Abstract
Adherence to topical treatments is low and is known to be influenced by the vehicle properties. Betamethasone dipropionate (BD) is an anti-inflammatory steroid, used in psoriasis treatment in the form of an ointment, cream, or solution. The aim of this work was to develop a new vehicle for BD, focusing on the preferences of patients with psoriasis as a strategy to improve treatment adherence. Two vehicles with an aqueous external phase were explored: an emulgel and a hydrogel based on a cyclodextrin inclusion complex used to improve the aqueous solubility of BD. Since BD solubilization was not fully achieved in the hydrogel, only the emulgel was selected for further characterization. This new vehicle (emulgel) is characterized by its white, shiny appearance and good spreading properties. In comparison with petrolatum, a lower residue, higher evaporation rate, lower stickiness, and reduced ability to stain polyester fabric were observed. This vehicle also showed shear thinning behavior. The impact of this new vehicle on adherence to topical treatments should be further confirmed in clinical settings.
1. Introduction
Adherence to medication is widely recognized as a crucial issue to healthcare systems as poor adherence is associated with negative health outcomes and higher patient costs [1]. Psoriasis is a chronic inflammatory skin disease recognized to have poor treatment adherence [2,3,4]. Topical treatment has been a mainstay for the management of mild psoriasis but patient satisfaction and adherence are typically low for this therapy [5]. Some of the reasons for poor adherence include frustration with the low effectiveness of the treatment, the inconvenience of application, low cosmetic acceptability, and fear of side effects [2,6]. Besides the selection of the appropriate type of active substance and strength, it is also important that the medicinal product adequately addresses patients’ needs and preferences, ensuring optimal adherence, safety, and effectiveness. Patient-centric drug products play an essential role in patient satisfaction and adherence. Patient-centric pharmaceutical drug product design (PCDPD) describes an approach that directly aligns product characteristics with patient preferences in a targeted patient population [7]. A target product profile (TPP) is defined based on patient insights. Patient-reported outcome studies intend to measure patient feedback on their experiences with a specific drug treatment, providing unique information on the physical, functional, and psychological impacts of a treatment [7]. Incorporating patient preferences into the design of pharmaceutical drug products may enhance treatment adherence and optimize outcomes. Several studies aimed at understanding patient preferences regarding topical anti-psoriatic medicines are available in the literature [8,9,10,11,12,13,14,15,16,17,18,19,20,21], but only a limited number focused on the sensory attributes [9,18,19,22]. In general, low residue, minimum ability to stain clothes, low stickiness and good moisturizing properties are considered positive attributes [9]. Based on these attributes, other approaches have recently been developed, to the detriment of classic oily vehicles, such as hydrogel [10], foam [11], or cream [12].
Betamethasone dipropionate (BD) is a potent glucocorticoid, frequently used in the treatment of inflammatory skin disorders such as psoriasis. Several topical commercial formulations containing this drug are available, namely ointments, creams, and solutions, but no gel or vehicle with an external gelled phase (such as the emulgel) are on the market. In fact, a study reported that 82% of physicians thought that adherence could be markedly improved if psoriasis patients could use a non-alcoholic gel. Offering a BD aqueous gel could thus represent a relevant contribution to the improvement of patient satisfaction with anti-inflammatory topical treatments, especially in chronic diseases such as psoriasis. This is, however, technically challenging due to the high lipophilicity and low aqueous solubility of BD. Cyclodextrins (CDs) are cyclic oligosaccharides that have been recognized as promising pharmaceutical adjuvants due to their unique ability to form water-soluble inclusion complexes with various poorly soluble compounds. CDs are widely used in pharmaceuticals, drug delivery systems, cosmetics, and in the food and chemical industries due to their potential to enhance the aqueous solubility and dissolution rate of drugs; they also protect drugs from heat, light, hydrolysis, and oxidation, thereby improving the formulation stability [23]. An emulgel has the characteristics of both emulsions (with two immiscible phases) and hydrogels (with an aqueous phase gelled with a gelling agent) and promotes the stability of simple emulsions while maintaining their permeation and emollient characteristics [24].
The purpose of this study was to develop a new topical formulation containing BD with improved characteristics, focusing on patient preferences. Two vehicles with an aqueous external phase were explored: an emulgel and a hydrogel based on a CD inclusion complex. With both vehicles having an external gelled aqueous phase, they are expected to present improved sensory properties such as being greaseless, non-staining, and having better spreadability.
