Featured Application
An improvement in adhesion (gluing) in the printing production process.
Abstract
In this study, the effect of air plasma on the surface properties of printed and coated cardboard was investigated. The material was activated by low-pressure cold plasma for 1, 10, 20, and 30 s. Wettability changes on the surface were examined by contact angle measurements using the sessile droplet technique. The differences in the surface free energy were calculated using the Lifshitz–van der Waals/acid–base and Contact Angle Hysteresis approaches. Optical profilometry was used for the surface roughness evaluation and an X-ray photoelectron spectroscopy analysis was performed to find changes in surface chemistry. Adhesive strength tests were carried out to estimate the adhesion changes after the material’s modification. It was found that the water and formamide contact angles increased after the plasma treatment while the diiodomethane contact angle did not change. As a result of the modification, the surface free energy also increased significantly and the surface roughness increased. The pull-off tests confirmed the improvement in the material’s surface properties. Moreover, it was demonstrated that the optimal effect can be obtained after just 10 s of the plasma process.
1. Introduction
Plasma is the fourth state of matter, which is quite common in space and rare on the Earth. Research on the scientific application of plasma has been carried out for many years accompanied by attempts at technological application of the unique properties of the fourth state of matter. From the point of view of modifying the surface of a solid, the most useful plasma is low-pressure cold plasma, commonly called cold or non-thermal plasma. Its density is in the order of 1014–1018 electrons per m3. Its particularly important feature is the differentiated temperature of electrons (Te) and other components of the gas (Tg). Colloquially, it is said that in non-thermal plasma electrons are “hot” while the other plasma particles are “cold”. Usually, Te/Tg ≈ 10/100, which at the electron temperature Te = 104–105 K gives an average temperature equal to about 500 K for the ions and 300 K for the remaining particles. Thus, most of the electricity is used to produce high-energy electrons, not to heat the gas. In this way, the plasma energy is channeled into dissociation caused by collisions with electrons and gas ionization.
Figure 1 shows the energy transformation and the phenomena accompanying the generation of non-thermal plasma. Such plasma has no destructive properties and does not cause thermal damage, while the concentration and energy of the electrons are high enough to initiate many chemical processes [1,2]. The mechanisms between the plasma and the modified surface are very complex and include bombardment with high-energy particles and photons of vacuum ultraviolet radiation and chemical reactions that take place on the surface. As a result, four main processes take place on the plasma-treated surface: (1) plasma cleaning, in which the surface is physically cleaned by ion bombardment; (2) physical etching, in which spraying and properly selected gas and plasma parameters can have effects similar to those of mechanical “roughening”, e.g., a contact surface increase; (3) chemical etching, which can be used to obtain appropriate surface properties and the production of specific functional groups that enable specific interactions with other groups; and (4) plasma polymerization, which can be used to obtain a thin polymer layer with unique physicochemical properties [1,2,3,4,5]. The construction of better-quality plasma-generating equipment has allowed us to extend the use of surface activation to such materials as paper. Cornelius et al. [6] investigated the influence of atmospheric plasma on paper pulps and used dielectric barrier discharge plasma for the substrate activation. The electrodes used 4.8 kW of power, and audio frequency (5 kHz) plasma was used. Helium with the addition of oxygen, carbon tetrafluoride, and hexafluoropropylene gases at a flow of 10.00 L/min was used as a gas for the substrate activation. The plasma treatment time was 1 min. Generally, the plasma treatment resulted in a pulp density decrease. As was found during tests, plasma influenced the surface properties. The bulk properties of the paper sheets obtained from the activated pulp did not