Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application
Abstract
1. Introduction
2. Quercus suber and the Cork Industry
3. Cork By-Products and Applications
4. Bioactive Compounds on Cork and Cork By-Products
Biological Activity of Cork Extracts and Cork By-Products
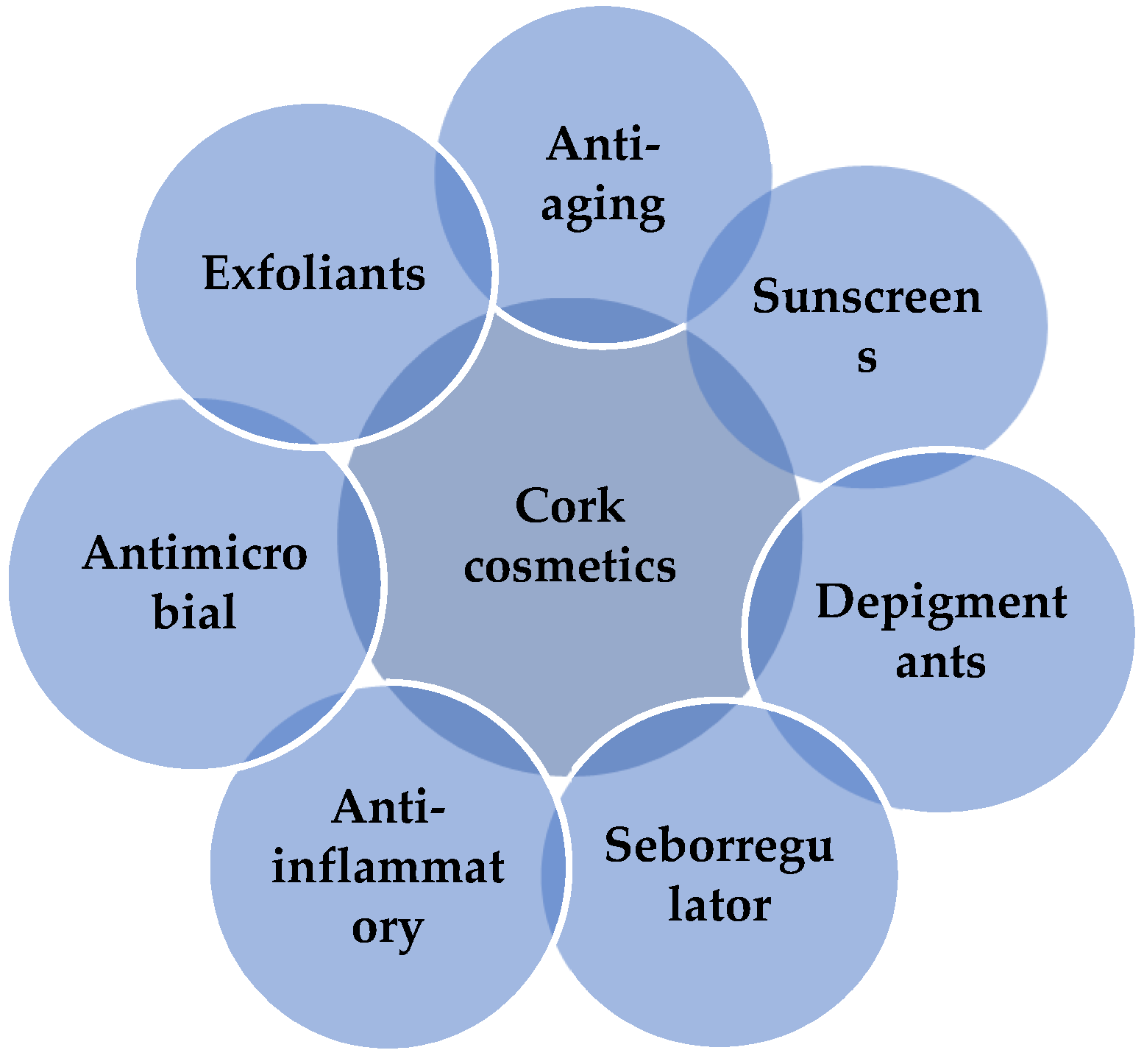
5. Current Cosmetic Applications of Cork
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hay, L.; Duffy, A.; Whitfield, R.I. The Sustainability Cycle and Loop: Models for a more unified understanding of sustainability. J. Environ. Manag. 2014, 133, 232–257. [Google Scholar] [CrossRef] [PubMed]
- Kurnaz, M.L.; Kurnaz, I.A. Commercialization of medicinal bioeconomy resources and sustainability. Sustain. Chem. Pharm. 2021, 22, 100484. [Google Scholar] [CrossRef]
- Mori, K.; Christodoulou, A. Review of sustainability indices and indicators: Towards a new City Sustainability Index (CSI). Environ. Impact Assess. Rev. 2012, 32, 94–106. [Google Scholar] [CrossRef]
- Carriço, C.; Ribeiro, H.M.; Marto, J. Converting cork by-products to ecofriendly cork bioactive ingredients: Novel pharmaceutical and cosmetics applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Patnaik, A.; Tripathy, S.; Dash, A. Identifying the Features Influencing Sustainable Products: A Study on Green Cosmetic; Pant, P., Mishra, S.K., Mishra, P.C., Eds.; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2021; pp. 631–640. [Google Scholar]
- Bom, S.; Ribeiro, H.M.; Marto, J. Sustainability Calculator: A Tool to Assess Sustainability in Cosmetic Products. Sustainability 2020, 12, 15. [Google Scholar] [CrossRef]
- Born, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A step forward on sustainability in the cosmetics industry: A review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar]
- Kumar, S. Exploratory analysis of global cosmetic industry: Major players, technology and market trends. Technovation 2005, 25, 1263–1272. [Google Scholar] [CrossRef]
- Environmental Sustainability-The European Cosmetics Industry’s Contribution 2017–2019. Available online: https://cosmeticseurope.eu/files/3715/6023/8402/Environmental_Sustainability_Report_2019.pdf (accessed on 10 January 2022).
- NATRUE. Circular Beauty: Upcycled Ingredients in Cosmetic Products. Available online: https://www.natrue.org/circular-beauty-upcycled-ingredients-in-cosmetic-products/ (accessed on 10 January 2022).