The TPP for BD vehicles for psoriasis treatment was established based on minimum residue, fast absorption, allowing the patient to dress shortly after applying the medicine, reduced staining of clothes, and moisturizing properties.
2. Materials and Methods
2.1. Materials
Betamethasone dipropionate was purchased from Fagron Ibérica (Barcelona, Spain) and Carbopol® 980 was obtained from Noveon (Chaussee de Wavre, Brussels). Sodium hyaluronate, almond oil, Tween® 80, glycerin, methylparaben, propylene glycol, petrolatum, and triethanolamine were purchased from Acofarma (Madrid, Spain). Methyl-β-cyclodextrin (Me-β-CD) and (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD) were obtained from Merck Life Science (Algés, Portugal). Schönberg polymethyl methacrylate plates were purchased from Rotoquímica (Porto, Portugal). Deionized water (conductivity < 0.1 µS cm−1) was used throughout all the experiments.
2.2. Methods
2.2.1. Phase Solubility Studies
The solubilizing potential and complexing tendency of Me-β-CD and HP-β-CD with BD in aqueous solution were evaluated from the phase solubility studies performed by the method reported by Higuchi and Connors [25].
The excess amount of BD (20 mg) was added to 25 mL aqueous solutions containing increasing amounts of Me-β-CD and HP-β-CD (0–20 mmol L−1). The suspensions were shaken on a rotary shaker (Ika KS 4000i, Staufen, Germany) at 25 ± 2 °C for 48 h until reaching the equilibrium. All suspensions were filtered through a 0.45 µm membrane filter (Millipore) and properly diluted, and the concentration of BD was determined by spectrophotometry (Shimadzu UV-Vis Spectrophotometer, UV-1700, Tokyo, Japan) at 241.5 nm. The UV absorption of HP-β-CD was negligible at the assay wavelength.
The phase solubility diagrams were obtained plotting the equilibrium concentrations of BD against the concentration of each CD. The apparent stability constant, 𝐾𝑆, was calculated from the straight line of the phase solubility diagram, assuming a 1:1 stoichiometry, using
where 𝑆0 represents the intrinsic solubility of BD.
𝐾𝑆 = slope/𝑆0 (1 − slope)
Each experiment was performed in triplicate, with the mean (±SD) being reported.
2.2.2. UV/Visible Spectroscopy
Spectrophotometric measurements were performed to quantify BD in its free and CD-complexed form. Given the poor water solubility of BD, standard curves were prepared in water/ethanol (v/v = 8:2). Spectrophotometric scans were performed between 200 and 400 nm to monitor the UV spectra of BD. The absorbance maximum of 241.5 nm was used to quantify BD concentration.
2.2.3. Preparation of the HP-β-CD Hydrogel
Based on the phase solubility studies, BD and HP-β-CD were mixed in equimolar amounts in water and stirred for 3 days. Carbopol® 980 was dispersed in water and mixed for 4 h using a magnetic stirrer. An aqueous mixture containing sodium hyaluronate, glycerin, and methylparaben solution was prepared in the same conditions. These two mixtures were homogenized in the Microcaya Unguator (Bilbao, Spain) for 5 min. Triethanolamine was added dropwise to the mixture, while stirring, until it reached a pH of 6. The HP-β-CD/BD solution was added and water q.s. to 100 g, mixing for 5 min until complete homogenization. The amounts are shown in Table 1.

Table 1.
Composition of the emulgel and hydrogel.
2.2.4. Preparation of the Emulgel
The emulgel was prepared with the ingredient composition shown in Table 1, which corresponds to the formulation with the best features among the test formulations prepared. Carbopol® 980 was dispersed in water and mixed for 4 h using a magnetic stirrer. An aqueous mixture containing sodium hyaluronate, glycerin, and methylparaben solution was prepared in the same conditions. These two mixtures were homogenized in the Microcaya Unguator (Bilbao, Spain) for 5 min. Triethanolamine was added dropwise to the mixture, while stirring, until it reached a pH of 6. Almond oil, Tween® 80, and BD were mixed and stirred until complete dissolution. This oil phase was mixed with the previous aqueous phase, using mechanical stirring for 5 min, to obtain the emulgel.