differ from those obtained from the untreated pulp. Yang et al. [7] reported on a new method for the preparation of a durable biphilic surface on cellulose filter paper by an atmospheric air plasma treatment. The filter paper, which was pre-applied by different solutions, was placed in the discharge area and treated using a dielectric barrier discharge plasma apparatus at a control voltage of 30 V for 5 min. After the treatment, the paper was used for a multiple-layer silica coating (the first layer contained hydrophobic particles and the second layer contained hydrophilic ones). The obtained materials were used for water/oil emulsion separation. It was proved that the atmospheric air plasma influenced the reaction between the silanes, silica particles, and cellulose substrate. Generally, the plasma treatment resulted in high separation efficiency as well as a coating with excellent durability and stability. Pykönen et al. [8] investigated hydrophobic plasma coatings on 90 g/m2 pigment-coated paper. They carried out their research in a laboratory as well as in a pilot-scale installation. Ethylene was used for the hydrocarbon coating and hexamethyldisiloxane (HMDSO) and perfluorohexane were used for the organosilicon coating at pressures of 49, 25, and 140 mTorr. The power of the radio frequency generator was 20–40 W, and the treatment time was 1–2 min. In the case of the pilot-scale installation, atmospheric plasma made by a 400-W generator using HMDSO and acetylene was applied and the treatment line speed was 4–6 m/min. The plasma coating’s uniformity appeared to be a critical factor influencing mottling in the offset printing. An interesting application of plasma was proposed by Vohrer et al. [9], where it was used for paper restoration. Many paper documents are collected and stored in libraries. These materials can be contaminated by microbes as well as oxidized or acidified. Aged groundwood paper and a specially designed after-glow plasma chamber were used for the experiments. In the upper part of the chamber, 13.56 MHz RF plasma was generated. The upper chamber was separated from the lower chamber so that the plasma did not interact directly with the sample. After the plasma treatment, no visible degradation of the paper was found. The hydrogen plasma restored the original tensile strength, while the oxygen plasma caused greater inhibition of microbial activity.
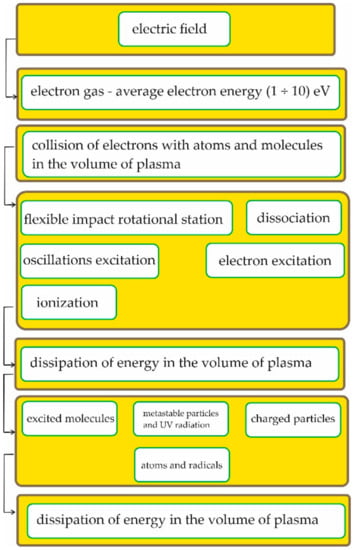
Figure 1.
Energy transformation and the phenomena accompanying the generation of non-thermal plasma.
In our previous paper [10], plasma treatment was investigated in the case of the gluing of paper composite materials. A paper sheet was glued to metallized polyester foil and the effect of plasma treatment was investigated for the packaging industry. In this paper, the influence of plasma treatment on 270 g/m2 paper was studied. The main goal was to determine whether plasma treatment can promote the gluing of offset printing paper sheets. Gluing these types of materials is a difficult task because the joints are usually weak and do not withstand extended use. The sheets are used as material for the production of cosmetic packages, and the edge that is glued is usually roughened, which leads to the loss of a large amount of material.
2. Materials and Methods
2.1. Materials and Reagents
The material used for the research was obtained from the El-Press company (Lublin, Poland), who uses it in the production of packaging. A large sheet of GC1-type cardboard with a grammage of 270 g/m2 was printed on one side by the offset technique and then protected by a UV coating (lacquer). For the experiment, 2 × 2 cm samples were cut out and the printed side was used.
Water (Milli-Q™, Merck, Darmstadt, Germany), diiodomethane (for synthesis, Sigma-Aldrich, St. Louis, MO, USA), and formamide (AR grade, POCH S.A., Lublin, Poland) were used for the contact angle measurements.
2.2. Sample Modification
The samples were activated by cold plasma using the low-pressure Pico system (Diener Electronic HmbH, Ebhausen, Germany). The pressure in the chamber was fixed to 0.2 mbar and air was used as the carrier gas with the flow set to 20 sccm. The power of the generator was set at 100%, and the process lasted for 1, 10, 20, or 30 s. The full record of the samples and its parameters is shown in the Table 1 below.

Table 1.
Parameters of the plasma activation of the samples.
