Abstract
(1) Background: Chorda tympani (CT) manipulation during stapes surgery affects its functions. We hypothesized that this alters tongue morphology and sensory functions. (2) Methods: Patients undergoing stapes surgery were tested 1 day preoperatively, 1 and 6 months postoperatively. Narrow band imaging contact endoscopy (NBI) was used to determine the number of fungiform papillae (Npapillae) and the total score of blood vessel morphology (NBItotal). The taste was tested with taste strips. General sensation was tested with a static two-point discrimination. Tests were performed on ipsilateral and contralateral side of the tongue. (3) Results: 52 otosclerosis patients were included in the study. There was a statistically significant decrease of NBItotal (p = 0.005), Npapillae (p = 0.009), sensation of sweet (p = 0.003), salty (p = 0.035), sour (p = 0.036), and bitter taste (p = 0.013) within the test side during the follow-up. A statistically significant impact on presence of dysgeusia for sweet was found 1 month postoperatively (p < 0.005). Postoperative decrease in two-point discrimination score did not reach a statistical significance (p = 0.056). (4) Conclusions: CT manipulation affects fungiform papillae density, vascular patterns and taste sensation. The general sensation of the tongue is not influenced by CT manipulation.
1. Introduction
Stapes surgery remains the procedure of choice for hearing rehabilitation in otosclerosis [1], even though this disease was described almost 400 years ago [2] Otosclerosis is a rather common cause of adult conductive hearing loss, with a clinical prevalence estimated at 0.3% to 0.38% [3].
During the stapes surgery, the chorda tympani (CT) nerve is manipulated, leaving the operated patient with potential consequences.
CT is considered as a “taste nerve”; however, other functions have also been discovered. The CT nerve is said to be responsible for non-gustatory information such as texture, temperature [4], and pain [5], for conveying the general sensations from the tongue [6] and for having parasympathetic fibers for submandibular and sublingual glands that stimulate salivary flow [5].
In humans, taste receptor cells are found on the tongue, palate, and other parts of the pharynx and epiglottis [7]. They are innervated by the branches of facial, glossopharyngeal and vagal nerves [8]. Taste receptor cells form taste buds that are organized in macroscopically visible structures named papillae. Papillae are named regarding their shape: foliate (can be found on the back lateral sides of the tongue), vallate (the largest among papillae, found on the dorsum of the posterior tongue) and mushroom-shaped fungiform papillae (FP) (located at the first 2/3 of the tongue). FP have complex sensory functions, including responses to chemical, thermal and mechanical stimuli [9].
FP of anterior 2/3 of the tongue are innervated by CT, a branch of the facial nerve [7]. However, according to animal studies, they could be dually innervated, including the trigeminal nerve fibers [10]. As described by Purohit et al., 2007 [11], the CT also innervates the contralateral side of the anterior 2/3 of the tongue, explaining the normal taste and FP morphology after ipsilateral CT injury. On the other hand, the number and morphology of papillae are expected to reflect the viability of the appropriate nerve. Sollars et al., 2000 [12] reported that neural damage leads to a decrease in the number of papillae. For that reason, the degeneration process of papillae after severing the CT nerve remains unclear [13].
Taste perception is reported to be influenced by the density and morphology of papillae. Consequently, the visualization of FP is essential [14,15], being the most anatomically accessible. This has been in the focus of scientific interest for many years, yet the techniques have evolved. In vivo techniques allow the visualization of papillae number [12], structure, and vascularization [14]. Some techniques of papillae quantification require the usage of food dye [16]. Very few studies concentrate on postoperative FP morphology [17]. Only one study [18] applies narrow-band imaging (NBI), a noninvasive technique to visualize FP vascular patterns of the anterior 2/3 of the tongue. NBI is a relatively new method of blood vessel visualization that illuminates the structures in narrowed red, green, and blue spectrum bands with interference filters. That increases the contrast between the epithelial surface and the subjacent vascular network [19]. It has been essential in evaluating different organ systems and has an enormous added value in assessing a lesion’s malignant potential [20]. To obtain detailed images of epithelium, NBI can be performed with the contact endoscopy technique [21,22].
The alterations in taste are not the only complaints after the stapes surgery. A tingling sensation and tongue numbness are common postoperative findings. The CT nerve is believed to confer the general sensory fibers to the anterior 2/3 of the tongue. A static two-point discrimination test proved to be a sensitive and specific tool for the tongue general sensation assessment [6].
Although there is a lot of evidence of CT function impairment in relation to stapes surgery, many questions remain unanswered. For that reason, this prospective clinical study aims to determine the effect of CT manipulation during stapes surgery on tongue morphological characteristics and sensory functions in 6 months postoperative follow-up. The study hypothesizes that CT manipulation affects tongue FP vascular patterns and density of FP, the taste, and the general sensation of the tongue.
2. Materials and Methods
The Slovenian National Medical Ethics Committee approved this prospective non-randomized clinical study (No. 0120-544/2019/6, date: 6 February 2020). It was registered on ref. [23] (NCT04881513). Written informed consent was obtained from each patient.
2.1. Enrollment
Patients undergoing stapes surgery for otosclerosis were enrolled between February 2020 and June 2021 during their regularly scheduled check-ups at five otologists (SB, IF, KJ, MH, and AM) at the tertiary otorhinolaryngology referral center.