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Sousa, V.B.; Leal, S.; Quilho, T.; Pereira, H. Characterization of Cork Oak (Quercus suber) Wood Anatomy. IAWA J. 2009, 30, 149–161. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The polyphenolic profile of Oak (Quercus) species: A phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Bugalho, M.N.; Caldeira, M.C.; Pereira, J.S.; Aronson, J.; Pausas, J.G. Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front. Ecol. Environ. 2011, 9, 278–286. [Google Scholar] [CrossRef]
- Petroselli, A.; Vessella, F.; Cavagnuolo, L.; Piovesan, G.; Schirone, B. Ecological behavior of Quercus suber and Quercus ilex inferred by topographic wetness index (TWI). Trees-Struct. Funct. 2013, 27, 1201–1215. [Google Scholar] [CrossRef]
- Gil, L.; Varela, M.C. EUFORGEN-Technical Guidelines for Genetic Conservation and Use for Cork Oak (Quercus suber); EUFORGEN: Barcelona Spain, 2008; p. 6. [Google Scholar]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Joffre, R.; Rambal, S.; Ratte, J.P. The dehesa system of southern Spain and Portugal as a natural ecosystem mimic. Agrofor. Syst. 1999, 45, 57–79. [Google Scholar] [CrossRef]
- Gil, L. Cork: Sustainability and New Applications. Front. Mater. 2015, 1, 38. [Google Scholar] [CrossRef]
- Gil, L. Cork: A strategic material. Front. Chem. 2014, 2, 16. [Google Scholar] [CrossRef]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Quercus suber in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 164–165. [Google Scholar]
- Branco, D.G.; Santiago, C.; Lourenço, A.; Cabrita, L.; Evtuguin, D.V. Structural Features of Cork Dioxane Lignin from Quercus suber L. J. Agric. Food Chem. 2021, 69, 8555–8564. [Google Scholar] [CrossRef]
- Pereira, H. Chemical composition and variability of cork from Quercus suber L. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Villaverde, J.J.; Sousa, A.F.; Coelho, J.F.J.; Neto, C.P.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of industrial cork by-products. Ind. Crops Prod. 2013, 47, 262–269. [Google Scholar] [CrossRef]
- Reis, S.F.; Lopes, P.; Roseira, I.; Cabral, M.; Mateus, N.; Freitas, V. Recovery of added value compounds from cork industry by-products. Ind. Crops Prod. 2019, 140, 111599. [Google Scholar] [CrossRef]
- Madureira, J.; Melo, R.; Botelho, M.L.; Leal, J.P.; Fonseca, I.M. Effect of ionizing radiation on antioxidant compounds present in cork wastewater. Water Sci. Technol. 2013, 67, 374–379. [Google Scholar] [CrossRef]
- Mislata, A.M.; Puxeu, M.; Ferrer-Gallego, R. Aromatic potential and bioactivity of cork stoppers and cork by-products. Foods 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Castola, V.; Marongiu, B.; Bighelli, A.; Floris, C.; Laï, A.; Casanova, J. Extractives of cork (Quercus suber L.): Chemical composition of dichloromethane and supercritical CO2 extracts. Ind. Crops Prod. 2005, 21, 65–69. [Google Scholar] [CrossRef]
- Araújo, A.R.; Pereira, D.M.; Aroso, I.M.; Santos, T.; Batista, M.T.; Cerqueira, M.T.; Marques, A.P.; Reis, R.L.; Pires, R.A. Cork extracts reduce UV-mediated DNA fragmentation and cell death. RSC Adv. 2015, 5, 96151–96157. [Google Scholar] [CrossRef]
- Khennouf, S.; Benabdallah, H.; Gharzouli, K.; Amira, S.; Ito, H.; Kim, T.H.; Yoshida, T.; Gharzouli, A. Effect of tannins from Quercus suber and Quercus coccifera leaves on ethanol-induced gastric lesions in mice. J. Agric. Food Chem. 2003, 51, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Sousa, A.; Mateus, N.; Cabral, M.; Freitas, V. Analysis of phenolic compounds in cork from Quercus suber L. by HPLC-DAD/ESI-MS. Food Chem. 2011, 125, 1398–1405. [Google Scholar] [CrossRef]
- Conde, E.; Cadahia, E.; García-Vallejo, M.C.; de Simon, B.F.; Adrados, J.R.G. Low molecular weight polyphenols in cork of Quercus suber. J. Agric. Food Chem. 1997, 45, 2695–2700. [Google Scholar] [CrossRef]
- Bejarano, I.; Godoy-Cancho, B.; Franco, L.; Martínez-Cañas, M.A.; Tormo, M.A. Quercus Suber L. Cork Extracts Induce Apoptosis in Human Myeloid Leukaemia HL-60 Cells. Phytother. Res. 2015, 29, 1180–1187. [Google Scholar] [CrossRef]
- Carriço, C.; Pinto, P.; Graça, A.; Gonçalves, L.M.; Ribeiro, H.M.; Marto, J. Design and characterization of a new Quercus suber-based pickering emulsion for topical application. Pharmaceutics 2019, 11, 131. [Google Scholar] [CrossRef]
- Hunt, K.J.; Hung, S.K.; Ernst, E. Botanical extracts as anti-aging preparations for the skin: A systematic review. Drugs Aging 2010, 27, 973–985. [Google Scholar] [CrossRef]
- Coquet, C.; Bauza, E.; Oberto, G.; Berghi, A.; Farnet, A.M.; Ferré, E.; Peyronel, D.; Dal Farra, C.; Domloge, N. Quercus suber cork extract displays a tensor and smoothing effect on human skin: An in vivo study. Drugs Exp. Clin. Res. 2005, 31, 89–99. [Google Scholar]
- Diaz-Maroto, I.J.; Diaz-Maroto, M.C. Cork from Quercus suber L.: Forest certification system for sustainable management of cork oak forests. Wood Res. 2020, 65, 855–864. [Google Scholar] [CrossRef]
- Montero, I.; Miranda, T.; Sepúlveda, F.J.; Arranz, J.I.; Nogales, S. Analysis of pelletizing of granulometric separation powder from cork industries. Materials 2014, 6, 6686–6700. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Pereira, D.; Gago, A.; Proença, J. Experimental characterisation of cork agglomerate core sandwich panels for wall assemblies in buildings. J. Build. Eng. 2016, 5, 194–210. [Google Scholar] [CrossRef]
- Gil, L. Cork powder waste: An overview. Biomass Bioenergy 1997, 13, 59–61. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Ferreira, C.I.; Pereira, J.C.; Correia, P.; Silva, S.P.; Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Use of cork powder and granules for the adsorption of pollutants: A review. Water Res. 2012, 46, 3152–3166. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.M.; Correlo, V.M.; Chagas, J.A.M.; Mano, J.F.; Reis, R.L. Cork based composites using polyolefin’s as matrix: Morphology and mechanical performance. Compos. Sci. Technol. 2010, 70, 2310–2318. [Google Scholar] [CrossRef]
- Gil, L. New cork-based materials and applications. Materials 2015, 8, 625–637. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Cardoso, B.; Mestre, A.S.; Carvalho, A.P.; Pires, J. Activated Carbon Derived from Cork Powder Waste by KOH Activation: Preparation, Characterization, and VOCs Adsorption. Ind. Eng. Chem. Res. 2008, 47, 5841–5846. [Google Scholar] [CrossRef]