2.3. Analysis of Samples
2.3.1. Optical Profilometry
The topography of the initial and plasma-treated samples was investigated by optical profilometry using a ContourGT 3D Optical Profiler (Bruker, Billerica, MA, USA) and Vision64® Software in order to evaluate the roughness parameters. A sample area with dimensions of 0.9 × 1.3 mm was probed, which corresponds to the surface area occupied by the 6-µL droplet used for the contact angle measurements.
2.3.2. X-ray Photoelectron Spectroscopy
An Ultra-High Vacuum analytical system (Prevac, Rogów, Poland) equipped with a Scienta MX 650 X-ray source and an R4000 high-resolution electron energy analyzer was used to record the XPS spectra in order to study the changes in the surface’s chemical composition after the plasma modification.
2.3.3. Contact Angle Measurements
The contact angle measurements were made at room temperature using the sessile drop method. A DigiDrop Contact Angle Meter (GBX, Romans-sur-Isère, France) apparatus and WinDrop++ software were applied as follows. A 6-µL droplet was settled onto the examined surface and the advancing contact angle was measured, then 2 µL of water was sucked from the droplet into the syringe and the receding contact angle was measured. Then, the advancing and receding contact angles for each surface were averaged from 10 measurements and the equilibrium contact angles were calculated using the Tadmor approach [11]:
where is the equilibrium contact angle, is the advancing contact angle, and is the receding contact angle. and are given by:
2.3.4. Surface Free Energy Determination
The Lifshitz–van der Waals/acid–base (LWAB) approach proposed by van Oss, Chaudhury, and Good [12] was used for the surface free energy determination based on the equilibrium contact angles as follows:
where is the surface free energy, is the Lifshitz–van der Waals component, is the acid (electron-acceptor) component, and is the base (electron-donor) component.
In addition, the contact angle hysteresis (CAH) method proposed by Chibowski [13] was used to compare the approaches as well as the surface properties:
where is the surface free energy, is the surface tension of the test liquid, is the advancing contact angle, and is the receding contact angle.
2.3.5. Adhesive Strength Tests
The force required for the detachment of two components joined by an adhesive can be determined by the peel adhesion test. We carried out 180-degree peel adhesion tests as follows. Two samples (a non-modified sample and a sample modified by plasma) with dimensions of 1 cm × 9.7 cm were glued together with Jowacoll® 764.60 glue (Jowat, Sady near Poznań, Poland), which is a one-component copolymer dispersion commonly used by printing companies. The adhesion area was equal to 9.7 cm2 and the adhesive was applied with a brush. The amount of applied glue was about 0.07 g, which corresponds to an approximately 0.7 mm glue layer. Then, the end of one sample was mounted in a holder while that of the other sample was attached to a dynamometer so that the force could act along the joint at an angle of 180 degrees downwards toward the fixed sample as illustrated in Figure 2.
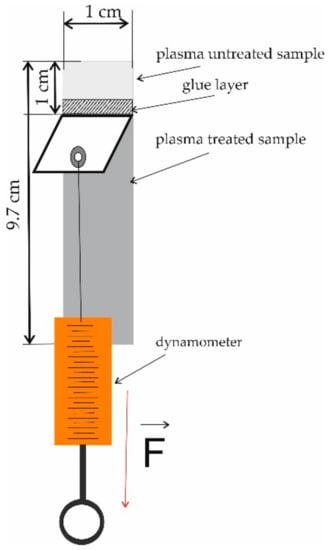
Figure 2.
Scheme of the adhesive strength test.
Then, strength was gradually applied to the dynamometer until the weld was broken or the sample was destroyed. Each set contained three repetitions. The maximum measuring range in the apparatus is up to 1 N, which is perfectly sufficient for printing applications.