2.1.1. Inclusion Criteria
- Age > 18 years;
- Patients with clinical otosclerosis diagnosed by the ENT specialist-otosurgeon.
2.1.2. Exclusion Criteria
- A recent modification of regular therapy [24];
- Recent onset of smoking [25];
- COVID-19 infection [26];
- History of taste disturbance at the enrolment;
- History of smell disturbance at the enrolment;
- History of facial palsy at the enrolment;
- Diseases of the oral cavity.
Patients were assessed by two-point discrimination, tongue morphology, and taste 1 day prior to surgery, 1 month (first check-up) and 6 months postoperatively (second check-up). Two-point discrimination was tested 6 h postoperatively, as well. The ipsilateral (i.e., ipsilateral to stapes surgery or test) and contralateral (i.e., contralateral to stapes surgery or control) sides of the tongue were tested. The stapes surgery was conducted under local anesthesia with an endaural approach.
2.2. Tongue Morphology Assessment with Narrow Band Imaging
The patient was instructed to keep the tongue out and still as much as possible. The FP blood vessels were visualized on the test and control side of the middle lateral area of the anterior 2/3 of the tongue using NBI contact endoscopy technique with a 0° otological endoscope (Olympus-Gyrus ACMI, Hamburg, Germany) and compatible equipment (Olympus Visera Pro PRO CLV S40 light source, and Olympus Visera Pro OTV -S7 PRO HD endoscopic camera system, Olympus, Tokyo, Japan). The videos of contact endoscopy were recorded using high-definition technique in an upscaled resolution of 1920 × 1080 pixels at 50 frames per second (Sony, Tokyo, Japan) symmetrically on the test and control side, avoiding the tongue tip. Endoscopy was performed by two independent observers (NBU and MH) with the standardized protocol.
Then, two independent observers (NBU and RŠ) performed analyses of recorded videos to determine blood vessel morphology and the number of FP. In unclear cases, the consensus was reached with a third researcher (SB). The morphological blood vessel evaluation was performed on both sides of the tongue from the recorded videos with the five-point scoring system (Table 1) [13,17].

Table 1.
NBI Morphological Blood Vessel Evaluation Grading System.
The number of fungiform papillae (Npapillae) was counted in the areas with the richest blood vessel morphology under the still picture from the recorded video (Figure 1). The total score of blood vessel morphology (NBItotal) was obtained by summing the points of papillae grades (Table 1) in the 20 mm² areas (using the special plasticized paper with the 20 mm² holes cut out (Figure 1A) of the richest vascular morphology graded. The mean score of blood vessel morphology (NBImean) was calculated by dividing the NBItotal by the Npapillae in the 20 mm² areas. All the evaluations were done using the contact endoscopy technique exclusively for all the measurements and regarded the unoperated side as the control side.
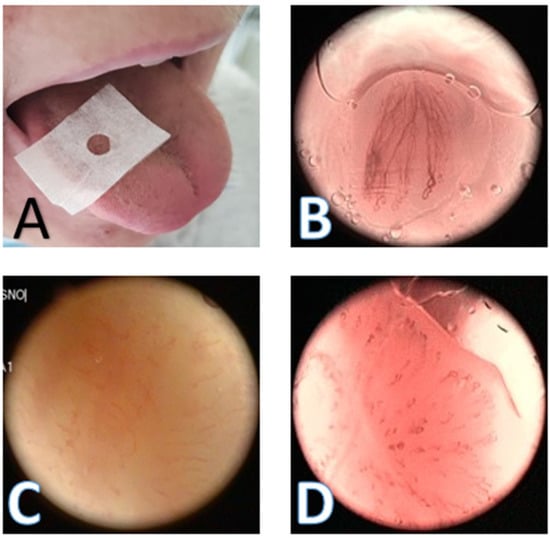
Figure 1.
NBI contact endoscopy. (A) The tongue with the special plasticized paper with the 20 mm² holes positioned in the anterior 2/3 of the lateral tongue; (B) the NBI contact endoscopy picture of FP type A; (C) the contact endoscopy picture of FP type D without the use of the NBI; and (D) the contact endoscopy picture of FP type D with the use of the NBI; NBI—Narrow band imaging; FP—Fungiform papillae.
2.3. The Taste Assessment
The sense of taste was tested using taste strips (Burghart Messtechnik GmbH, Tinsdaler Weg 175, Wedel, Germany). The participants were instructed not to eat, chew bubble gum, or smoke, and to drink only water 1 h before testing. Each side of the tongue was tested separately. During the testing, the tongue was out. To avoid the activation of other gustatory nerves, patients were instructed to define only tastes they sensed with their tongues before swallowing. Taste strips were placed on one side of the tongue and moved gently anteroposteriorly (regarding the size of the tongue) for up to 5 s, as needed. The sweet, sour, salty, and bitter tastes were tested in four concentrations, beginning with lower concentrations in a randomized manner following the producer’s instruction manual. Each correct answer yielded 1 point, with a maximum possible sum score of 4 points per taste tested. Tasteless strips were offered as well. After each strip, the patient rinsed the mouth with water. The anterior 1/3 of the tongue was avoided because of the possibility of dual innervation [10,27].