2.2.5. Characterization of the Emulgel
The prepared emulgel formulation was visually inspected and its textural properties and flow behavior were determined. Its evaporation rate and ability to stain clothes were also evaluated. Petrolatum (which is the main component of ointments) was similarly evaluated in all tests for comparison purposes.
Texture Analysis
The textural properties of the emulgel were measured using a TA-XT2iHR texturometer (Stable Micro Systems, Godalming, UK) in the compression mode, using a TTC spreadability rig (velocity 3 mm/s; penetration distance 23 mm). Measurements were performed in triplicate at 20 °C. The parameters maximum force (correlated with firmness) and negative area (correlated with stickiness) [26] were calculated from the texturogram.
Flow Analysis
Rheological measurements were performed on a rheometer (Malvern, Worcestershire, UK) fitted with a cone–plate configuration with a 0.14 mm gap. The samples were placed in appropriate amounts to entirely fill the space between the cone and dish. The data were collected using the rSpace software (Kinexus 1.75: PSS0211-17). Flow analysis was performed in the shear rate range from 0.5 to 250 s−1, with 10 samples per decade.
Measurements were performed in triplicate at 20 °C. The power law model was fitted to the results and its parameters, consistency coefficient (K) and power law index (n), were calculated with the Solver add-in Excel program (Microsoft Office™, 2016):
Evaluation of the Evaporation Rate and Residue
Evaporation rate and residue were determined after the application of the emulgel on polymethyl methacrylate plates (1.5 mg/cm2) and weighing the plates every 5 min over 1 h.
Evaluation of the Ability to Stain Clothes
Ability to stain clothes was assessed by spreading the emulgel on the roughness surface of polymethyl methacrylate plates (1.5 mg/cm2) and then covering the plates with a polyester fabric. A new plate was placed on the top of the fabric for 30 min. Then, the plates and the fabric were weighed to evaluate the amount of product that evaporated from the plate and the amount of product that was transferred to the fabric. Photographic records were obtained using a smartphone camera.
Evaluation of the Physical Stability and pH
Physical stability was evaluated by centrifugation (Eppendorf Centrifuge 5804, Eppendorf, Hamburg, Germany). The centrifugation test was carried out by placing 5 gof emulgel in a 14 mL centrifuge tube which was centrifuged for 30 min at 3500 rpm. At the end, tubes were examined macroscopically to determine the presence of any possible phase separation.
The pH was measured using a Hanna HI 207 pH meter (Hanna Instruments, Woonsocket, RI, USA) at room temperature.
3. Results
In this study, new topical formulations containing BD, based on vehicles with an aqueous external phase, were designed in order to improve sensory characteristics, focusing on patient preferences.
3.1. HP-β-CD Hydrogel
The specific recognition and binding of CDs and various guest molecules have been used as a strategy to alter the release profiles of soluble and poorly soluble active ingredients from hydrogels. Although the natural CDs are hydrophilic, their aqueous solubility is limited, as is the case with β-CD. The physicochemical properties and, consequently, the inclusion capacity of native CDs have been improved over time through the chemical modification of their hydroxyl groups, giving rise to different CD derivatives, such as Me-β-CD and HP-β-CD. These substituted CDs have better water solubility and are suitable for the formation of inclusion complexes with poorly water-soluble “guest” molecules. As the incorporation of a BD–cyclodextrin inclusion complex into the hydrogel matrix can enhance the aqueous solubility of the corticosteroid drug, the solubilizing potential and complexing ability of Me-β-CD and HP-β-CD with BD was evaluated using a phase solubility method [25]. Phase-solubility study is a very efficient method and involves an examination of the effect of cyclodextrin on the substrate (BD). Thus, the equilibrium concentrations of BD were plotted against the concentration of each CD and in this way the phase solubility diagrams were established. The aqueous solubility of BD increased linearly as a function of CDs showing, in both cases, an 𝐴𝐿-type solubility profile [26]. As the slope of the plot is less than unity, a 1:1 molecular complex is formed for the BD and CDs [26]. The determination of the stability constant (𝐾𝑆) is a useful index to estimate the binding strength of the host–guest and the changes in the physicochemical properties of the guest in the complex. The 𝐾𝑆 value of the complexation was calculated from the phase solubility diagram according to (1) (see Materials and Methods). The stability constants, 𝐾𝑆, calculated for Me-β-CD and HP-β-CD inclusion complexes, were 6603.7 ± 325.0 M−1 and 5990.0 ± 275.4 M−1, respectively. Phase solubility studies indicate that the solubility of BD is considerably improved in the presence of CDs. Considering the data found and that HP-β-CD is nowadays one of the most versatile excipients among the cyclic oligosaccharides, being used in oral, dermal, and parenteral formulations, this CD was selected for the preparation of the hydrogel. Although encapsulation in cyclodextrin has been shown to be effective in increasing BD solubility, it was not possible to achieve the active pharmaceutical ingredient dosage commonly found in marketed formulations for topical application. In fact, during the hydrogel preparation, complete solubilization of BD was not obtained (it was only possible to dissolve about 15 mg of the 64 mg of BD), meaning that HP-β-CD complexation capacity does not sufficiently enhance the solubility of the poorly soluble drug. New techniques and approaches are currently under study in order to improve the aqueous solubility of BD.
