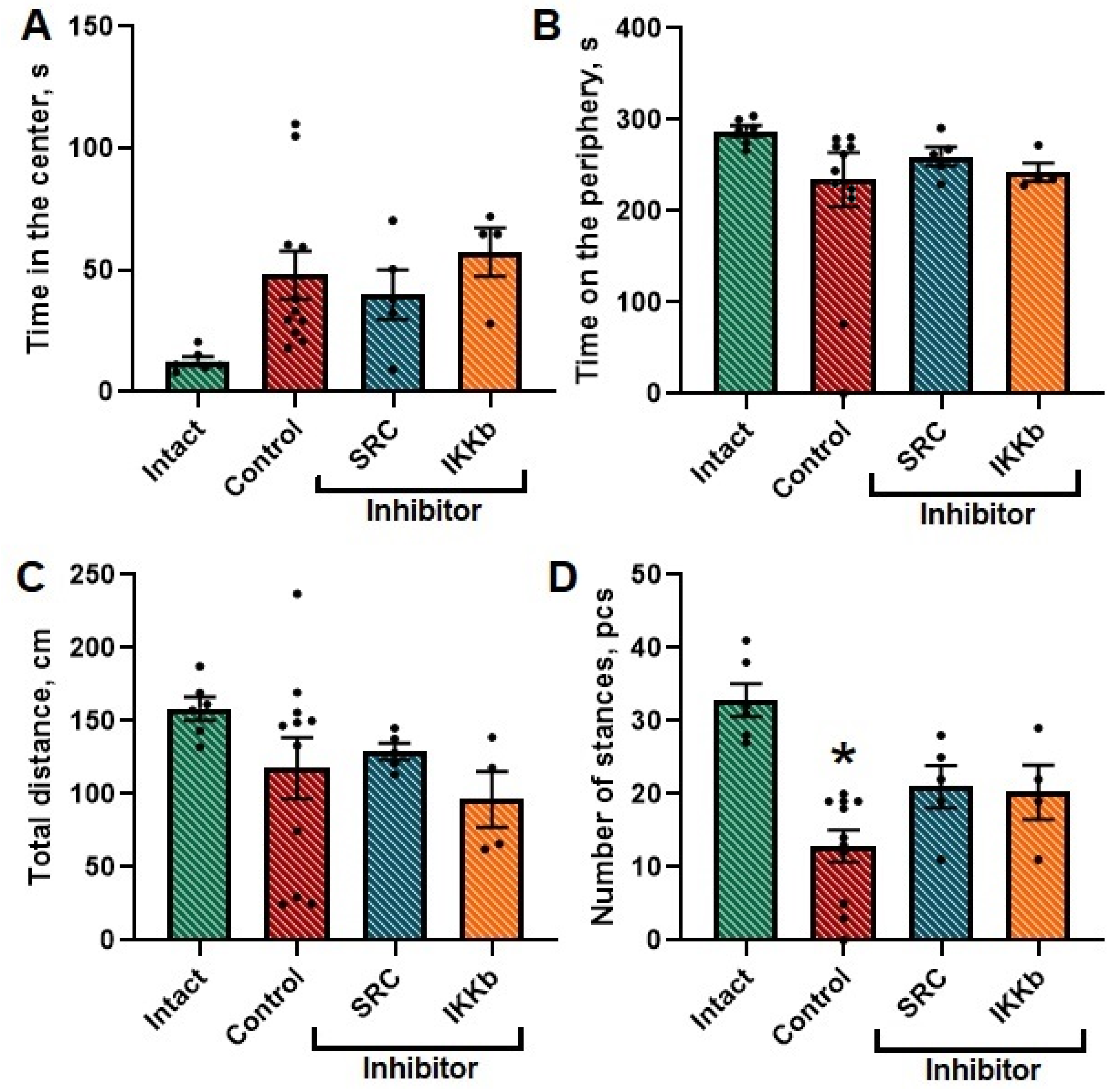
The next step in assessing the neuroprotective effect of the selected kinases was to study the resistance of laboratory animals to ischemic damage. Ischemic stroke was modeled by performing unilateral carotid artery occlusion in mice. The survival rate of control animals was 83%. The survival rate of animals injected with SRC kinase inhibitor did not differ from the Control group. In IKKβ kinase blockade, the survival rate was 66.6%.