The dysgeusia was assessed with the same taste strips. The dysgeusia for sweet was present if a patient tasted sweet with any other taste strip (i.e., bitter, salty, or sour). Patients with no sensation of sweet taste, whether true or false (i.e., dysgeusia), were excluded from the analysis. The same method was applied to analyze bitter, salty, and sour dysgeusia.
2.4. Two-Point Discrimination
Patients were assessed for static two-point discrimination with the tongue out with a Discrim-a-gon 2-point Discriminator 12-1492 (D1) (3 B Scientific, Libertyville, IL, USA). The two-disc set was used with the distances between the rods from 1 to 15 mm. The patient’s tongue was touched for 3 s with the testing rods at 90 degrees to the tongue surface in the anterior 2/3 of the tongue in the lateral middle part symmetrically on both sides, avoiding the tongue tip and beginning with the rods that are more apart and proceeding to closer positioned rods (from 15 mm to 1 mm), taking care not to touch the tongue with three rods at the same time. Patients were asked to indicate whether they felt the touch of one or two rods by showing the number 1 or 2 with their fingers. When they showed one finger, we applied the one-rod-size up and down to confirm the result one more time. The smallest distance between the two rods that the patient sensed was taken as the definite result of tongue two-point discrimination testing. The procedure was then repeated on the other half of the tongue. The cooperation was checked from time to time by applying one rod to the tongue.
2.5. Surgical Procedure
After preparing the operative field, the mixture of local anesthetic (Bupivakain Grindeks 5 mg/mL sol., AS Grindeks, Krustpils iela 53, Riga, LV-1057, Latvia) and 0.4 mL of adrenaline (Suprarenin 1 mg/mL sol, Cheplapharm, Arzneimittel GmbH, Ziegelhof 24, Greifswald, Germany) was injected into the EAC meatus just lateral to the osseocartilaginous junction. The surgery was done using the operating microscope with the endaural approach. The tympanomeatal flap was elevated, and the bone covering the chorda tympani nerve was removed with the curette. With the use of the laser, the anterior and posterior crus of the stapes and the stapedius tendon were divided, and the footplate was perforated. Sometimes the footplate was perforated manually, using the perforator. The oval window was sealed with an autologous temporal muscle fascia or tragal perichondrium and a Schuknecht-type Teflon piston prosthesis (RN:2024-07-19, Medtronic Xomed, Jacksonville, FL, USA) was used. At the end of the surgical procedure, the surgeon graded the intraoperative CT manipulation from 1 to 5, as adapted from other authors [6]: (1) nonmanipulated, (2) minimal, (3) intermediate, (4) significant manipulation, and (5) transected.
2.6. Statistical Analysis
Statistical analysis was performed using Microsoft Excel for Windows and for Mac (Microsoft Corp., Redmond, WA, USA) and IBM SPSS (version 23, IBM Corp., Armonk, NY, USA). p < 0.05 was considered as statistically significant.
3. Results
In total, 52 patients (35% male, 65% female) aged 47 ± 9.4 years (48.2 ± 11.5 male, 45.7 ± 9.3 female) were enrolled in the study. Four patients skipped the first check-up (Mdn = 33 days) and four the second one (Mdn = 181 days).
Of them, 11 patients (21%: 5 female, 6 male) received regular therapy. One female patient received oral contraceptive, two levothyroxine sodium, and one bisoprolol and one patient received rosuvastatin, ezetimibe, bisoprolol, and acetylsalicylic acid. Two male patients received enalapril, two adalimumab, one secukinumab, and one patient received bupropion and pregabalin. In none of these patients did the treatment regimen change.
3.1. Tongue Morphology Assessment with NBI
Results of statistical analysis of NBItotal, Npapillae, and NBImean are summarized in Table 2, Table 3, Table 4, Figure 2 and Figure 3.

Table 2.
The NBItotal.

Table 3.
The Npapillae.

Table 4.
The NBImean.
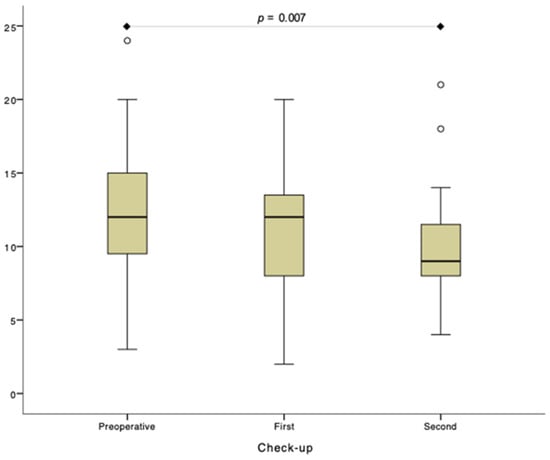
Figure 2.
The total score of blood vessel morphology analysis (NBItotal) within the test side. First check-up was performed 1 month and second check-up 6 months postoperatively.