3.2. Emulgel
The emulgel prepared had a shiny, white and homogeneous appearance (Figure 1). The emulgel’s pH was 6.97 ± 0.01, since the emulgel was neutralized to obtain the semisolid texture typical of gels containing polyacrylic acids. This result is beneficial regarding skin compatibility. Skin pH is around 5.5, and formulations with a pH in the range of 4–8 are considered appropriate for topical application. Regarding physical stability, no phase separation was observed after centrifugation. This result regarding physical stability, although preliminary, is important, and should be confirmed in long-term stability studies.
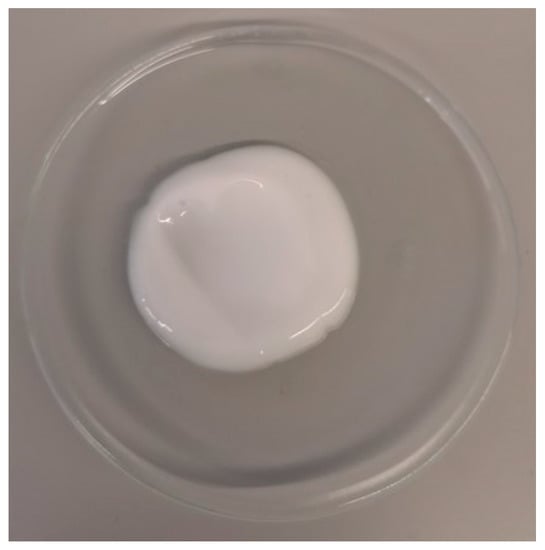
Figure 1.
Emulgel appearance.
The developed emulgel presented much lower firmness and adhesiveness than petrolatum (Figure 2).
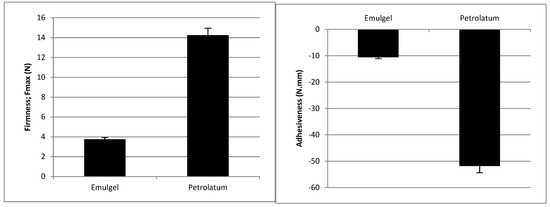
Figure 2.
Firmness and adhesiveness of the emulgel and petrolatum at 20 °C. (mean values ± SD, n = 3).
A decrease in the apparent viscosity of the emulgel was observed, with the increase in the shear rate (Figure 3) suggesting a non-Newtonian behavior, namely shear thinning. The power law model showed a good fit to the results (R ≥ 0.98) and confirmed the shear thinning behavior (n < 1) since the flow index was 0.201 ± 0.01. The consistency coefficient was 94.6 ± 5.3 Pa.sn.
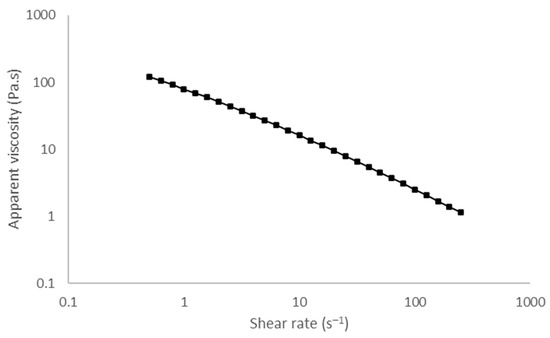
Figure 3.