Ischemic damage was shown to cause a decrease in the level of orientation-exploratory activity in mice (the number of stances (upright postures) in the Intact group was 33 ± 4.53, in the Control group—18.09 ± 3.94). In the group with the administration of studied kinase blockers, this parameter did not differ from the corresponding parameters in intact animals. No significant differences were found for the rest of the studied parameters (
Figure 5).
We analyzed the memory traces within the reproduction of navigation learning in the Morris water maze. The results of animal training in the Morris maze are shown in
Supplementary Figure S1. Our research has shown that ischemia decreases the spatial learning abilities of mice. There were no mistakes in the Intact group during the training in the maze since the third session. In the Ischemia group, two animals failed one attempt to find the hidden platform on the third learning session and one attempt to find the hidden platform on the fifth learning session. In the SRC inhibitor” group there were no failed attempts on the fourth and fifth session. In the IKKβ inhibitor group, two animals had a wrong attempt on the third learning session and one animal failed on the fourth and fifth learning sessions. It was shown that the delayed coefficient of memory retention in the Control group after ischemia was significantly decreased compared to intact animals (Intact—42.62 ± 1.12, Control—29.48 ± 2.15). IKKβ inhibitor treatment also led to the delayed coefficient of memory retention decreasing. In SRC kinase blockade, the values of the delayed coefficient of retention did not differ from the Intact group (
Table 3).
Thus, inhibition of SRC kinase increased the animals’ resistance to hypoxic and ischemic damage and supported their mnestic abilities. In contrast, the use of an IKKβ kinase inhibitor negatively affected the survival rate of animals in AHH and ischemia modeling.