- Duarte, A.P.; Bordado, J.C. Cork–A Renewable Raw Material: Forecast of Industrial Potential and Development Priorities. Front. Mater. 2015, 2. [Google Scholar] [CrossRef]
- Novais, R.M.; Caetano, A.P.F.; Seabra, M.P.; Labrincha, J.A.; Pullar, R.C. Extremely fast and efficient methylene blue adsorption using eco-friendly cork and paper waste-based activated carbon adsorbents. J. Clean. Prod. 2018, 197, 1137–1147. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Mu, J.; Chu, D.; Zang, X. Converting industrial waste cork to biochar as Cu (II) adsorbent via slow pyrolysis. Waste Manag. 2020, 105, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Pires, R.A.; Mano, J.F.; Reis, R.L. Surface properties of extracts from cork black condensate. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 2010, 64, 217–222. [Google Scholar] [CrossRef][Green Version]
- Ponce-Robles, L.; Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Trinidad-Lozano, M.J.; Yuste, F.J.; Malato, S. Cork boiling wastewater treatment and reuse through combination of advanced oxidation technologies. Environ. Sci. Pollut. Res. 2017, 24, 6317–6328. [Google Scholar] [CrossRef] [PubMed]
- Madureira, J.; Pimenta, A.I.; Popescu, L.; Besleaga, A.; Dias, M.I.; Santos, P.M.P.; Melo, R.; Ferreira, I.C.F.R.; Cabo Verde, S.; Margaça, F.M.A. Effects of gamma radiation on cork wastewater: Antioxidant activity and toxicity. Chemosphere 2017, 169, 139–145. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Miranda, I.; Sousa, V.B.; Pereira, H. Chemical composition of barks from Quercus faginea trees and characterization of their lipophilic and polar extracts. PLoS ONE 2018, 13, 18. [Google Scholar] [CrossRef]
- Touati, R.; Santos, S.A.O.; Rocha, S.M.; Belhamel, K.; Silvestre, A.J.D. The potential of cork from Quercus suber L. grown in Algeria as a source of bioactive lipophilic and phenolic compounds. Ind. Crops Prod. 2015, 76, 936–945. [Google Scholar] [CrossRef]
- Lopes, M.H.; Gil, A.M.; Silvestre, A.J.D.; Neto, C.P. Composition of Suberin Extracted upon Gradual Alkaline Methanolysis of Quercus suber L. Cork. J. Agric. Food Chem. 2000, 48, 383–391. [Google Scholar] [CrossRef]
- Santos, S.; Cabral, V.; Graça, J. Cork Suberin Molecular Structure: Stereochemistry of the C18 Epoxy and vic-Diol ω-Hydroxyacids and α,ω-Diacids Analyzed by NMR. J. Agric. Food Chem. 2013, 61, 7038–7047. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Neto, C.P.; Gandini, A. Chapter 14-Cork and Suberins: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 305–320. [Google Scholar]
- Menager, C.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. Suberin from Cork as a Tough Cross-Linker in Bioepoxy Resins. ACS Appl. Polym. Mater. 2021, 3, 6090–6101. [Google Scholar] [CrossRef]
- Pinto, P.C.R.O.; Sousa, A.F.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Eckerman, C.; Holmbom, B. Quercus suber and Betula pendula outer barks as renewable sources of oleochemicals: A comparative study. Ind. Crops Prod. 2009, 29, 126–132. [Google Scholar] [CrossRef]
- Križková, L.V.; Lopes, M.H.; Polónyi, J.; Belicová, A.; Dobias, J.; Ebringer, L. Antimutagenicity of a suberin extract from Quercus suber cork. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 1999, 446, 225–230. [Google Scholar] [CrossRef]
- Pereira, H. The Rationale behind Cork Properties: A Review of Structure and Chemistry. Bioresources 2015, 10, 6207–6229. [Google Scholar] [CrossRef]
- Marques, A.V.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Pereira, H. Ferulates and lignin structural composition in cork. Holzforschung 2016, 70, 275–289. [Google Scholar] [CrossRef]
- Jové, P.; Olivella, M.À.; Cano, L. Study of the variability in chemical composition of bark layers of cork from different production areas. BioResources 2011, 6, 1806–1815. [Google Scholar]
- Fernandes, E.M.; Aroso, I.M.; Mano, J.F.; Covas, J.A.; Reis, R.L. Functionalized cork-polymer composites (CPC) by reactive extrusion using suberin and lignin from cork as coupling agents. Compos. B Eng. 2014, 67, 371–380. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-Lignin Biodegradable and Flexible UV Protection Film. ACS Sustain. Chem. Eng. 2016, 5, 625–631. [Google Scholar] [CrossRef]
- Asensio, A. Polysaccharides from the Cork of Quercus suber, II. Hemicellulose. J. Nat. Prod. 1988, 51, 488–491. [Google Scholar] [CrossRef]
- Sousa, A.F.; Pinto, P.C.R.O.; Silvestre, A.J.D.; Neto, C.P. Triterpenic and Other Lipophilic Components from Industrial Cork Byproducts. J. Agric. Food Chem. 2006, 54, 6888–6893. [Google Scholar] [CrossRef]
- Castola, V.; Bighelli, A.; Rezzi, S.; Melloni, G.; Gladiali, S.; Desjobert, J.M.; Casanova, J. Composition and chemical variability of the triterpene fraction of dichloromethane extracts of cork (Quercus suber L.). Ind. Crops Prod. 2002, 15, 15–22. [Google Scholar] [CrossRef]
- Moiteiro, C.; Curto, M.J.M.; Mohamed, N.; Bailén, M.; Martínez-Díaz, R.; González-Coloma, A. Biovalorization of Friedelane Triterpenes Derived from Cork Processing Industry Byproducts. J. Agric. Food Chem. 2006, 54, 3566–3571. [Google Scholar] [CrossRef]
- Teplova, V.V.; Isakova, E.P.; Klein, O.I.; Dergachova, D.I.; Gessler, N.N.; Deryabina, Y.I. Natural Polyphenols: Biological Activity, Pharmacological Potential, Means of Metabolic Engineering (Review). Appl. Biochem. Microbiol. 2018, 54, 221–237. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Chapter 12-Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 253–271. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Lourenço, A.; Barreiros, S.; Paiva, A.; Simões, P. Valorization of Cork Using Subcritical Water. Molecules 2020, 25, 4695. [Google Scholar] [CrossRef] [PubMed]
- Şen, A.; Miranda, I.; Santos, S.; Graça, J.; Pereira, H. The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind. Crops Prod. 2010, 31, 417–422. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Batista, M.; Rosete, M.; Ferreira, I.; Ferreira, J.; Duarte, C.; Matias, A.; Poejo, J.; Crespo, J.; Valério, R.; Fraga, M.; et al. Extracto Hidro-Glicólico de Cortiça, Processo para a sua Preparação, Formulações Compreendendo o Referido Extracto e sua Utilização. 2015. Available online: https://patentimages.storage.googleapis.com/9f/01/2a/9d81e00f9817b0/WO2015152746A1.pdf (accessed on 4 January 2022).