Viscosity curve of the emulgel. The shear rate ranges from 0.5 to 250 s−1 at 20 °C.
Regarding evaporation results, after 1 h, only 30% of the emulgel remained on the plate, in opposition to 100% for petrolatum (Figure 4).

Figure 4.
Evaporation rate obtained for (○) emulgel and (■) petrolatum. Results are expressed as mean values ± SD (n = 3).
Regarding the evaluation of the emulgel’s ability to stain clothes, a relevant improvement was observed in comparison with petrolatum (Table 2, Figure 5).

Table 2.
Evaluation of the ability to stain clothes.
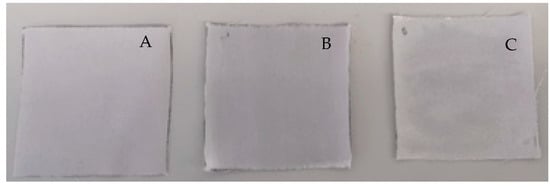
Figure 5.
Ability to stain clothes: (A) control (only fabric); (B) petrolatum; (C) emulgel.
4. Discussion
The composition of the emulgel was established considering multiple properties in order to match TPP. Hyaluronic acid was incorporated to ensure moisturization, which is an essential attribute for psoriasis management [27]. Adding an oily phase also has the ability to reduce transepidermal water loss thus contributing to skin moisturization via a passive mechanism, unlike hyaluronic acid, which retains water. Humectants such as glycerin also reinforce the ability to increase water content in the upper skin layers. Moisturization stood out as a preferred attribute of psoriasis patients in a previous study [9] which could be explained by the typical dryness of psoriatic lesions and the recognized contribution of moisturizers and emollients to disease improvement [28,29]. The use of a non-irritant surfactant (Tween® 80) has the advantage of avoiding skin irritation, especially considering the application on inflamed skin, as in psoriasis.
Texture analysis can be used to predict real life usage [30]. This technique has been extensively employed in the food sector [31] but seldom applied in the pharmaceutical industry [32,33,34,35,36]. These mechanical measurements serve as a tool to predict product performance and are relevant for understanding treatment preference and adherence. Our group studied the topical formulations for the treatment of psoriasis using this technique [37]. A wide range of results was described which supports the possibility of preferences for a subset of products with similar textural properties. Psoriasis patients have rated their satisfaction for topical formulations according to their past experience and reported higher satisfaction for products which were then analyzed using the spreadability test and exhibited low firmness and stickiness [37]. The superiority of a topical calcipotriene/betamethasone dipropionate cream was demonstrated in two phase 3 clinical trials comparing efficacy, safety, and quality of life (QoL) and treatment preferences against the same actives in an oily vehicle [12,38]. Furthermore, skin feel appreciation of topical formulations has been correlated with descriptive sensory attributes using partial least squares (PLS) regression, and it was found to be higher for formulations with lower stickiness and residue [13]. The developed emulgel presented much lower firmness and stickiness than petrolatum (Figure 2) and, in view of the aforementioned findings, it is expected to exhibit high patient acceptability. Flow analysis also revealed that the emulgel has shear thinning behavior (Figure 3). This behavior is favorable for skin application since the force required to allow the spreading of the formulation drops when it is applied to the skin. Skin feel has been described as an important feature for satisfaction with treatment. In fact, about 79% of a panel of patients with psoriasis identified “pleasant consistency”, and 83% picked “easy to apply” as preferred attributes; both are related to the sensory/mechanical profile [9].
Regarding evaporation results, after 1 h, only 30% of the emulgel remained on the plate, in opposition to 100% for petrolatum (Figure 3). A minimum residue is therefore expected upon topical application, which is regarded as a positive attribute [13]. As the emulgel has a high evaporation rate, it will evaporate more rapidly from the patient’s skin, allowing them to dress faster, compared with the application of ointments containing petrolatum. The preference for a low skin residue that will contribute to a minimum set period before dressing was put in evidence in a previous study addressing topical formulations for psoriasis [9]. The quick evaporation observed is also beneficial since the drug’s concentration on the skin surface will increase, which favors its absorption.
A relevant improvement was observed with respect to fabric staining, in comparison with petrolatum (Table 2, Figure 4). The lower amount transferred to the fabric results in lower staining, which is regarded as a critical attribute of topical medicines. In fact, 90% of a panel of psoriasis patients surveyed in a previous study identified “does not leave stains” as a relevant attribute of topical anti-psoriatic formulations [9].