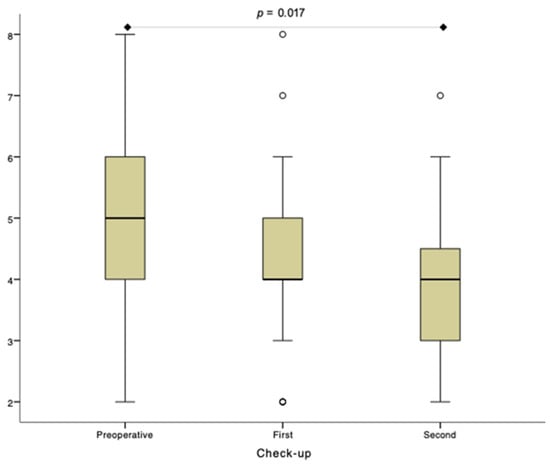
Figure 3.
The number of fungiform papillae (Npapillae) analysis within the test side. First check-up was performed 1 month and second check-up 6 months postoperatively.
3.1.1. The Total Score of Blood Vessel Morphology
Summarized data are presented in Table 2 and Figure 2. Results of detailed between-group and within-group statistical analysis are provided in Supplementary File S1.
Due to outliers in the preoperative and second check-up group, the Friedman test was run. There was a statistically significant difference in NBItotal between the preoperative and second check-up (p = 0.007). No statistically significant difference was observed between preoperative and first check-ups and between first and second check-ups (p > 0.05). p—p-value.
3.1.2. The Number of Fungiform Papillae
Summarized data are presented in Table 3 and Figure 3. Results of detailed between-group and within-group statistical analysis are provided in Supplementary File S2.
Due to outliers in the first and second check-up group, the Friedman test was run. There was a statistically significant difference in the Npapillae between the preoperative and second check-up (p = 0.017). No statistically significant difference was observed between preoperative and first check-ups and between first and second check-ups (p > 0.05). p—p-value.
3.1.3. The Mean Score of Blood Vessel Morphology
Summarized data are presented in Table 4. Results of detailed between-group and within-group statistical analysis are provided in Supplementary File S3.
3.2. The Taste Assessment
Summarized data are presented in Table 5. Results of detailed between-group and within-group statistical analysis are provided in Supplementary File S4.

Table 5.
Taste Strips Sum Score.
Dysgeusia Analysis

Table 6.
The Test Side.

Table 7.
The Control Side.
3.3. Two-Point Discrimination
Summarized data are presented in Table 8. Results of detailed between-group and within-group statistical analysis are provided in Supplementary File S5.

Table 8.
Two-Point Discrimination.
3.4. CT Manipulation
The data about the CT manipulation during stapes surgery are presented in Table 9. The statistical analyses were performed to test the assumptions prior to the planned correlation tests. Multiple scatter graphs were created to plot the grade of CT manipulation as an ordinal independent variable and one continuous dependent variable (difference between two check-ups in NBItotal, Npapillae, NBImean, taste strips sum score for each taste, and two-point discrimination). Visual inspection of these scatter graphs did not show any monotonic or non-monotonic relationship of the data. For that reason, statistical correlation tests were not performed. The detailed data are available in Supplementary File S6.

Table 9.
The Grade of Chorda Tympani Manipulation.
4. Discussion
This prospective tertiary center clinical study evaluated the influence of stapes surgery in a group of adult otosclerosis patients on FP density and blood vessel morphology with NBI contact endoscopy, taste function with taste strips, and tongue general sensation with static two-point discrimination in 6 months follow-up. Stapes surgery was proved to cause the decrease in the Npapillae, the NBItotal and sensation of the sweet, sour, salty, and bitter taste. Increased likelihood of dysgeusia for sweet taste was detected. Stapes surgery did not influence the static two-point discrimination scores. Therefore, the hypothesis that CT manipulation affects tongue FP vascular patterns and density of FP and the taste was confirmed. We rejected the hypothesis that the general sensation of the tongue was influenced by CT manipulation during stapes surgery.
4.1. NBI
Studies confirm the change of FP blood vessel morphology after the middle ear surgery [13]. Our research has proved the usefulness of contact endoscopy in tongue papillae assessment in combination with the NBI technology. To our knowledge, this is the first prospective NBI contact endoscopy study to compare the FP blood vessel morphology before and after surgery to be applied to both sides of the tongue of the same patient. Unlike some other NBI studies [18], we have used contact endoscopy exclusively for all the measurements. We have found that observational distances of 2, 5, 10, and 20 mm used by Shimada et al. [18] are difficult to obtain. Furthermore, they have a negative impact on the accuracy of the measurements. Contact endoscopy provides more detailed views and annulates the influence of tongue movements on the picture quality. We have also found the 0° otoendocope to be the most suitable instrument for the FP blood vessel assessment as it is small and easy to handle. Instead of taking pictures, we have filmed a larger portion of the tongue and analyzed the areas with the richest vasculature.
Our study has shown that stapes surgery negatively impacts the number of FP and the blood vessel morphology, which is consistent with Saito et al. (2001, 2014). Regarding some studies, taste buds disappear 50 days after severing the CT nerve [11]. Whitehead and colleagues showed that the hamster’s taste buds persisted 330 days after transection of the CT [28]. In contrast, in our group of patients, the number of FP decreased statistically significantly on the operated side during follow-up of 6 months (Figure 3 and Table 3). That could be explained by the human taste buds being more sensitive to CT manipulation than those of hamsters and the turnover of hamster taste buds being much slower. As there was no change in the FP number during the 1-month postoperative follow up, we can conclude that human FP persisted for at least 30 days after the CT nerve manipulation. That could indicate the human FP turnover time is between 1 and 6 months, which is consistent with the study by Perea-Martinez et al. [29] that estimates the half-life of cells in mammalian taste buds to be 8–22 days depending on the cell type. There was no decrease in NBImean, which could be explained by the reduction in the number of the papillae and the total score of blood vessel morphology. As the number of papillae has not changed on the other non-operated, our study does not confirm the contralateral CT influences on the anterior tongue as reported by others [10].