- Santos, S.A.O.; Pinto, P.C.R.O.; Silvestre, A.J.D.; Neto, C.P. Chemical composition and antioxidant activity of phenolic extracts of cork from Quercus suber L. Ind. Crops Prod. 2010, 31, 521–526. [Google Scholar] [CrossRef]
- Santos, S.A.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.F.; Coelho, E.; Evtuguin, D.V.; Coimbra, M.A.; Lopes, P.; Cabral, M.; Mateus, N.; Freitas, V. Migration of Tannins and Pectic Polysaccharides from Natural Cork Stoppers to the Hydroalcoholic Solution. J. Agric. Food Chem. 2020, 68, 14230–14242. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Fernandes, I.; Cruz, L.; Mateus, N.; Cabral, M.; Freitas, V. Antioxidant and Biological Properties of Bioactive Phenolic Compounds from Quercus suber L. J. Agric. Food Chem. 2009, 57, 11154–11160. [Google Scholar] [CrossRef]
- Azevedo, J.; Fernandes, A.; Oliveira, J.; Brás, N.F.; Reis, S.; Lopes, P.; Roseira, I.; Cabral, M.; Mateus, N.; Freitas, V. Reactivity of Cork Extracts with (+)-Catechin and Malvidin-3-O-glucoside in Wine Model Solutions: Identification of a New Family of Ellagitannin-Derived Compounds (Corklins). J. Agric. Food Chem. 2017, 65, 8714–8726. [Google Scholar] [CrossRef]
- Gabrielli, M.; Fracassetti, D.; Tirelli, A. Release of phenolic compounds from cork stoppers and its effect on protein-haze. Food Control 2016, 62, 330–336. [Google Scholar] [CrossRef]
- Mota-Panizio, R.; Hermoso-Orzáez, M.J.; Carmo-Calado, L.; Lourinho, G.; de Brito, P.S.D. Biochemical Methane Potential of Cork Boiling Wastewater at Different Inoculum to Substrate Ratios. Appl. Sci. 2021, 11, 3064. [Google Scholar] [CrossRef]
- Igueld, S.B.; Abidi, H.; Trabelsi-Ayadi, M.; Chérif, J.K. Study of physicochemicals characteristics and antioxidant capacity of cork oak acorns (Quercus Suber L.) grown in three regions in Tunisia. J. Appl. Pharm. Sci. 2015, 5, 26–32. [Google Scholar] [CrossRef]
- Aroso, I.M.; Fernandes, E.M.; Pires, R.A.; Mano, J.F.; Reis, R.L. Cork extractives exhibit thermo-oxidative protection properties in polypropylene–cork composites and as direct additives for polypropylene. Polym. Degrad. Stab. 2015, 116, 45–52. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Aroso, I.M.; Araújo, A.R.; Fernandes, J.P.; Santos, T.; Batista, M.T.; Pires, R.A.; Mano, J.F.; Reis, R.M. Hydroalcoholic extracts from the bark of Quercus suber L. (Cork): Optimization of extraction conditions, chemical composition and antioxidant potential. Wood Sci. Technol. 2017, 51, 855–872. [Google Scholar] [CrossRef]