The results obtained for the emulgel correspond to the TPP previously defined and thus the PCDPD strategy was successfully implemented. Additionally, all the experimental results of this work point to the high acceptability of the developed emulgel by psoriasis patients. However, this vehicle may not fit every patient’s preference since a global profile is difficult to establish [13]. Offering a personalized product for each patient is also not feasible from the industrial point of view. This goal can, however, be easily accomplished with compounding practices in pharmacy settings. Patient-centric compounding of dermatological products associates both flexibility and personalization, thus representing an invaluable treatment option to improve patient satisfaction and ultimately clinical outcomes. The potential to improve care through compounding medicines based on patient preferences has been, however, largely ignored. Standard questionnaires are needed to objectively evaluate preferences regarding topical products, and to support the personalized design of the topical medicines, optimizing sensory appreciation. To further confirm the role of PCDPD on treatment adherence, studies with different vehicles in real clinical settings are required to draw conclusions on whether the use of topical formulations designed to match patient preferences, such as the developed emulgel, translates into higher treatment adherence.
5. Conclusions
An HP-β-CD hydrogel and an emulgel have been prepared as vehicles for BD for psoriasis treatment. Although encapsulation in cyclodextrin has been shown to be effective in increasing solubility, complete solubilization of BD was not achieved and further studies were only performed with the emulgel. The latter had a white, shiny appearance, and was physically stable. Lower residue, lower stickiness, and reduced ability to stain polyester fabric were observed in comparison with petrolatum. These results match the TPP established based on psoriasis patient preferences described in the literature. This insight supports a higher treatment satisfaction with the emulgel than with ointments, which can have a positive effect on medication adherence and, therefore, on the clinical outcomes of the anti-inflammatory therapy. The impact of this new vehicle on adherence to the topical treatment of psoriasis should be further confirmed in real clinical settings.
In the dermatological field, patient-reported preferences represent invaluable data to guide the development process in the pharmaceutical industry but also in compounding pharmacies. Patient-centric pharmaceutical drug product design can be thus regarded as a promising strategy to be taken into account in a global strategy to promote adherence to topical medication.
Author Contributions
Conceptualization, J.G. and I.F.A.; Funding acquisition, J.M.S.L.; Investigation, D.F.d.S., S.M. and E.M.G.; Methodology, R.S.O., D.F.d.S. and E.M.G.; Writing—original draft, R.S.O., S.M., J.G. and I.F.A.; Writing—review and editing, R.S.O., J.M.S.L., J.G. and I.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by national funds under the project 47239-Cork2Cosmetic (NORTE-01-0247-FEDER-047239). S. Mota acknowledges the research fellowship (NORTE-01-0247-FEDER-047239), fully supported by national funding from project 47239-Cork2Cosmetic (NORTE-01-0247-FEDER-047239).
Acknowledgments
This work was supported by national funds from FCT-Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020, UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences-UCIBIO. The projects UIDB/00081/2020 and I LA/P/0056/2020 were funded by FCT/MCTES (PIDDAC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Sousa Lobo, J.M.; et al. Patient Centric Pharmaceutical Drug Product Design-The Impact on Medication Adherence. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Oliveira, C.; Teixeira, M.; Rita Gaio, A.; Lobo, J.M.S.; de Almeida, I.F.M.; Almeida, V. Development and Validation of a Novel Questionnaire for Adherence with Topical Treatments in Psoriasis (QATOP). Am. J. Clin. Dermatol. 2017, 18, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Storm, A.; Andersen, S.E.; Benfeldt, E.; Serup, J. One in 3 prescriptions are never redeemed: Primary nonadherence in an outpatient clinic. J. Am. Acad. Dermatol. 2008, 59, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Imafuku, S.; Abe, M.; Shintani, Y.; Onozuka, D.; Hagihara, A.; Katoh, N.; Murota, H.; Takeuchi, S.; Sugaya, M.; et al. Poor adherence to medication as assessed by the Morisky Medication Adherence Scale-8 and low satisfaction with treatment in 237 psoriasis patients. J. Dermatol. 2015, 42, 367–372. [Google Scholar] [CrossRef]