3. Results and Discussion
The water, formamide, and diiodomethane equilibrium contact angles measured on the examined samples are presented in Figure 3. On the initial (non-modified) sample, the water contact angle was equal to about 67.2 degrees. The air plasma modification caused a decrease in the contact angle value, and this effect can be seen in all samples. For the one-second plasma treatment, the water contact angle decreased by about 20 degrees, while for longer modification times its value decreased to less than 10 degrees and the surface became more hydrophilic. However, the effect obtained for modification times longer than 10 s was found to be comparable. This is consistent with the results obtained by Pykönen et al., who reported that, in the case of plasma hydrophobization of paper, modification times longer than 5 s did not affect the material’s surface properties significantly [8]. A similar tendency can be observed in the case of the second polar liquid (formamide). However, Figure 2 shows that the plasma modification of the samples did not affect the diiodomethane contact angles significantly. This was due to the different types of interactions between the test liquid and the surface. Zhao et al. reported that the diiodomethane surface tension only has a non-polar (dispersive) component; thus, the liquid interacts with the surface only in a dispersive way [14]. Based on this, it can be concluded that the air plasma modification did not affect this kind of interaction.
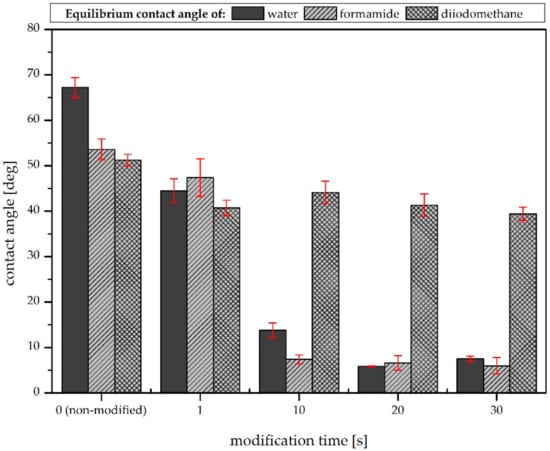
Figure 3.
Surface wettability (water, formamide, and diiodomethane equilibrium contact angles) versus modification time.
Figure 4 shows that the surface free energy of the material increased as the plasma modification time increased regardless of the approach used to estimate its value. In the case of the CAH theory and the use of water as a probe liquid, the surface free energy increased as the modification time increased up to 10 s; longer treatment times did not affect the energy values. Similar results were obtained using formamide as the test liquid. In the case of diiodomethane, no significant changes in the energy between the samples were observed.
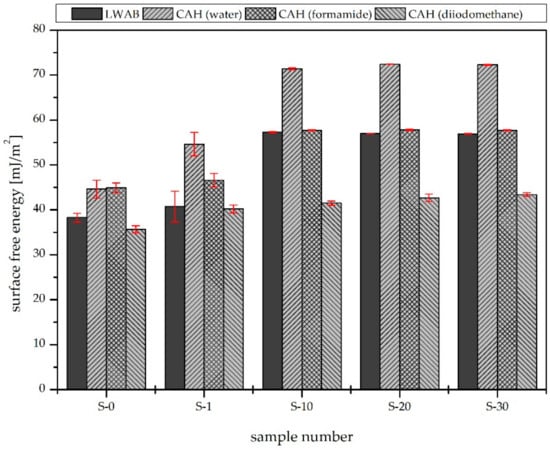
Figure 4.
Surface free energy calculated using the LWAB and CAH approaches.
The LWAB approach shows increasing surface free energy values that are compatible with the CAH results for the polar liquids (water and diiodomethane). More details are provided in Figure 5, which shows the dispersive and polar components of the surface free energy. It can be observed that the air plasma treatment only changed the polar component of the material’s surface free energy significantly. A similar effect was observed in the case of air plasma modification of a polymer [15]. This might be due to the introduction of hydrophilic groups, such as C=O, –OH, and –COO, on the surface [16], which was confirmed by the XPS analysis. According to the LWAB theory, the polar component of the surface free energy is composed of acidic and basic constituents [17].
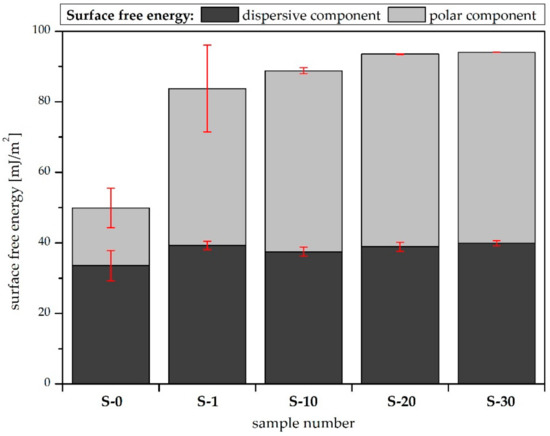
Figure 5.