Histological samples were analyzed to study the dynamics of morphological changes in the brain tissue.
The nervous tissue of intact animals was normal. The cells had a round or oval shape; the membrane had no ruptures; the cell diameter was 5.2 ± 0.6 µm, nuclear diameter was 2.2 ± 0.3 µm. The nuclei had an elongated oval shape, clearly defined. The cytoplasm of the cells was homogeneous, the contours of the cells were even, and the processes were quite numerous.
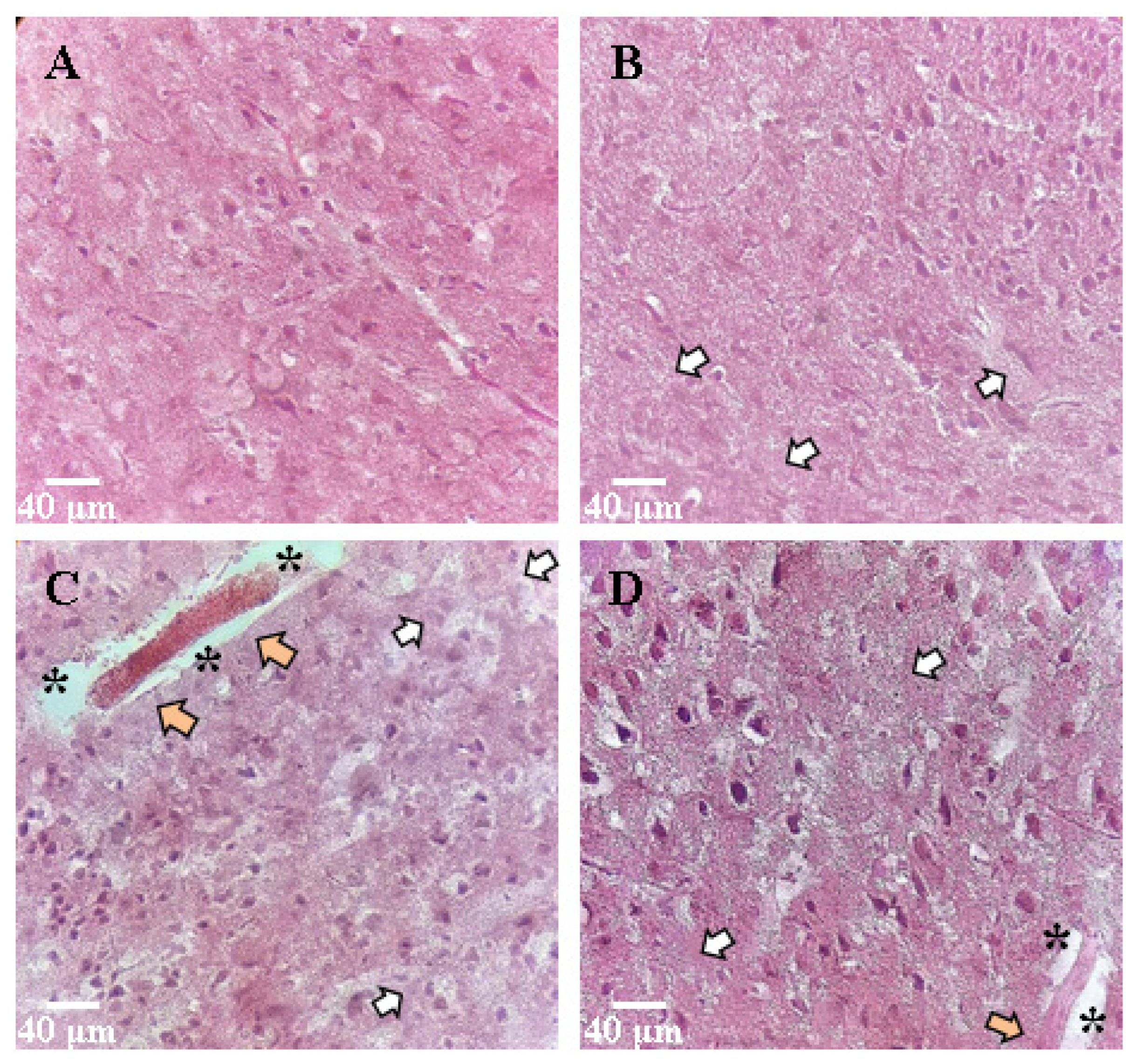
During the study of the morphology of the sensorimotor cortex and the region of the ventricles of the brain, when modeling hypoxia as a factor of ischemia and ischemic damage, pronounced changes were observed (
Figure 6). There was an expansion of the pericapillary spaces and hyperchromic neurons (small clusters of two to three cells in 10 fields of view). Tissue edema was pronounced. A significant part of the cells had an irregular shape with uneven contours; nuclear pycnosis was noted (about five cells in 10 fields of view). The cell size and nuclear diameter increased compared to the Intact group and was 8.0 ± 0.9 and 4.4 ± 0.6 μm, respectively (
Table 4).
The group with blockade of SRC kinase showed slight morphological changes when modeling AHH relative to the Control. Near the inflammation focus, there were few altered neurons. Moreover, there were cells with distinct nucleus and soma contours (10 ± 1.5%). A decrease in perivascular edema indicates a neuroprotective effect of the studied inhibitor. The cell size in the SRC kinase inhibitor group was 6.9 ± 0.6 µm.
IKKβ kinase blockade increased the share of dark-colored deformed cells. Binuclear neurons were found in small numbers (about nine to ten cells were found in 10 fields). There were 1.5–2 times more cells with large nuclei and blurred contours of the nuclear envelope than in the Intactgroup. The cell diameter was 9.5 ± 1.2 µm, and the nuclear diameter 4.7 ± 0.4 µm (
Table 4). There was significant tissue edema.