- Passi, S.; Nazzaro-Porro, M. Molecular basis of substrate and inhibitory specificity of tyrosinase: Phenolic compounds. Br. J. Dermatol. 1981, 104, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Srivastava, S.; Mishra, N.; Yadav, N.P. New Perspectives on Antiacne Plant Drugs: Contribution to Modern Therapeutics. BioMed Res. Int. 2014, 2014, 301304. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, S.; Begum, S.F.M.; Rajesh, G. Antimicrobial characterisation combining spectrophotometric analysis of different oak species. Int. J. Herb. Med. 2016, 4, 32–35. [Google Scholar]
- Rattanata, N.; Klaynongsruang, S.; Daduang, S.; Tavichakorntrakool, R.; Limpaiboon, T.; Lekphrom, R.; Boonsiri, P.; Daduang, J. Inhibitory Effects of Gallic Acid Isolated from Caesalpinia mimosoides Lamk on Cholangiocarcinoma Cell Lines and Foodborne Pathogenic Bacteria. Asian Pac. J. Cancer Prev. 2016, 17, 1341–1345. [Google Scholar] [CrossRef]
- Goncalves, F.; Correia, P.; Silva, S.P.; Almeida-Aguiar, C. Evaluation of antimicrobial properties of cork. FEMS Microbiol. Lett. 2016, 363, 6. [Google Scholar] [CrossRef]
- Birkenstock-Natural Cosmetics. Available online: https://www.birkenstock.com/gb/natural-cosmetics/ (accessed on 4 January 2022).
- CosIng-Cosmetics. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.simple (accessed on 4 January 2022).
- ACTISCRUB™ Cork. Available online: https://cosmetics.specialchem.com/product/i-lubrizol-actiscrub-cork (accessed on 4 January 2022).
- Suberlift™ Biofunctional. Available online: https://cosmetics.specialchem.com/product/i-ashland-suberlift-biofunctional (accessed on 4 January 2022).
- DIAM Oléoactif®. Available online: https://cosmetics.specialchem.com/product/i-hallstar-diam-oleoactif (accessed on 4 January 2022).
- The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation-11th Revision. Available online: https://ec.europa.eu/health/system/files/2021-04/sccs_o_250_0.pdf (accessed on 6 January 2022).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council. Available online: https://ec.europa.eu/health/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 6 January 2022).
- Technical Document on Cosmetic Claims. Available online: https://ec.europa.eu/docsroom/documents/24847 (accessed on 6 January 2022).

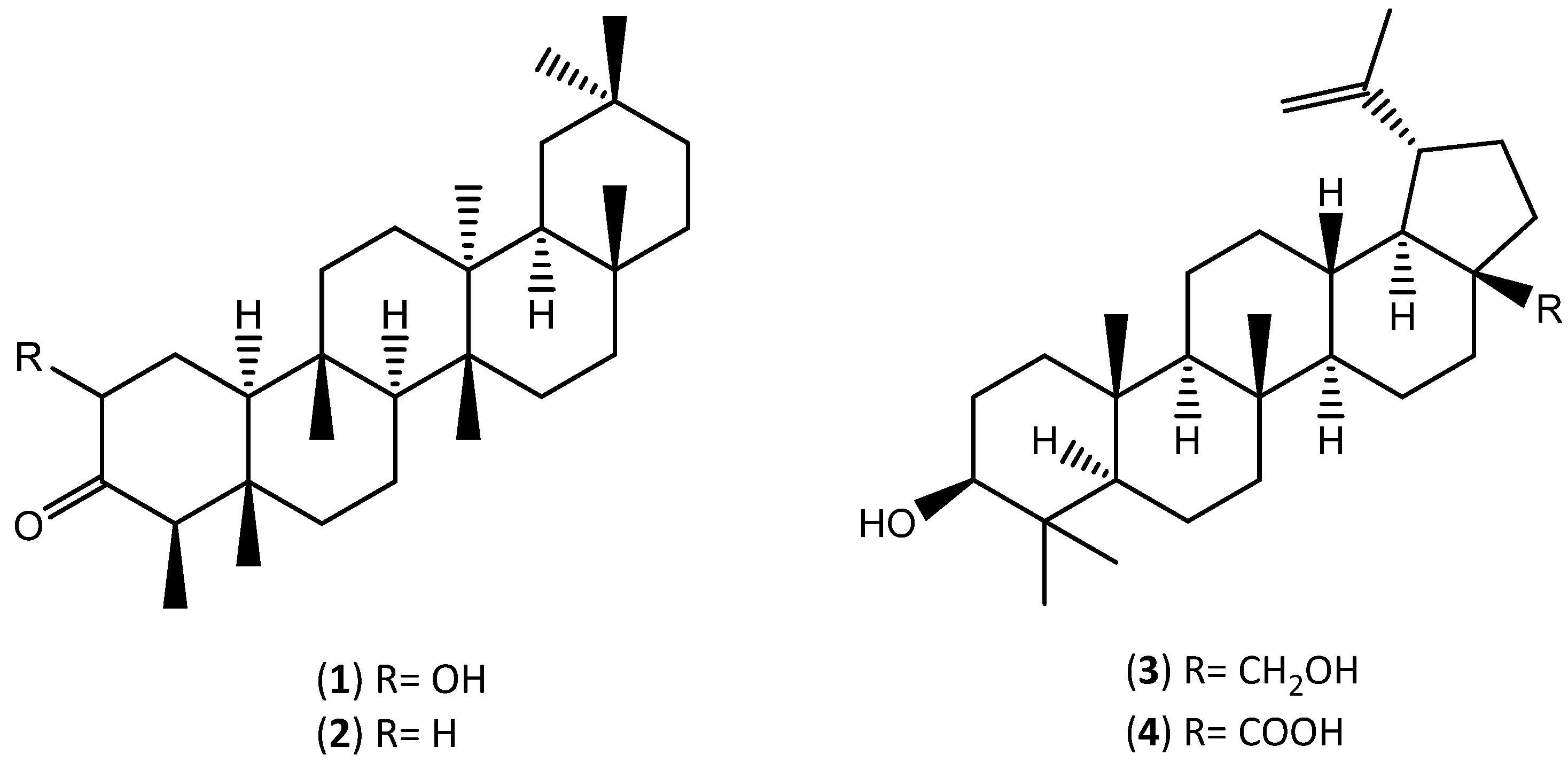
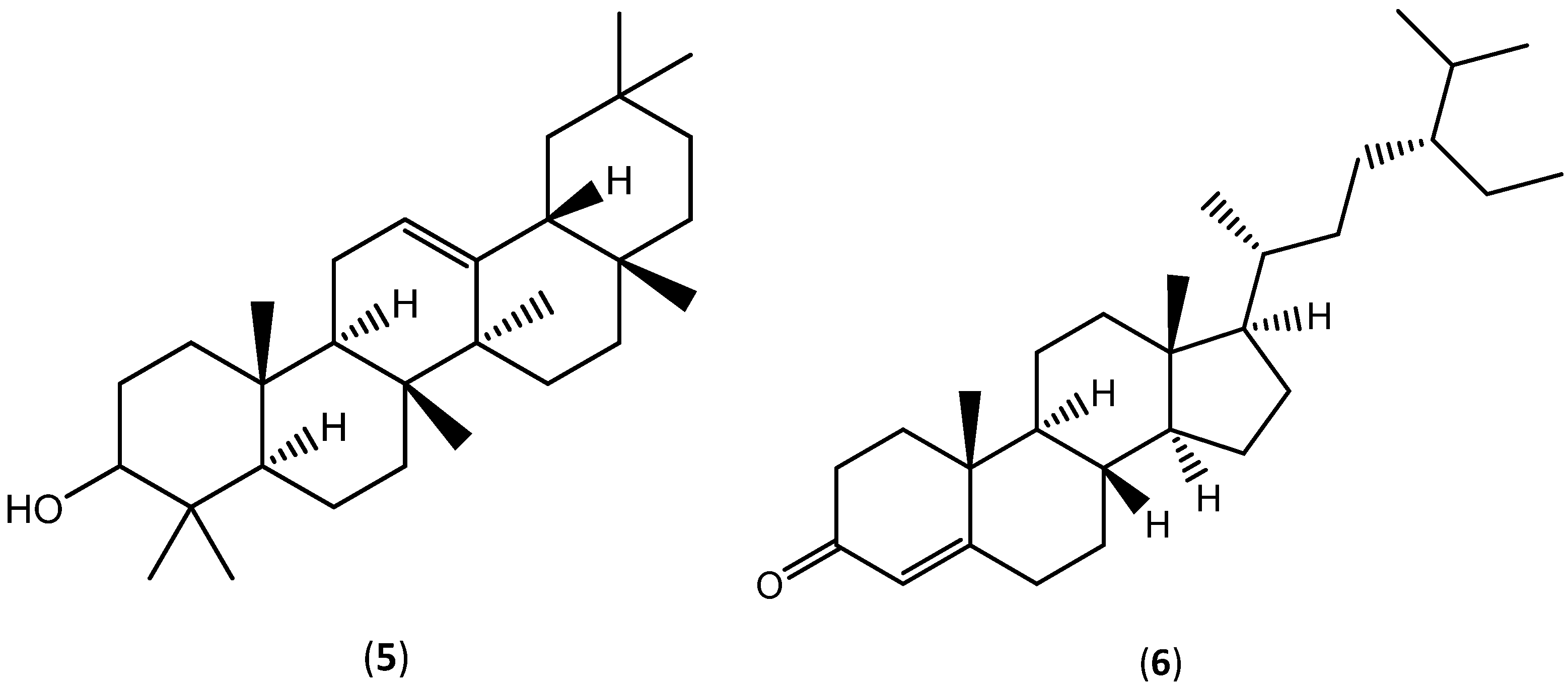
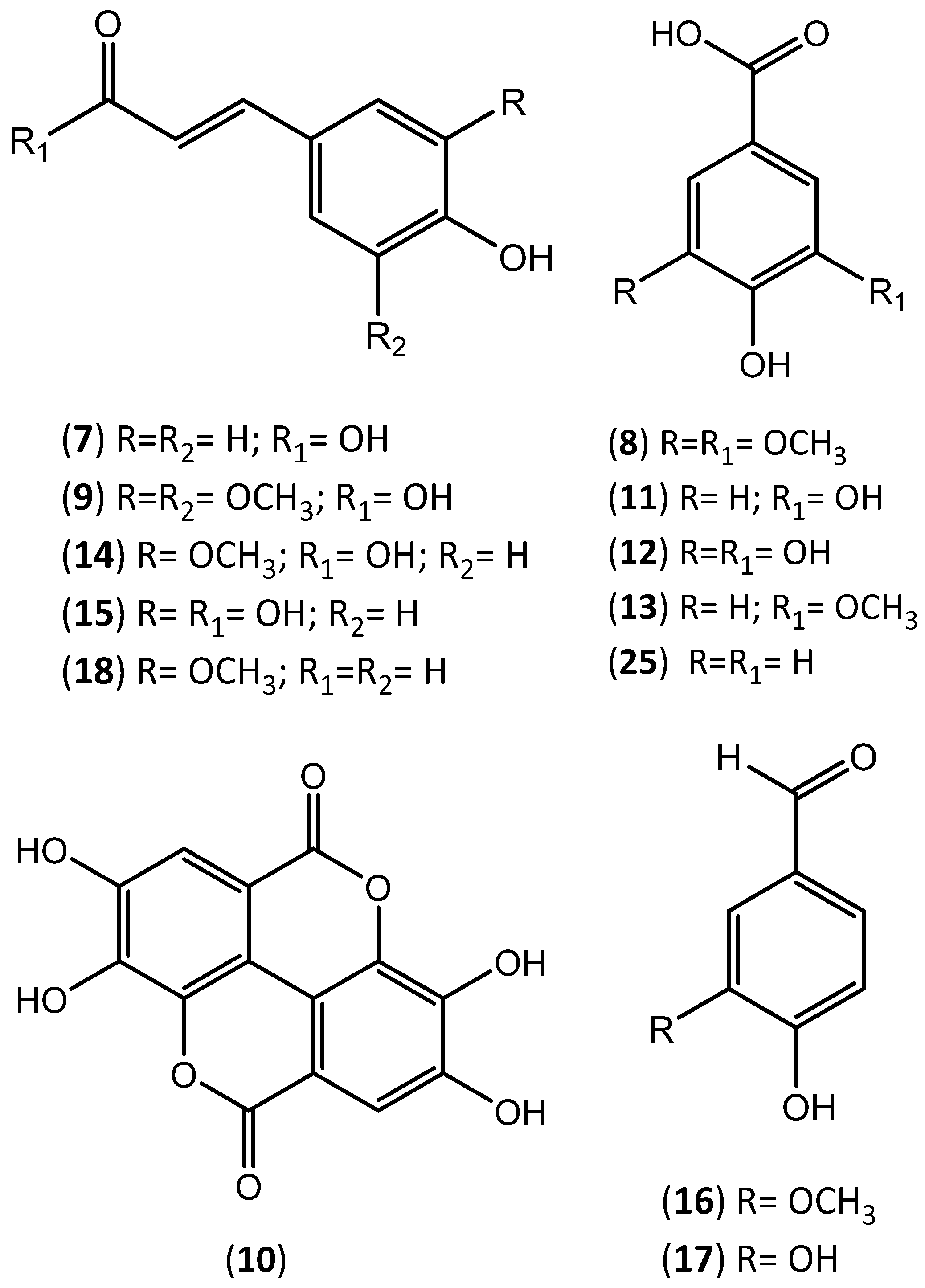
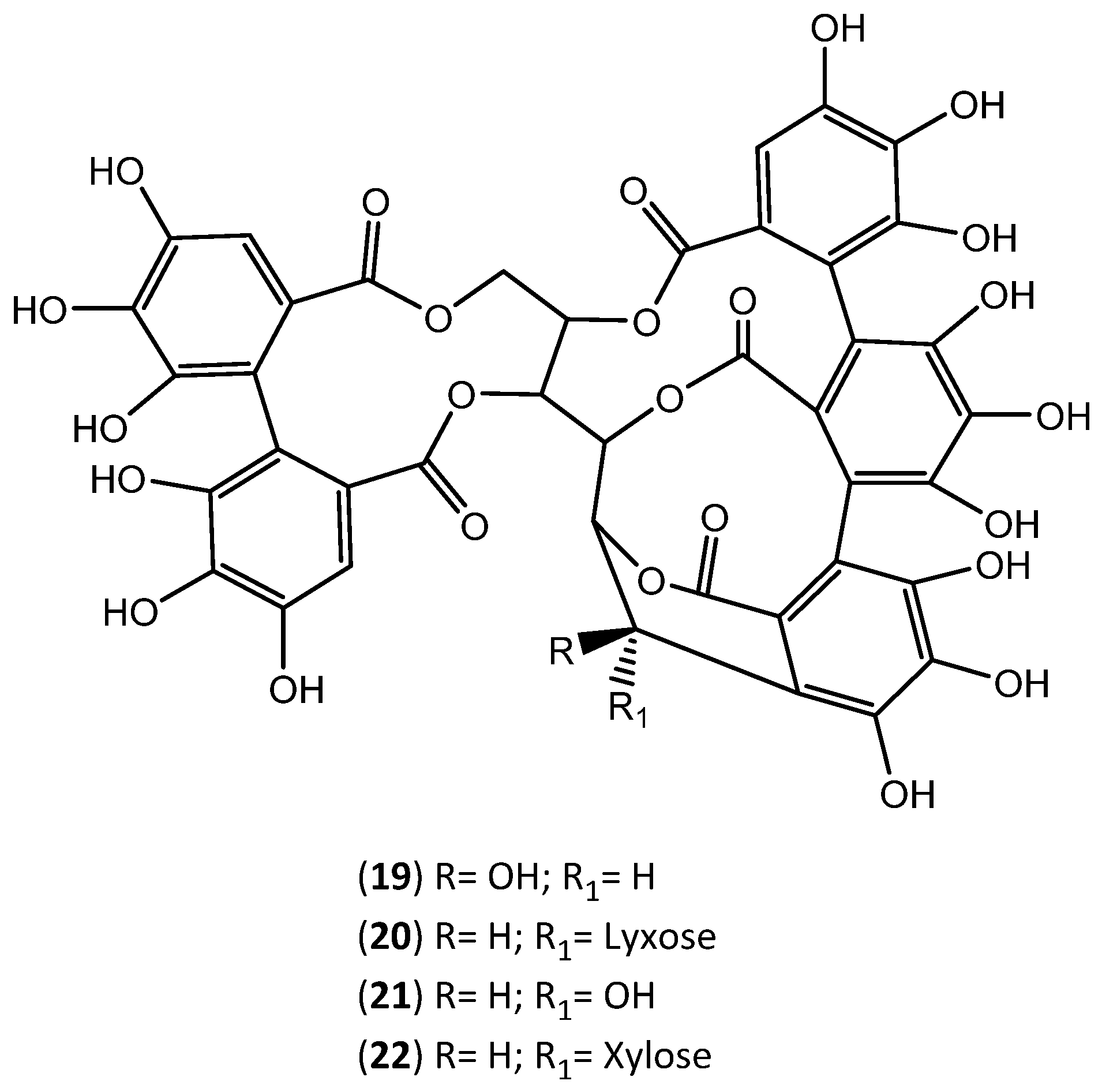
| Extraction Solvent and Source Material | Composition | Quantification | Biological Activity | References | |
|---|---|---|---|---|---|
| methanol/water (80:20); diethylether granulated cork from Spain | Ellagic acid (10) | 228.4 | µg of compound/g of dry cork | -------- | [32] |
| Protocatechuic acid (11) | 48.8 | ||||
| Vanillic acid (13) | 27.4 | ||||
| Gallic acid (12) | 18.3 | ||||
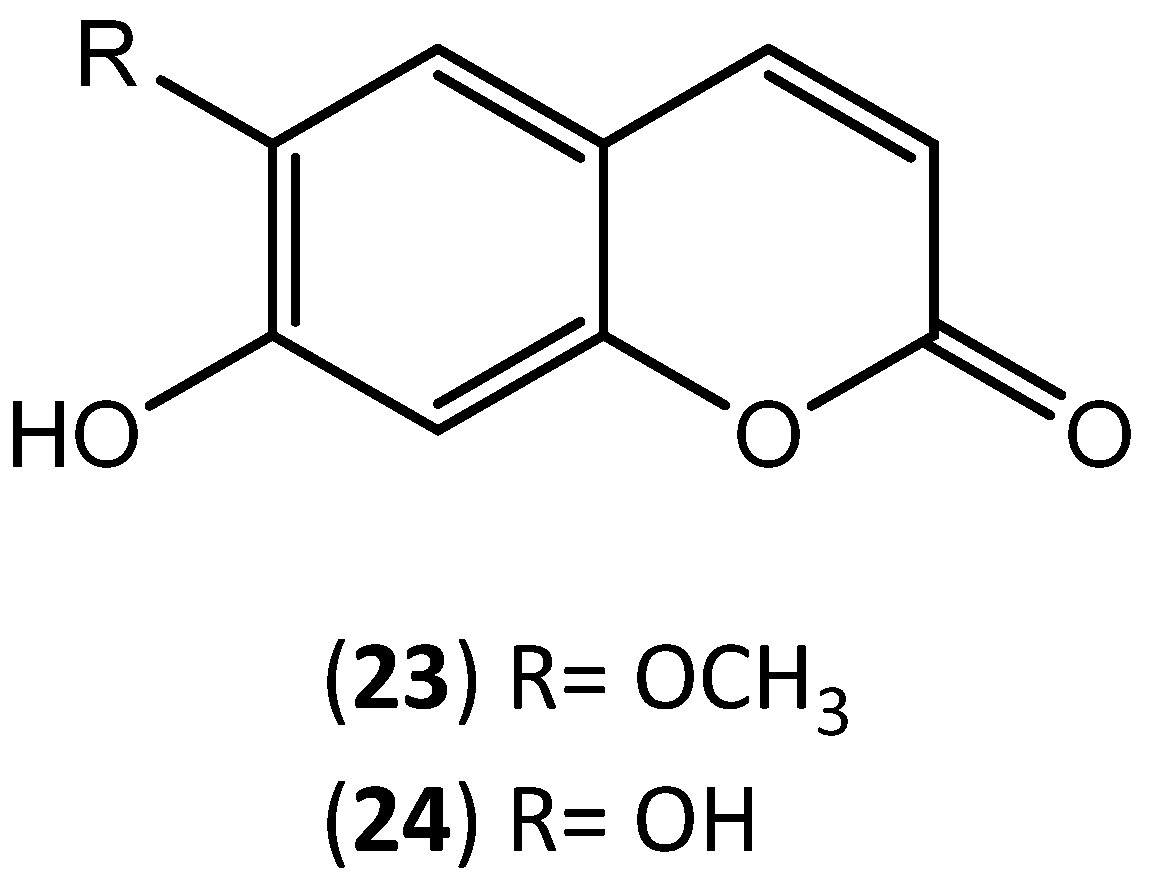
| Scopoletin (23) | 12.7 | ||||
| Vanillin (16) | 16.1 | ||||
| Coniferaldehyde (18) | 11.2 | ||||
| Protocatechuic aldehyde (17) | 8.1 | ||||
| Caffeic acid (15) | 12.1 | ||||
| Ferulic acid (14) | 10.7 | ||||