Components of the surface free energy calculated using the LWAB approach.
In practice, the acidic constituent (the electron-acceptor parameter) achieves a negligible value, so it seems that air plasma modification only influences the electron-donor parameter (the basic constituent) [10]. On the other hand, studies have reported that, in the case of the LWAB approach, some of the major problems are the systematic overestimation of the electron-donor component of the surface free energy [18] and the fact that the method does not provide comprehensive values of the free energy components [19].
These results lead to the conclusion that the modification of the material with air plasma caused significant changes in the surface chemical groups that can interact in a polar way. However, more details were provided by the X-ray photoelectron spectroscopy (XPS) analysis, which is discussed in the next section of this paper. Furthermore, it should be kept in mind that both chemical (functional groups) and physical (topography) factors influence the surface wettability [20].
The two samples (unmodified (S-0) and modified for 10 s (S-10)) with the most visible changes were taken for further investigations.
The surface height maps are presented in Figure 6. Based on them, the roughness parameters of the samples were calculated and are shown in Table 2.
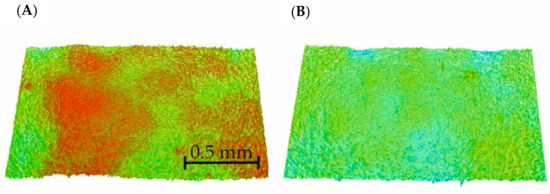
Figure 6.
Roughness map of the selected samples: (A) non–modified sample (S-0) and (B) sample modified for 10 s (S-10).

Table 2.
Roughness parameters of the selected samples.
The air plasma modification of the material surface caused an increase in the roughness (Table 2). This may improve the gluing effect because surface development promotes adhesion [21].
The X-ray photoelectron spectroscopy analysis showed differences in the surface groups and their chemistry. After the air plasma modification, there was an increase in the oxygen content on the sample surface. This was due to the generation of C=O and C–OH species during the plasma process, which was confirmed by the data obtained from the high-resolution XPS spectra (Table 3), in which an increasing number of carbonyl and hydroxyl groups can be seen. Despite the reduction in the nitrogen content (because of the oxidation properties of air plasma), imines appeared.

Table 3.
The results of XPS analysis of the non-modified sample and the sample modified for 10 s.
The O/C ratio of the non-modified sample is equal to 0.291, which corresponds to the range 0.31 to 0.4, which is typical of lignin [22]. In the case of the sample treated by the air plasma for 10 s, the O/C ratio increased almost 2-fold, which confirms the strong surface oxidation mostly to unsaturated hydrocarbons, in particular to produce the C=O and O–C=O species [23].
However, it should be emphasized that despite the relative simplicity of the modification process, the interactions between the plasma and the material surface are rather complex [24], particularly in the case of the composite material. Therefore, it is not possible to propose a scheme for the specific reaction that occurs during plasma surface modification and only the states before and after the treatment were compared without taking into account the course of the process itself.
The force values necessary to detach the samples are shown in Figure 7. In the case of the two unmodified sheets glued together (at 0 s—the reference test), the joint was broken at about 0.3 N.
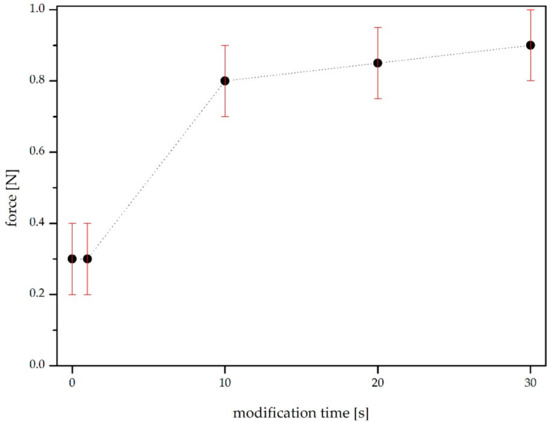
Figure 7.