4.2. Taste
The taste strips are an excellent clinical tool for taste assessment [30]. Taste dysfunction after middle ear surgery is said to be dependent on the degree of CT manipulation [30]. Ciofalo et al. (2015) presented the results of 30 otosclerosis patients whose taste assessment on both sides of the tongue revealed no changes in the taste scores on the contralateral side of the tongue and the declining taste scores on the operated side in the early postoperative period. They have found the improvement of taste scores 6 to 12 months after surgery and, unfortunately, did not collect the data about postoperative dysgeusia [31]. Mueller et al. found a significant change in the taste strip scores on the operated side in patients with the considerable manipulation of the CT nerve, with no significant differences in patients with minor manipulation or on the contralateral side of the tongue [30]. These results are consistent with ours since we have found statistically significant differences in the test strips scores for all tastes after the stapes surgery.
On the other hand, the taste scores on the control side remained unchanged throughout the follow-up period. In our study, the only patient with the sectioned nerve had lost all the taste sensitivity on the sectioned nerve side. Some authors found the possible preservation of taste during the whole-mouth testing, regardless of the CT manipulation, which is explained by releasing CT’s inhibitory effect on the glossopharyngeal nerve [32,33]. Regardless of the sectioning of the CT nerve, Celis-Blaubach et al., 1970 [34] found the preserved ability to taste the acid in 43.5% of cases, which was explained by the possibility of acid fibers following a different pathway. To avoid the influence of other nerves, we have used the separate taste testing of both halves of the tongue, avoiding the tongue tip region, and instructed the patients to report the tastes felt with the tongue before swallowing the saliva.
Some authors speculate that a part of the taste afferent fibers innervating the anterior part of the tongue may bypass the tympanic cavity [35] and lead to the preservation of taste function [31] after the middle ear surgery. Our study indicates that there are probably no afferent CT taste fibers that would be bypassing the tympanic cavity or innervating the contralateral side of the tongue as well.
There are only a handful of studies exploring the characteristics of post-stapedectomy dysgeusia. Regarding a review of the stapes surgery complications by Bartel et al., 2021 [36] the rate of postoperative dysgeusia is said to be 10%, with no detailed description of the dysgeusia type. Molinari et al. [37] found only 3/48 (6.25%) of patients having a subjective (no objective taste testing performed) persistent dysgeusia 6 months after endoscopic stapes surgery, regardless of the preservation of the CT nerve. In microscopic surgical approaches, the percentage of dysgeusia is said to be even higher, up to 44.1% [38]. Regarding the postsurgical dysgeusia type, the most pronounced early postoperative changes were documented in a sweet and bitter taste, with the late recovery primarily of bitter taste [39]. Other studies found the most alterations in the sweet and salty taste qualities [40]. Guder et al. [41] found taste impairment on the ipsilateral side of the tongue after 3 days and the improvement within 3 months of surgery, not explicitly concentrating on individual tastes. We have found that stapes surgery has a statistically significant impact on the presence of dysgeusia for sweet taste up to 1 month postoperatively.
4.3. General Sensation
Somatosensory receptors of the tongue have small receptive fields, which is why they have a superior two-point discrimination ability compared to other regions of the body [42]. The tongue tip is one of the most sensitive tactile sites of the body [9]. Previous studies advocated that there might be a relation between FP density and tactile perception [43]. The studies addressing the tongue general sensation after the stapes surgery are scarce. We have found the decrease in the static two-point discrimination testing after otosclerosis surgery that was statistically insignificant (p = 0.056), as Perez et al., 2006 [6] showed when considering the late-stage follow-up period. Inconsistently, in our patient with the transected nerve, the sensory function regarding the static two-point discrimination test remained unchanged in the 6-month postoperative period. It is interesting that the same findings considering the patient with the transected nerve were found by Perez et al., 2006 [6].
One possible explanation could be that the CT nerve carries only a small number of sensory fibers to the tongue. That’s why some patients complain of tongue numbness or tingling sensations for some weeks after surgery, and then eventually those sensations disappear. Perez et al. (2006) also found the static two-point discrimination recovered during the follow-up period [6]. The main sensory nerve of the anterior 2/3 of the tongue is the trigeminal nerve. There are said to be CT and trigeminal nerve interactions that can occur at the periphery or at the central level [44]. Results of our study do not support the theories of CT having the fibers for the tongue general sensation. Due to these inconsistencies and the small body of literature that includes animal studies [12,28], retrospective studies [45], small groups [13,41], and unilateral tongue analysis [18], there is an urge for multicentric prospective randomized controlled clinical trials.