- Schaarschmidt, M.L.; Umar, N.; Schmieder, A.; Terris, D.D.; Goebeler, M.; Goerdt, S.; Peitsch, W.K. Patient preferences for psoriasis treatments: Impact of treatment experience. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 187–198. [Google Scholar] [CrossRef]
- Fouere, S.; Adjadj, L.; Pawin, H. How patients experience psoriasis: Results from a European survey. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 2–6. [Google Scholar] [CrossRef]
- Stegemann, S.; Ternik, R.L.; Onder, G.; Khan, M.A.; van Riet-Nales, D.A. Defining Patient Centric Pharmaceutical Drug Product Design. AAPS J. 2016, 18, 1047–1055. [Google Scholar] [CrossRef]
- Svendsen, M.T.; Feldman, S.R.; Tiedemann, S.N.; Sorensen, A.S.S.; Rivas, C.M.R.; Andersen, K.E. Psoriasis patient preferences for topical drugs: A systematic review. J. Dermatol. Treat. 2019, 1–6. [Google Scholar] [CrossRef]
- Vasconcelos, V.; Teixeira, A.; Almeida, V.; Teixeira, M.; Ramos, S.; Torres, T.; Sousa Lobo, J.M.; Almeida, I.F. Patient preferences for attributes of topical anti-psoriatic medicines. J. Dermatol. Treat. 2019, 30, 659–663. [Google Scholar] [CrossRef]
- Rana, K.; Pani, T.; Jha, S.K.; Mehta, D.; Yadav, P.; Jain, D.; Pradhan, M.K.; Mishra, S.; Kar, R.; Srivastava, A.; et al. Hydrogel-mediated topical delivery of steroids can effectively alleviate psoriasis via attenuating the autoimmune responses. Nanoscale 2022, 14, 3834–3848. [Google Scholar] [CrossRef]
- Fabbrocini, G.; De Simone, C.; Dapavo, P.; Malagoli, P.; Martella, A.; Calzavara-Pinton, P. Long-term maintenance treatment of psoriasis: The role of calcipotriol/betamethasone dipropionate aerosol foam in clinical practice. J. Dermatol. Treat. 2022, 1–8, ahead of print. [Google Scholar] [CrossRef]
- Pinter, A.; Green, L.J.; Selmer, J.; Praestegaard, M.; Gold, L.S.; Augustin, M.; Trial Investigator Group. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.; Jakobsen, H.B. Patient Preferences for Topical Psoriasis Treatments are Diverse and Difficult to Predict. Dermatol. Ther. 2016, 6, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Boeri, M.; Saure, D.; Schuster, C.; Hill, J.; Guerreiro, M.; Klein, K.; Hauber, B. Impact of clinical and demographic characteristics on patient preferences for psoriasis treatment features: Results from a discrete-choice experiment in a multicountry study. J. Dermatol. Treat. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bolt, T.; Kobayashi, H.; Mahlich, J. Patient and Physician Preferences for Therapy Characteristics for Psoriasis: A Discrete Choice Experiment in Japan. PharmacoEconomics-Open 2019, 3, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Felix, K.; Unrue, E.; Inyang, M.; Cardwell, L.A.; Oussedik, E.; Richardson, I.; Feldman, S.R. Patients preferences for different corticosteroid vehicles are highly variable. J. Dermatol. Treat. 2020, 31, 147–151. [Google Scholar] [CrossRef]
- Florek, A.G.; Wang, C.J.; Armstrong, A.W. Treatment preferences and treatment satisfaction among psoriasis patients: A systematic review. Arch. Dermatol. Res. 2018, 310, 271–319. [Google Scholar] [CrossRef]
- Hong, C.H.; Papp, K.A.; Lophaven, K.W.; Skallerup, P.; Philipp, S. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: Randomized phase IIIb PSO-INSIGHTFUL study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1876–1883. [Google Scholar] [CrossRef]
- Eastman, W.J.; Malahias, S.; Delconte, J.; DiBenedetti, D. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis 2014, 94, 46–53. [Google Scholar]