| Aesculetin (24) | 7.5 | ||||
| Sinapaldehyde | 4.5 | ||||
| supercritical CO2 granulated cork | Friedelin (2) | 30.6 | mg of compound/extract | -------- | [28] |
| Sitost-4-en-3-one (6) | 22.5 | ||||
| β-Sitosterol | 6.59 | ||||
| Betulinic acid (4) | 4.93 | ||||
| Betulin (3) | 3.13 | ||||
| dichloromethane granulated cork | Friedelin (2) | 30.2 | mg of compound/extract | -------- | [28] |
| Sitost-4-en-3-one (6) | 4.1 | ||||
| Betulinic acid (4) | 10.5 | ||||
| Betulin (3) | 3.9 | ||||
| Protocatechuic aldehyde (17) | |||||
| Vanillin (16) | |||||
| Protocatechuic acid (11) | |||||
| Gallic acid (12) | |||||
| Conyferaldehyde (18) | |||||
| Caffeic acid (15) | |||||
| Ferulic acid (14) | |||||
| Ellagic acid (10) | |||||
| Ellagic acid-pentose | |||||
| Ellagic acid-deoxyhexose | |||||
| wine solution (12% ethanol, 5.0 g/L tartaric acid, pH = 3.2); ethyl acetate granulated cork | Ellagic acid-hexose | -------- | --------- | [31,83,84] | |
| Valoneic acid dilactone | |||||
| HHDP-glucose | |||||
| Valoneic acid | |||||
| Dehydrated tergallic-C-glucoside HHDP-galloyl-glucose | |||||
| Trigalloy-glucose | |||||
| Di-HHDP-glucose | |||||
| HHDP-digalloyl-glucose | |||||
| Tetragalloyl-glucose | |||||
| Castalagin (19) | |||||
| Vescalagin (21) | |||||
| Di-HHDP-galloyl-glucose | |||||
| Trigalloyl-HHDP-glucose | |||||
| Pentagalloyl-glucose | |||||
| Mongolicain A and B | |||||
| water; water/ethanol (50:50) granulated cork | Castalagin (19) | 46.9 | mg of compound/g extract | Antioxidant activity (DPPH (EC50) = 5.32 ± 0.45 µg of extract/mL; ORAC = 2.11 ± 0.24 mgTeq/gextract) | [29] |
| Ellagic acid (10) | 26.7 | ||||
| Vescalagin (21) | 22.4 | ||||
| Gallic acid (12) | 2.9 | ||||
| dichloromethane; methanol/water cork powder | Betulinic acid (4) | 11719 | mg of compound/kg of cork powder | -------- | [67] |
| Cerin (1) | 2060 | ||||
| Friedelin (2) | 2009 | ||||
| Ellagic acid (10) | 1347 | ||||
| Betulin (3) | 875 | ||||
| β-Sitosterol | 254 | ||||
| Ursolic acid | 104 | ||||
| Lupeol | 60 | ||||
| subcritical water granulated cork | Gallic acid (12) | 4.9 ± 0.9 | mg of compound/g extract) | Antioxidant activity (EC50 = 0.25 mg extract/mg DPPH) | [76] |
| Ferulic acid (14) | 0.6 ± 0.1 | ||||
| Caffeic acid (15) | 0.5 ± 0.1 | ||||
| Ellagic acid (10) | 6800–8200 | Antioxidant activity (ORAC = 22,603 ± 2097 ymolET/L; HORAC = 15,712 ± 1419 µmolEAC/L; HOSC = 22,678 ± 3225 pmolET/L; DPPH = 1.68 (IC50) mL/L; O2− = 11.08 (IC50) mL/L) Antiaging activity: inhibition of MMP-1, MMP-3, MMP-9 activity; inhibition of ROS formation in keratinocytes and fibroblasts. Depigmenting activity: inhibition of tyrosinase activity; inhibition of melanin production in melanocytes. Anti-inflammatory activity: inhibition of NO production; reduction of IL-6, TNF-α, CCL5 levels; reduction of the activation of NF-kB. Inhibition of lipid accumulation in keratinocytes (inhibition of SREBP-1 gene expression) | |||