4.4. CT Manipulation
The data about the influence of the degree of CT manipulation on morphological and functional changes of the tongue FP are scarce. Mueller et al., 2008 [29] have found more prominent taste change in the group of patients with significant CT manipulation during surgery, while Guder et al., 2012 [41] found the changes in taste even after minor CT manipulation. We did not find any relationship between the degree of CT manipulation during surgery and the severity of taste disturbance after stapes surgery, the change in number and morphology of FP or the change in two-point discrimination score. Our data were too scattered. The reason could lie in the subjectiveness of the grading system.
4.5. Perspective of CT Preservation
The preservation of the CT nerve could lower the incidence of the morphology and FP function change after stapes surgery. The endoscopic approach is said to enable the surgeon to reach better views of the operation field and avoid the manipulation of the CT nerve [38,46]. Injury to the nerve was said to be more than three times more likely to happen with the microscopic approach [47]. In our department, all surgeons use the microscopic approach in stapes surgery and should be stimulated to start applying other CT preservation techniques. On the other hand, Szmuda et al., 2020 [48] explored the new techniques of intraoperative nerve preservation. They have tested the preoperative visualization of the facial nerve by magnetic resonance-based diffusion tensor imaging-fiber tracking with neuronavigation system integration in patients with cerebellopontine angle tumors. Similar methods could be used in planning the stapes surgery in order to preserve the facial nerve and its branches, including CT.
4.6. Advantages and Limitations
Additional advantages of our study are its prospective design and relatively long and regular follow-up period, despite the epidemiological restrictions. Another advantage is taking the contralateral side of the tongue as a control, which annulates the effect of less-known between-subject differences that could influence the taste, tongue general sensation, and FP number and morphology [47]. Moreover, there were no within-subject (i.e., between sides) differences of these measurements at the enrolment.
The disadvantages are a relatively low number of patients included in the final analysis, which is also a consequence of the COVID-19 pandemic, which resulted in patients being rescheduled or lost to follow-up because of COVID-19.
5. Conclusions
That prospective, 6-month follow-up clinical study confirms the negative impact of stapes surgery on sweet, salty, sour, and bitter taste and tongue morphology with taste strips and narrow-band imaging contact endoscopy (NBI). This trial provides the sound basis for the routine clinical use of NBI in papillae evaluation in other medical conditions. It offers physiological insights into chorda tympani functions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app12073248/s1, Supplementary File S1: Results of detailed between-group and within-group statistical analysis of the total score of blood vessel morphology. Supplementary File S2: Results of detailed between-group and within-group statistical analysis of the number of fungiform papillae. Supplementary File S3: Results of detailed between-group and within-group statistical analysis of the mean score of blood vessel morphology. Supplementary File S4: Results of detailed between-group and within-group statistical analysis of the taste assessment. Supplementary File S5: Results of detailed between-group and within-group statistical analysis of the two-point discrimination. Supplementary File S6: Chorda tympani manipulation analyses.
Author Contributions
Conceptualization: N.B.U. and S.B.; methodology: N.B.U., S.B., J.U. and R.Š.; software: N.B.U., J.U. and D.V.; validation: N.B.U., R.Š. and S.B.; formal analysis: N.B.U. and D.V.; investigation: N.B.U., D.V.,N.S., M.H., I.F. and S.B.; resources: N.B.U., M.H., I.F. and S.B.; data curation: N.B.U., D.V., J.U., R.Š. and S.B.; writing—original draft preparation: N.B.U. and S.B.; writing—review and editing: N.B.U., D.V., N.S., S.B.; visualization: N.B.U. and D.V.; supervision: S.B.; project administration: N.B.U. and S.B.; funding acquisition: S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received a grant from the University Medical Centre Ljubljana (no. 20210092) and the Slovenian Research Agency (no. P3-0374).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The Slovenian National Medical ethical committee approved this prospective non-randomized clinical study (No. 0120-544/2019/6, 13 December 2018). It was registered at ref. [23] (NCT04881513).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be found at the Department of Otorhinolaryngology and Cervicofacial Surgery, University Medical Centre Ljubljana, Zaloska 2, 1000 Ljubljana, Slovenia.