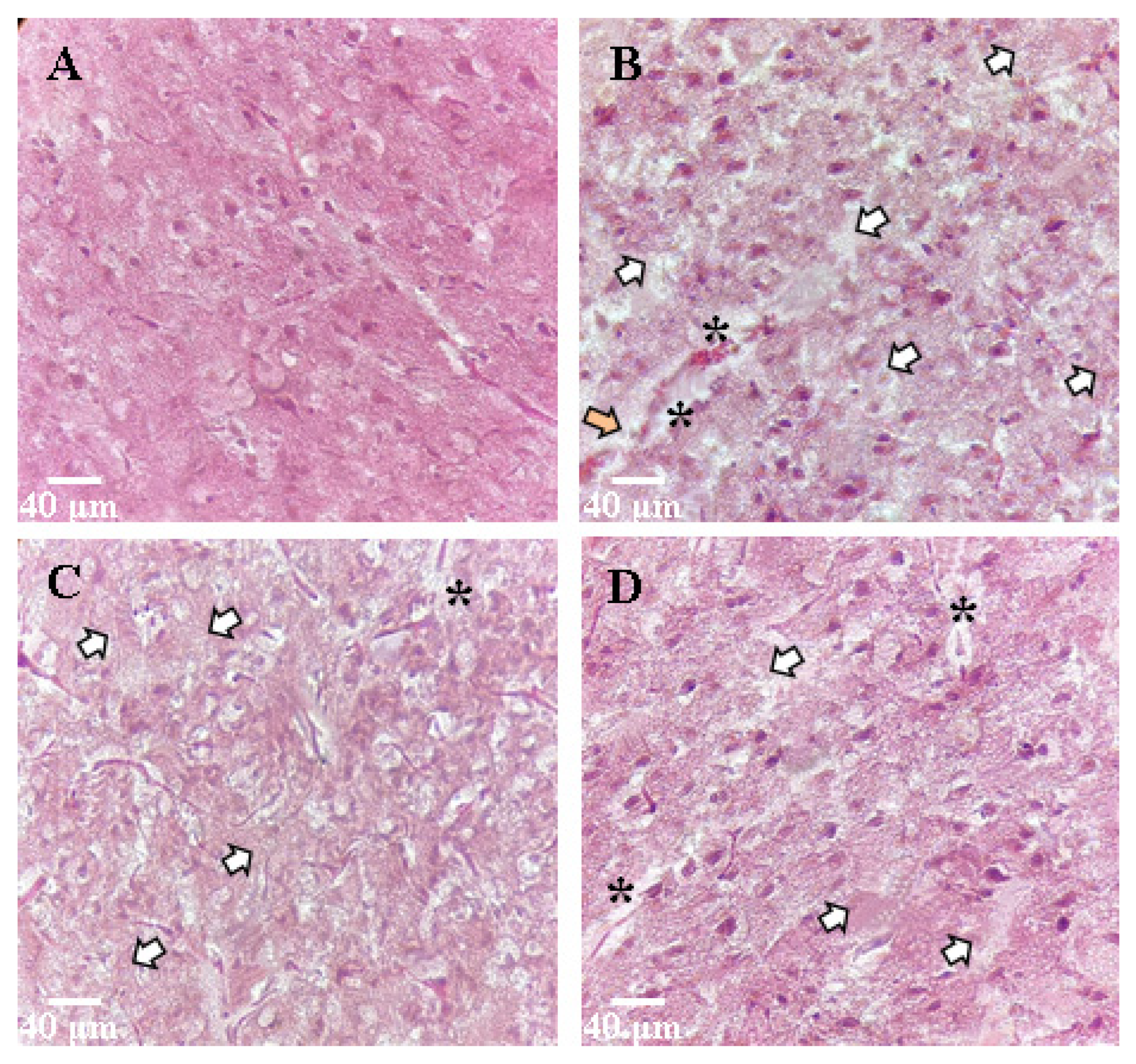
Ischemic damage to the brain tissue was expressed in active cellular infiltration, plethora, and edema of the nervous tissue. In the region of the ventricles, there were areas of tissue expansion. Thinning of the vessel walls was noted. Light phenotype neurons predominated due to swelling (about four to five cells in 10 fields of view); cell diameter increased to 9.1 ± 0.9 µm, and nuclear diameter increased to 4.6 ± 0.4 µm (
Figure 7).