- Feldman, S.R.; Housman, T.S. Patients’ vehicle preference for corticosteroid treatments of scalp psoriasis. Am. J. Clin. Dermatol. 2003, 4, 221–224. [Google Scholar] [CrossRef]
- Housman, T.S.; Mellen, B.G.; Rapp, S.R.; Fleischer, A.B., Jr.; Feldman, S.R. Patients with psoriasis prefer solution and foam vehicles: A quantitative assessment of vehicle preference. Cutis 2002, 70, 327–332. [Google Scholar]
- Puig, L.; Carrascosa, J.M.; Belinchon, I.; Fernandez-Redondo, V.; Carretero, G.; Ruiz-Carrascosa, J.C.; Careaga, J.M.; de la Cueva, P.; Garate, M.T.; Ribera, M.; et al. Adherence and patient satisfaction with topical treatment in psoriasis, and the use, and organoleptic properties of such treatments: A Delphi study with an expert panel and members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Actas Dermo-Sifiliogr. 2013, 104, 488–496. [Google Scholar] [CrossRef]
- Rincon-Lopez, J.; Almanza-Arjona, Y.C.; Riascos, A.P.; Rojas-Aguirre, Y. Technological evolution of cyclodextrins in the pharmaceutical field. J. Drug Deliv. Sci. Technol. 2021, 61, 102156. [Google Scholar] [CrossRef] [PubMed]
- Ajazuddin; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release Off. J. Control. Release Soc. 2013, 171, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Berardesca, E.; Darlenski, R. Psoriasis and dry skin: The impact of moisturizers. In Treatment of Dry Skin Syndrome: The Art and Science of Moisturizers; Springer: Berlin/Heidelberg, Germany, 2012; pp. 285–293. [Google Scholar]
- Draelos, Z.D. Moisturizing cream ameliorates dryness and desquamation in participants not receiving topical psoriasis treatment. Cutis 2008, 82, 211–216. [Google Scholar]
- Oliveira, S.M.; Almeida, I.F.; Costa, P.C.; Barrias, C.C.; Ferreira, M.R.P.; Bahia, M.F.; Barbosa, M.A. Characterization of Polymeric Solutions as Injectable Vehicles for Hydroxyapatite Microspheres. Aaps Pharmscitech 2010, 11, 852–858. [Google Scholar] [CrossRef][Green Version]
- Chen, J. Recognition of the great successes of food texture research. J. Texture Stud. 2019, 50, 187–192. [Google Scholar] [CrossRef]
- Neves, N.; Campos, B.B.; Almeida, I.F.; Costa, P.C.; Cabral, A.T.; Barbosa, M.A.; Ribeiro, C.C. Strontium-rich injectable hybrid system for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 818–827. [Google Scholar] [CrossRef]
- Almeida, I.F.; Maleckova, J.; Saffi, R.; Monteiro, H.; Goios, F.; Amaral, M.H.; Costa, P.C.; Garrido, J.; Silva, P.; Pestana, N.; et al. Characterization of an antioxidant surfactant-free topical formulation containing Castanea sativa leaf extract. Drug Dev. Ind. Pharm. 2015, 41, 148–155. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Barrias, C.C.; Almeida, I.F.; Costa, P.C.; Ferreira, M.R.; Bahia, M.F.; Barbosa, M.A. Injectability of a bone filler system based on hydroxyapatite microspheres and a vehicle with in situ gel-forming ability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Lawlor, M.S.; Woolfson, A.D. Examination of the flow rheological and textural properties of polymer gels composed of poly(methylvinylether-co-maleic anhydride) and poly(vinylpyrrolidone): Rheological and mathematical interpretation of textural parameters. J. Pharm. Sci. 2002, 91, 2090–2101. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm. Res. 1997, 14, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Teixeira, M.; Almeida, V.; Almeida, I.F.; Alves, M.M.; Sousa-Lobo, J.M. Mechanical properties of topical products containing bethametasone: Implications for adherence to treatment in psoriasis. Int. J. Cosmet. Sci. 2012, 34, 384. [Google Scholar]
- Almeida, I.F.; Gaio, A.R.; Bahia, M.F. Estimation of hedonic responses from descriptive skin sensory data by chi-square minimization. J. Sens. Stud. 2006, 21, 2–19. [Google Scholar] [CrossRef]
- Armstrong, A.; Pinter, A.; Selmer, J.; Præstegaard, M.; Reich, A.; Koo, J. Pooled Analysis Demonstrating Superior Patient- Reported Psoriasis Treatment Outcomes for Calcipotriene/Betamethasone Dipropionate Cream Versus Suspension/Gel. J. Drugs Dermatol. 2022, 21, 242–248. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).