Acknowledgments
To Klemen Jenko and Aleš Matos for inclusion of their patients in this trial.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McElveen, J.T.; Kutz, J.W. Controversies in the Evaluation and Management of Otosclerosis. Otolaryngol. Clin. N. Am. 2018, 51, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, R.; McElveen, J.T.; Eshraghi, A.A. History of Otosclerosis and Stapes Surgery. Otolaryngol. Clin. N. Am. 2018, 51, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Declau, F.; Van Spaendonck, M.; Timmermans, J.P.; Michaels, L.; Liang, J.; Qiu, J.P.; Van de Heyning, P. Prevalence of Otosclerosis in an Unselected Series of Temporal Bones. Otol. Neurotol. 2001, 22, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McManus, L.J.; Dawes, P.J.D.; Stringer, M.D. Clinical anatomy of the chorda tympani: A systematic review. J. Laryngol. Otol. 2011, 125, 1101–1108. [Google Scholar] [CrossRef]
- Perez, R.; Fuoco, G.; Dorion, J.M.; Ho, P.H.; Chen, J.M. Does the chorda tympani nerve confer general sensation from the tongue? Otolaryngol.-Head Neck Surg. 2006, 135, 368–373. [Google Scholar] [CrossRef]
- Bear, M.F.; Connors, B.W.; Paradiso, M.A. Neuroscience Exploring the Brain, 15th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; ISBN 978-0781778176. [Google Scholar]
- Northcutt, R.G. Taste buds: Development and evolution. Brain. Behav. Evol. 2004, 64, 198–206. [Google Scholar] [CrossRef]
- Yokota, Y.; Bradley, R.M. Geniculate Ganglion Neurons are Multimodal and Variable in Receptive Field Characteristics. Neuroscience 2017, 367, 147–158. [Google Scholar] [CrossRef]
- Berteretche, M.-V.; Eloit, C.; Dumas, H.; Talmain, G.; Herman, P.; Tran Ba Huy, P.; Faurion, A. Taste deficits after middle ear surgery for otosclerosis: Taste somatosensory interactions. Eur. J. Oral Sci. 2008, 116, 394–404. [Google Scholar] [CrossRef]
- Purohit, N.; Pratap, R.; Chawla, O.P. Bitter sweet tympani. J. Laryngol. Otol. 2007, 121, e3. [Google Scholar] [CrossRef]
- Sollars, S.I.; Bernstein, I.L. Neonatal chorda tympani transection permanently disrupts fungiform taste bud and papilla structure in the rat. Physiol. Behav. 2000, 69, 439–444. [Google Scholar] [CrossRef]
- Saito, T.; Ito, T.; Ito, Y.; Kato, Y.; Manabe, Y.; Narita, N. Degeneration Process of Fungiform Taste Buds After Severing the Human Chorda Tympani Nerve—Observation by Confocal Laser Scanning Microscopy. Otol. Neurotol. 2015, 36, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Ali, S.; Jameela, R.V.; Muhamood, M.; Haqh, M.F. Impact of fungiform papillae count on taste perception and different methods of taste assessment and their clinical applications: A comprehensive review. Sultan Qaboos Univ. Med. J. 2019, 19, e184–e191. [Google Scholar] [CrossRef] [Green Version]
- Negoro, A.; Umemoto, M.; Fukazawa, K.; Terada, T.; Sakagami, M. Observation of tongue papillae by video microscopy and contact endoscopy to investigate their correlation with taste function. Auris Nasus Larynx 2004, 31, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-H.; Zhang, H.-Y.; Wang, X.-F.; Zhan, Y.-H.; Deng, S.-P.; Qin, Y.-M. The Relationship between Fungiform Papillae Density and Detection Threshold for Sucrose in the Young Males. Chem. Senses 2008, 34, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Shahbake, M.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Rapid quantitative assessment of fungiform papillae density in the human tongue. Brain Res. 2005, 1052, 196–201. [Google Scholar] [CrossRef]
- Shimada, T.; Kamada, H.; Hoshino, R.; Okamiya, T.; Takahashi, K.; Chikamatsu, K. Development of a new method using narrow band imaging for taste assessment. Laryngoscope 2013, 123, 2405–2410. [Google Scholar] [CrossRef]
- Kuznetsov, K.; Lambert, R.; Rey, J.-F. Narrow-Band Imaging: Potential and Limitations. Endoscopy 2006, 38, 76–81. [Google Scholar] [CrossRef]
- Šifrer, R.; Rijken, J.A.; Leemans, C.R.; Eerenstein, S.E.J.; van Weert, S.; Hendrickx, J.-J.; Bloemena, E.; Heuveling, D.A.; Rinkel, R.N.P.M. Evaluation of vascular features of vocal cords proposed by the European Laryngological Society. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Šifrer, R.; Šereg-Bahar, M.; Gale, N.; Hočevar-Boltežar, I. The diagnostic value of perpendicular vascular patterns of vocal cords defined by narrow-band imaging. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1715–1723. [Google Scholar] [CrossRef]
- Just, T.; Pau, H.W.; Witt, M.; Hummel, T. Contact endoscopic comparison of morphology of human fungiform papillae of healthy subjects and patients with transected chorda tympani nerve. Laryngoscope 2006, 116, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- The Change of the Structure and Function of Taste Buds After the Otosclerosis Surgery. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04881513?cond=otosclerosis&draw=3&rank=12 (accessed on 12 January 2022).