Force needed to detach the modified sample from the unmodified one.
The tests confirm that the air plasma modification improved the adhesion of the examined materials, which is consistent with the surface free energy analysis results because the relation between the SFE of the paper and the surface tension of the glue is decisive in a good adhesive bond—an increase in the energy will improve the adhesion [25].
The one-second treatment did not produce a significant change, while the ten-second treatment increased the durability of the joint more than 2-fold. The force necessary to unglue the materials was equal to 0.8 N. In the case of using the paper in the technological process for the production of packaging, this is a sufficient and satisfactory change. A further increase in the modification time did not significantly increase the joint’s durability, and at 0.9 N the material was destroyed. Thus, the prolongation of the modification time to longer than 10 s is not profitable from a technological point of view.
4. Conclusions
In this paper, a simple method for the preprocessing of paper before gluing for the printing industry was proposed. A significant improvement in adhesion was achieved using air plasma surface modification. During the plasma treatment, which leads to surface oxidation, both C=O and C–OH bonds are created. It was found that the 10-second modification process produced the best effects in terms of material properties versus modification time. The effect was due to the change in the surface interactions and thus the surface free energy of the material. In addition, the air plasma treatment increased the material’s roughness, which also improved the adhesion.
Author Contributions
Conceptualization and methodology, K.T. and M.C.; sample preparation and modification, K.T. and M.C.; investigation, K.T., M.C. and S.P.-H.; data collection and analysis, Ł.W.; writing—original draft preparation, M.C. and K.T.; writing—review and editing, K.T. and M.C.; supervision, K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the sources mentioned in the references. All the data collected by the authors during the experiments are presented in this article.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Celiński, Z. Plazma; PWN: Warszawa, Poland, 1980; pp. 27–36. [Google Scholar]
- Stryczewska, H.D. Atmospheric pressure cold plasmas. Generation, modeling and applications. Prace Nauk. Politech. Śląskiej. Elektr. 2011, 1, 41–61. [Google Scholar]
- Salapare III, H.S.; Tiquio, M.G.J.P.; Ramos, H.J. RF plasma treatment of Neptune grass (Posidonia oceanica): A facile method to achive superhydrophilic surface for dye adsorption from aqueous solution. In Advances in Contact Angle, Wettability and Adhesion, 1st ed.; Mittal, K.L., Ed.; John Wiley: Hoboken, NJ, USA, 2015; Volume 2, pp. 305–332. [Google Scholar]
- Vandenabele, C.R.; Lucas, S. Technological challenges and progress in nanomaterials plasma surface modification—A review. Mater. Sci. Eng. R Rep. 2020, 139, 100521. [Google Scholar] [CrossRef]
- Tabares, F.L.; Junkar, I. Cold plasma systems and their application in surface treatments for medicine. Molecules 2021, 26, 1903. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.; Saquing, C.; Venditti, R.; McCord, M.; Bourham, M. The effect of atmospheric pressure plasma on paper and pulps. Bioresources 2017, 12, 8199–8216. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Zhao, Z.; He, Z.; Lin, T.; Zhao, Y.; Li, G.; Qu, J.; Huang, L.; Peng, X.; et al. A facile, clean construction of biphilic surface on filter paper via atmospheric air plasma for highly efficient separation of water-in-oil emulsions. Sep. Purif. Technol. 2021, 255, 117672. [Google Scholar] [CrossRef]