In histological specimens with an SRC kinase inhibitor, the edema was comparable with that of the Control group. The cells of the upper layers of the cerebral cortex in the affected area were characterized by cells of normal size (cell diameter 4.7 ± 0.8 μm, nuclear diameter 1.9 ± 0.4 μm), but there were changes in cell shape: cell contours were uneven and poorly structured, or the cells had the shape of an irregular polygon. Neurons of the light phenotype prevailed mainly in the IKKβ inhibition group, relative to the SRC kinase inhibitor (about two to three cells in 10 fields of view). In the IKKβ inhibition group, pronounced tissue changes and significant edema were observed. The cell diameter was 9.5 ± 1.5 µm.
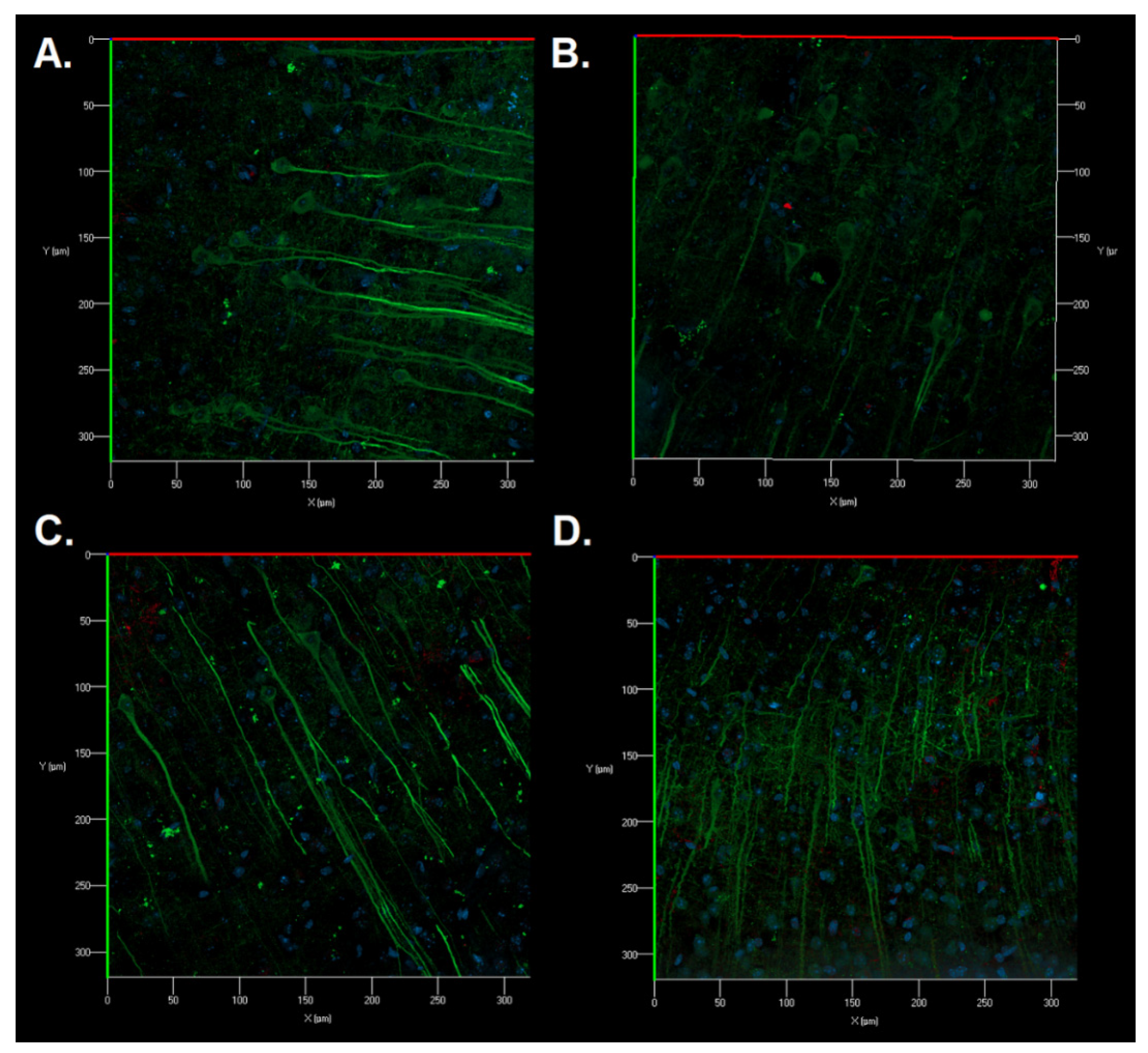
Immunocytochemical staining of brain sections was also carried out for a detailed study of the structural features of neurons and astrocytes of the sensorimotor cerebral cortex in the postischemic period. No significant morphological changes in the cell structure were revealed. Astrocytes had a typical morphology of protoplasmic astrocytes. The appearance of fragmented neuronal processes, a significant number of cellular fragments, and single binuclear neurons were found in the Control group after ischemic injury (
Figure 8B). Cellular fragments were also revealed in the SRC kinase inhibitor group; however, the neuronal processes were preserved (
Figure 8C). When IKKβ kinase was inhibited, a change in the structure of the cytoskeleton of neuronal dendrites was observed in some fields of vision: microtubules became of a tortuous structure (
Figure 8D).