| Roburin (22) and Grandinin (20) | 500–3200 | ||||
| water/propylene glycol (40:60) granulated cork | Castalagin (19) | 1800–2100 | µg of compound/g of dry cork | [79] | |
| Vescalagin (21) | 800–1900 | ||||
| Protocatechuic acid (11) | 100–130 | ||||
| Gallic acid (12) | 60–100 | ||||
| methanol/water (80:20); diethyl ether granulated cork from Portugal | Ellagic acid (10) | 2031.5 | mg of compound/kg of dry cork | Antioxidant activity (DPPH (IC50) = 2.79 ± 0.15 µg of extract/mL) | [80] |
| Caffeic acid (15) | 57.6 | ||||
| Salicylic acid | 32.7 | ||||
| Gallic acid (12) | 30.6 | ||||
| Eriodictyol | 27.4 | ||||
| Protocatechuic acid (11) | 17.5 | ||||
| Vanillin (16) | 14.3 | ||||
| Aesculetin (24) | 4.9 | ||||
| Naringenin | 2.6 | ||||
| Vanillic acid (13) | Trace | ||||
| p-coumaric acid (7) | Trace | ||||
| Ferulic acid (14) | Trace | ||||
| methanol granulated cork from Portugal | Ellagic acid (10) | 1576.9 | mg of compound/kg of dry cork | Antioxidant activity (DPPH (IC50) = 3.58 ± 0.20 µg of extract/mL) | [80] |
| Aesculetin (24) | 106.7 | ||||
| Protocatechuic acid (11) | 59.0 | ||||
| Gallic acid (12) | 48.1 | ||||
| Vanillin (16) | Trace | ||||
| Vanillic acid (13) | Trace | ||||
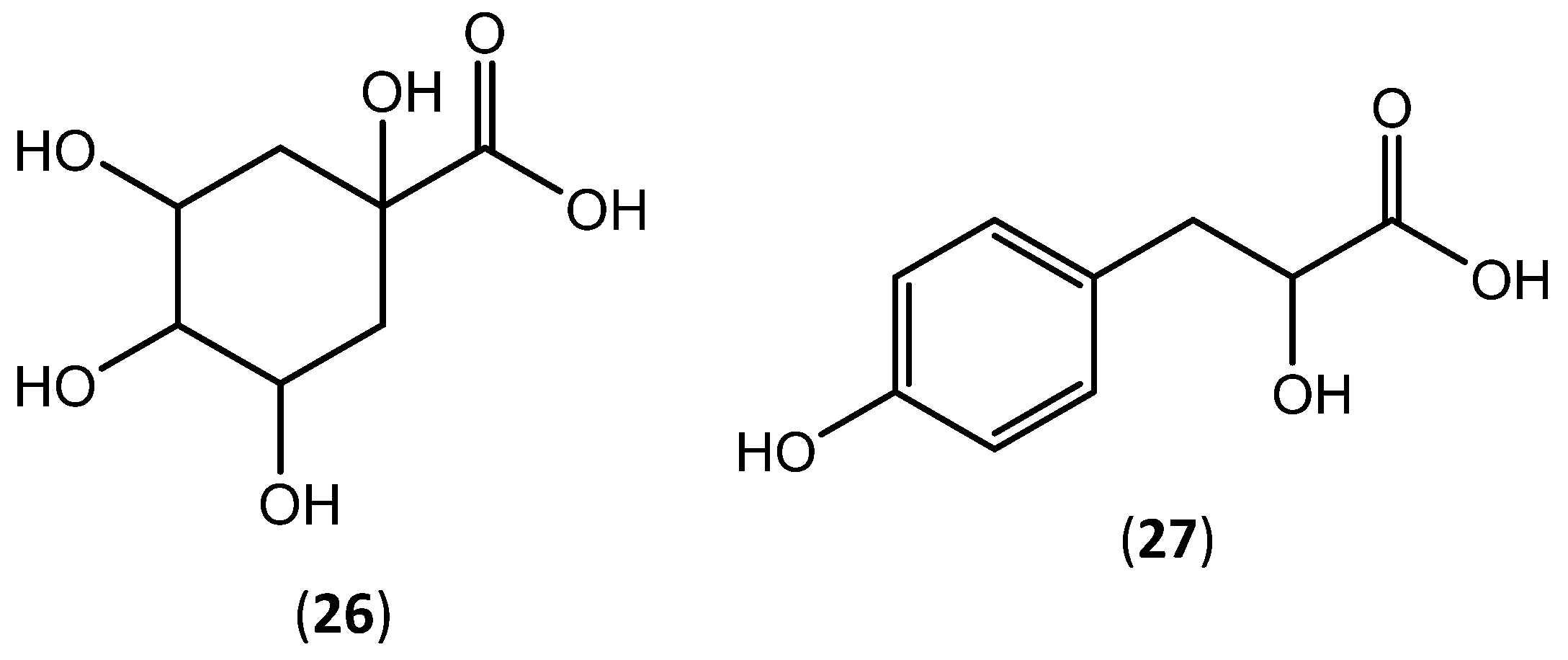
| Quinic acid (26) | Trace | ||||
| water granulated cork from Portugal | Ellagic acid (10) | 526.5 | mg of compound/kg of dry cork | Antioxidant activity (DPPH (IC50) = 5.84 ± 0.29 µg of extract/mL) | [80] |
| Gallic acid (12) | 241.6 | ||||
| Protocatechuic acid (11) | 118.3 | ||||
| Caffeic acid (15) | 12.9 | ||||
| p-hydroxybenzoic acid (25) | 1.0 | ||||
| p-hydroxyphenyllactic acid (27) | Trace | ||||
| Ellagic acid (10) | 1246.46 ± 0.18 | of dry cork | [24] | ||
| Ellagic acid-pentoside | 770.16 ± 0.15 | ||||
| Gallic acid (12) | 736.48 ± 1.63 | ||||
| Aesculetin (24) | 391.59 ± 1.10 | ||||
| methanol/water (50:50) granulated cork | Quinic acid (26) | 372.86 ± 1.94 | mg of compound/kg | Antioxidant activity (DPPH (IC50) = 4.77 ± 0.02 µg of extract/mL) | |
| Methyl gallate | 251.43 ± 0.06 | ||||
| Brevifolin-carboxylic acid | 102.03 ± 0.08 | ||||
| Protocatechuic acid (11) | 79.26 ± 0.10 | ||||
| Ferulic acid (14) | Trace | ||||
| Coniferyl aldehyde (18) | Trace | ||||
| p-hydroxyphenyllactic acid (27) | Trace | ||||
| Valoneic acid dilactone | 168.01 ± 0.70 | ||||
| Caffeic acid isoprenyl ester | 127.98 ± 0.28 | ||||
| Isorhamnetin-rhamnoside | Trace | ||||
| Eriodictyol | Trace | ||||
| Isorhamnetin | Trace | ||||
| methanol/water (50:50) cork powder (by-product) | Ellagic acid (10) | 527.59 ± 1.70 | mg of compound/kg of dry cork powder | Antioxidant activity (DPPH (IC50) = 3.33 ± 0.02 µg of extract/mL) | [24] |