- Pykönen, M.; Johansson, K.; Dubreuil, M.; Vangeneugden, D.; Ström, G.; Fardim, P.; Toivakka, M. Evaluation of Plasma-Deposited Hydrophobic Coatings on Pigment-Coated Paper for Reduced Dampening Water Absorption. J. Adhes. Sci. Technol. 2010, 24, 511–537. [Google Scholar] [CrossRef]
- Vohrer, U.; Trick, I.; Bernhardt, J.; Oehr, C.; Brunner, H. Plasma treatment—An increasing technology for paper restoration? Surf. Coat. Technol. 2001, 142–144, 1069–1073. [Google Scholar] [CrossRef]
- Terpiłowski, K. Apparent Surface Free Energy of Polymer/Paper Composite Material Treated by Air Plasma. Int. J. Polym. Sci. 2017, 2017, 9023197. [Google Scholar] [CrossRef] [Green Version]
- Tadmor, R. Line energy and the relation between advancing, receding, and young contact angles. Langmuir 2004, 20, 7659–7664. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Chibowski, E. Surface free energy of a solid from contact angle hysteresis. Adv. Colloid Interface Sci. 2003, 103, 149–172. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Abel, E.W. Effect of temperature on the surface free energy of amorphous carbon films. J. Colloid Interface Sci. 2004, 280, 174–183. [Google Scholar] [CrossRef]
- Baniya, H.B.; Guragain, R.P.; Subedi, D.P. Cold Atmospheric Pressure Plasma Technology for Modifying Polymers to Enhance Adhesion: A Critical Review. Rev. Adhes. Adhes. 2021, 9, 269–307. [Google Scholar] [CrossRef]
- Guragain, R.; Baniya, H.; Dhungana, S.; Chhetri, G.K.; Gautam, S.; Pandey, B.; Joshi, M.; Prasad, D.; Joshi, U.; Subedi, D.; et al. Improvement of Hydrophilicity of Polypropylene Film by Dielectric Barrier Discharge Generated in Air at Atmospheric Pressure. Rev. Adhes. Adhes. 2021, 9, 153–166. [Google Scholar]
- Chibowski, E.; Hołysz, L.; Szcześ, A. Wettability of Powders. In Adhesion in Pharmaceutical, Biomedical, and Dental Fields, 1st ed.; Mittal, K.L., Etzler, F.M., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 23–49. [Google Scholar]
- Della Volpe, C.; Siboni, S. Acid–base surface free energies of solids and the definition of scales in the Good–van Oss–Chaudhury theory. J. Adhes. Sci. Technol. 2000, 14, 235–272. [Google Scholar] [CrossRef]
- Chibowski, E.; Perera-Carpio, R. Problems of contact angle and solid surface free energy determination. Adv. Colloid Interface Sci. 2002, 98, 245–264. [Google Scholar] [CrossRef]
- Chodkowski, M.; Terpiłowski, K.; Pasieczna-Patkowska, S. Fabrication of transparent polysiloxane coatings on a glass support via the sol-gel dip coating technique and the effect of their hydrophobization with hexamethyldisilazane. Physicochem. Probl. Miner. Process. 2020, 56, 76–88. [Google Scholar] [CrossRef]
- Van Dam, J.P.B.; Abrahami, S.T.; Yilmaz, A.; Gonzalez-Garcia, Y.; Terryn, H.; Mol, J.M.C. Effect of surface roughness and chemistry on the adhesion and durability of a steel-epoxy adhesive interface. Int. J. Adhes. Adhes. 2020, 96, 102450. [Google Scholar] [CrossRef]
- Tóth, A.; Černáková, L.; Černák, M.; Kunovská, K. Surface analysis of groundwood paper treated by diffuse coplanar surface barrier discharge (DCSBD) type atmospheric plasma in air and in nitrogen. Holzforschung 2007, 61, 528–531. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Q.; Yao, C.; Zhang, P.; Shen, R.; Liu, H.; Lu, L.; Jiang, Y.; Yuan, X.; Miao, X.; et al. Long-lasting antifogging mechanism for large-aperture optical surface in low-pressure air plasma in-situ treated. Appl. Surf. Sci. 2022, 581, 152358. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Mozetič, M.; Primc, G. Surface modification of PS polymer by oxygen-atom treatment from remote plasma: Initial kinetics of functional groups formation. Appl. Surf. Sci. 2021, 561, 150058. [Google Scholar] [CrossRef]
- Bao, L.; Fan, H.; Chen, Y.; Yan, J.; Yang, T.; Guo, Y. Effect of surface free energy and wettability on the adhesion property of waterborne polyurethane adhesive. RSC Adv. 2016, 6, 99346–99352. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).