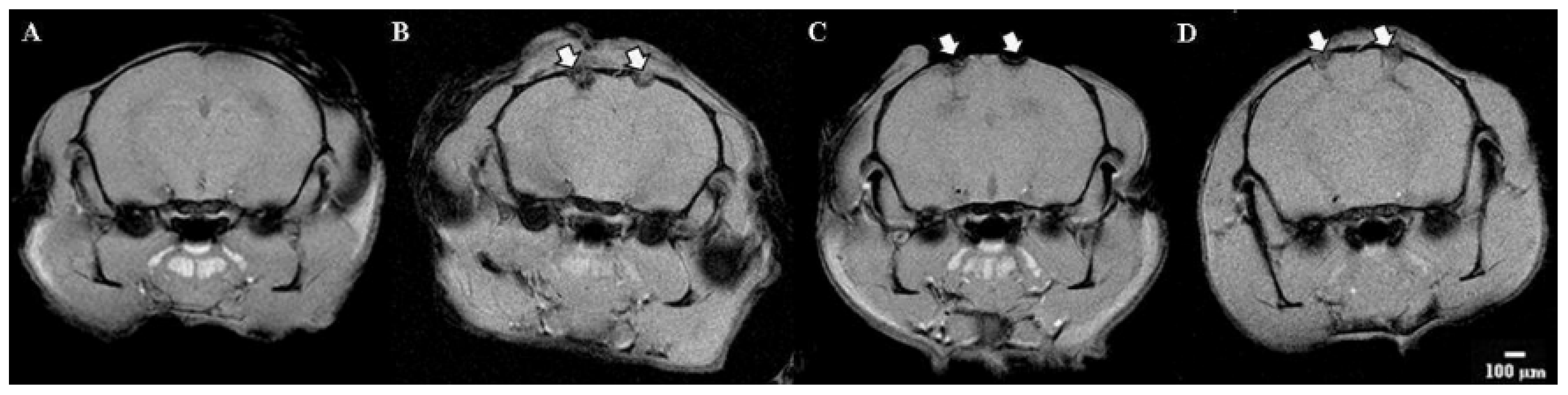
Visualization modeling of ischemia was performed on the 7th day using magnetic resonance imaging. An analysis of the structures of the brain was carried out. Particular attention is paid to the volume of the focus of ischemic inflammation.
Tomographic images of the brain of the group of Intact animals did not reveal any disorders—the cerebral hemispheres have a normal structure and intensity of the MR signal (differentiation of gray and white matter).
MRI images of the Control group demonstrate pronounced areas of edema in the areas of the sensorimotor cortex and ventricles of both the left and right hemispheres. The volume of the affected area was 28.5 ± 7.51 mm
3 (
Figure 9).