| Gallic acid (12) | 263.04 ± 0.52 | ||||
| Aesculetin (24) | 176.80 ± 0.60 | ||||
| Quinic acid (26) | 137.02 ± 0.50 | ||||
| Methyl gallate | 96.93 ± 0.56 | ||||
| Ellagic acid-pentoside | 46.18 ± 0.15 | ||||
| Valoneic acid dilactone | 46.05 ± 0.11 | ||||
| Protocatechuic acid (11) | 16.44 ± 0.01 | ||||
| Ferulic acid (14) | 14.77 ± 0.02 | ||||
| Coniferyl aldehyde (18) | Trace | ||||
| Caffeic acid isoprenyl ester | 82.47 ± 0.29 | ||||
| Brevifolin-carboxylic acid | 53.72 ± 0.15 | ||||
| Isorhamnetin-rhamnoside | Trace | ||||
| Isorhamnetin | Trace | ||||
| methanol/water (50:50)black condensate (by-product) | Coniferyl aldehyde (18) | 194.34 ± 0.56 | mg of compound/kg of dry black condensate | Antioxidant activity (DPPH (IC50) = 1.57 ± 0.01 µg of extract/mL) | [24] |
| Aesculetin (24) | 125.28 ± 0.65 | ||||
| Gallic acid (12) | 118.46 ± 0.61 | ||||
| Quinic acid (26) | 117.17 ± 0.30 | ||||
| Ellagic acid (10) | 52.52 ± 0.18 | ||||
| p-hydroxyphenyllactic acid (27) | 49.36 ± 0.12 | ||||
| p-coumaric acid (7) | 35.76 ± 0.22 | ||||
| Vanillin (16) | 32.47 ± 0.25 | ||||
| Caffeic acid (15) | 17.68 ± 0.05 | ||||
| Protocatechuic acid (11) | 9.97 ± 0.03 | ||||
| Ferulic acid (14) | Trace | ||||
| Eriodictyol | Trace | ||||
| water/ethanol granulated cork | Castalagin (19) | 47.2 | mg of compound/g of extract | Antioxidant activity (DPPH (EC50) = 7.9 ± 0.02 µg of extract/mL; ORAC = 1533 ± 147 mgTeq/gextract; FRAP = 1963 ± 126 mgTeq/gextract; TEAC = 802 ± 8 mgTeq/gextract) | [90] |
| Vescalagin (21) | 22.8 | ||||
| Ellagic acid (10) | 26.5 | ||||
| β-O-ethylvescalagin | 24.4 | ||||
| Gallic acid (12) | 0.6 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, S.; Pinto, C.; Cravo, S.; Rocha e Silva, J.; Afonso, C.; Sousa Lobo, J.M.; Tiritan, M.E.; Cidade, H.; Almeida, I.F. Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application. Appl. Sci. 2022, 12, 4604. https://doi.org/10.3390/app12094604
Mota S, Pinto C, Cravo S, Rocha e Silva J, Afonso C, Sousa Lobo JM, Tiritan ME, Cidade H, Almeida IF. Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application. Applied Sciences. 2022; 12(9):4604. https://doi.org/10.3390/app12094604
Chicago/Turabian StyleMota, Sandra, Cláudia Pinto, Sara Cravo, Joana Rocha e Silva, Carlos Afonso, José Manuel Sousa Lobo, Maria Elizabeth Tiritan, Honorina Cidade, and Isabel Filipa Almeida. 2022. "Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application" Applied Sciences 12, no. 9: 4604. https://doi.org/10.3390/app12094604
APA StyleMota, S., Pinto, C., Cravo, S., Rocha e Silva, J., Afonso, C., Sousa Lobo, J. M., Tiritan, M. E., Cidade, H., & Almeida, I. F. (2022). Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application. Applied Sciences, 12(9), 4604. https://doi.org/10.3390/app12094604