- Wang, T.; Glendinning, J.; Grushka, M.; Hummel, T.; Mansfield, K. Drug-Induced Taste Disorders In Clinical Practice And Preclinical Safety Evaluation. Toxicol. Sci. 2017, 156, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vennemann, M.M.; Hummel, T.; Berger, K. The association between smoking and smell and taste impairment in the general population. J. Neurol. 2008, 255, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M.; et al. More Than Smell—COVID-19 Is Associated With Severe Impairment of Smell, Taste, and Chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Ikeda, M.; Okuda, Y. Basis and Practice of Clinical Taste Examinations. Auris Nasus Larynx 1986, 13, S1–S15. [Google Scholar] [CrossRef]
- Whitehead, M.C.; Frank, M.E.; Hettinger, T.P.; Hou, L.T.; Nah, H.D. Persistence of taste buds in denervated fungiform papillae. Brain Res. 1987, 405, 192–195. [Google Scholar] [CrossRef]
- Perea-Martinez, I.; Nagai, T.; Chaudhari, N. Functional Cell Types in Taste Buds Have Distinct Longevities. PLoS ONE 2013, 8, e53399. [Google Scholar] [CrossRef] [Green Version]
- Mueller, C.A.; Khatib, S.; Naka, A.; Temmel, A.F.P.; Hummel, T. Clinical assessment of gustatory function before and after middle ear surgery: A prospective study with a two-year follow-up period. Ann. Otol. Rhinol. Laryngol. 2008, 117, 769–773. [Google Scholar] [CrossRef]
- Ciofalo, A.; Zambetti, G.; Romeo, M.; Vestri, A.R.; Iannella, G.; Re, M.; Magliulo, G. Taste and Olfaction in Middle Ear Surgery. Ann. Otol. Rhinol. Laryngol. 2015, 124, 312–316. [Google Scholar] [CrossRef]
- Lehman, C.D.; Bartoshuk, L.M.; Catalanotto, F.C.; Kveton, J.F.; Lowlicht, R.A. Effect of anesthesia of the chorda tympani nerve on taste perception in humans. Physiol. Behav. 1995, 57, 943–951. [Google Scholar] [CrossRef]
- Kveton, J.F.; Bartoshuk, L.M. The Effect of Unilateral Chorda Tympani Damage on Taste. Laryngoscope 1994, 104, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Celis-Blaubach, A. Chordal Dysgeusia. Arch. Otolaryngol.-Head Neck Surg. 1970, 92, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Compston, A. From The Archives. Brain 2013, 136, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, R.; Sanz, J.J.; Clemente, I.; Simonetti, G.; Viscacillas, G.; Palomino, L.; Asarta, I.; Lao, X. Endoscopic stapes surgery outcomes and complication rates: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2673–2679. [Google Scholar] [CrossRef] [PubMed]
- Molinari, G.; Reale, M.; Bonali, M.; Anschuetz, L.; Lucidi, D.; Presutti, L.; Alicandri-Ciufelli, M. Taste impairment after endoscopic stapes surgery: Do anatomic variability of chorda tympani and surgical technique matter?: Post-operative dysgeusia after EStS. Eur. Arch. Oto-Rhino-Laryngol. 2021. [Google Scholar] [CrossRef]
- Gulsen, S.; Karatas, E. Comparison of surgical and audiological outcomes of endoscopic and microscopic approach in stapes surgery. Pak. J. Med. Sci. 2019, 35, 1387–1391. [Google Scholar] [CrossRef] [Green Version]
- Skoloudik, L.; Krtickova, J.; Haviger, J.; Mejzlik, J.; Chrobok, V. Changes of taste perception after stapes surgery: A prospective cohort study. Eur. Arch. Oto-Rhino-Laryngol. 2021, 279, 175–179. [Google Scholar] [CrossRef]
- Landis, B.N.; Beutner, D.; Frasnelli, J.; Hüttenbrink, K.B.; Hummel, T. Gustatory function in chronic inflammatory middle ear diseases. Laryngoscope 2005, 115, 1124–1127. [Google Scholar] [CrossRef]
- Guder, E.; Böttcher, A.; Pau, H.W.; Just, T. Taste function after stapes surgery. Auris Nasus Larynx 2012, 39, 562–566. [Google Scholar] [CrossRef]
- Moritz, J.; Turk, P.; Williams, J.D.; Stone-Roy, L.M. Perceived Intensity and Discrimination Ability for Lingual Electrotactile Stimulation Depends on Location and Orientation of Electrodes. Front. Hum. Neurosci. 2017, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- Bangcuyo, R.G.; Simons, C.T. Lingual tactile sensitivity: Effect of age group, sex, and fungiform papillae density. Exp. Brain Res. 2017, 235, 2679–2688. [Google Scholar] [CrossRef] [PubMed]
- Boucher, Y.; Simons, C.T.; Faurion, A.; Azérad, J.; Carstens, E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Res. 2003, 973, 265–274. [Google Scholar] [CrossRef]
- Saito, T.; Manabe, Y.; Shibamori, Y.; Yamagishi, T.; Igawa, H.; Tokuriki, M.; Fukuoka, Y.; Noda, I.; Ohtsubo, T.; Saito, H. Long-term follow-up results of electrogustometry and subjective taste disorder after middle ear surgery. Laryngoscope 2001, 111, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, B.; Hunter, J.B.; Rivas, A. Endoscopic Stapes Surgery. Otolaryngol. Clin. N. Am. 2018, 51, 415–428. [Google Scholar] [CrossRef]
- Koukkoullis, A.; Tóth, I.; Gede, N.; Szakács, Z.; Hegyi, P.; Varga, G.; Pap, I.; Harmat, K.; Németh, A.; Szanyi, I.; et al. Endoscopic versus microscopic stapes surgery outcomes: A meta-analysis and systematic review. Laryngoscope 2020, 130, 2019–2027. [Google Scholar] [CrossRef] [Green Version]
- Szmuda, T.; Słoniewski, P.; Ali, S.; Pereira, P.M.G.; Pacholski, M.; Timemy, F.; Sabisz, A.; Szurowska, E.; Kierońska, S. Reliability of diffusion tensor tractography of facial nerve in cerebello-pontine angle tumours. Neurol. Neurochir. Pol. 2020, 54, 73–82. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).