Significant changes in brain tissue were noticeable when the IKKβ kinase inhibitor was applied. By day 7 of the postischemic period, the pronounced structural changes in the area of the cerebral cortex with a predominance of diffuse edema were observed. The edema smoothly passed to the ventricles and further into the deep layers of the nervous tissue. In this group, there was the largest volume of the affected area (38.6 ± 18.8 mm3), while the width of the affected area was also at 6–7 mm maximum.
The response of the nervous tissue to the SRC kinase inhibitor was similar to the IKKβ kinase blockade; however, in this group, there was a less diffuse influx of fluid into the area of ischemic injury. The volume of the affected area was 35.8 ± 6.2 mm3.
To sum up, the morphological analysis showed that the use of an SRC kinase inhibitor partially reduces the negative effect of hypoxic and ischemic damage on brain tissues. Blockade of the kinase IKKβ negatively affects the morphological structure of the cerebral cortex during the AHH modeling.
4. Discussion
Our previous work showed that the blockade of SRC and IKKβ kinases positively affects nerve cell viability when modeling ischemia factors [
8,
9]. However, IKKβ inhibition did not allow the main parameters of calcium activity to be maintained at the proper level, and SRC blockade only partially preserved the share of active cells. In this work, we investigated the effect of SRC and IKKβ kinase blockade in more detail and evaluated the effects of inhibition of the studied kinases in vivo.
In our work, we investigated the previously undescribed effect of SRC and IKKβ kinase inhibitors on neural network bioelectrical activity. It is important to evaluate not only cell viability but also the integrity of the neural network during the blockade of these kinases and modeling of ischemia factors. Nerve cells form neural networks providing intercellular communication, exchanging molecules and information, thereby exhibiting spontaneous electrical and chemical activity. The registration and analysis of signals between neurons allow the obtainment of necessary information about the properties of neural networks and their functional architecture [
17].
Using the multielectrode arrays for the extracellular action potentials’ registration we can assess the functional integrity of neural networks, which is an additional parameter for evaluating the efficiency of the adaptive capabilities of nerve cells. In addition to calcium imaging, the assessment of functional neural network activity using multielectrode arrays will create a comprehensive picture of the preservation of synaptic contacts and neuronal signaling.
Inhibition of IKKβ and SRC kinases cause a neuroprotection effect that contributes to the maintenance of viability. However, despite the preservation of neuronal viability, SRC kinase blockade partially maintains the number of spikes in a network impulse burst but does not allow for the preservation of the number of network bursts. IKKβ kinase blockade does not support the spontaneous bioelectrical activity of primary neuronal cultures in the late posthypoxic period.
Recent studies have shown that SRC kinase inhibition can attenuate hypoxia-induced overactivation of CaM kinase IV, which in turn attenuates apoptotic cell death and improves neurological status in modeling of hypoxia in piglets [
18]. SRC is also involved in receptor-mediated mitophagy (removal of unwanted or damaged mitochondria in response to energy demand) [
19]. There is evidence that kinases of the SRC family regulate the functioning of the NMDA receptor and are involved in the development of damage during ischemia/reperfusion. Blockade of p-SRC kinase improves neurological functions in hypoxic-ischemic injury [
20].
Our results obtained in vivo to verify the experiments on cell cultures are consistent with these data and indicate that the administration of an SRC kinase inhibitor to experimental animals increases the survival time of animals in the modeling of AHH, increases the share of high- and medium-resistant animals, and contributes to the reduction of morphological changes during hypoxic brain damage. Thus, studies in vivo confirmed the neuroprotective effect of SRC inhibition revealed in studies in vitro.
However, the protective effect of IKKβ kinase inhibition has not been confirmed in the in vivo study. This may be due to the fact that the action of this kinase is associated with the activation of the transcription factor NF-κB, which has highly diverse pleiotropic effects that may differ when implemented in cell culture and in vivo. It has been shown that the canonical NF-κB signaling pathway (NEMO-dependent pathway) mediated by kinase complexes consisting of the NEMO scaffold adapter protein and two kinases, IKKα and IKKβ [
21], can play an important role in the adaptation to ischemic brain damage by regulating the activity of transcription factors and modified expression of target genes and modulating many pathways involved in cerebral ischemia [
12].