Mass Cultivation of Microalgae: I. Experiences with Vertical Column Airlift Photobioreactors, Diatoms and CO2 Sequestration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photobioreactor Construction
2.2. Cultivation Environment and Cultivation Strategies
2.3. Physical Cultivation Environment, Inorganic Nutrients, Irradiance Measurements and Computations
2.4. Biomass Proxies, Growth Rates, Light Utilization and CO2 Uptake
2.5. Total Lipid, Lipid Class and Fatty Acids, Protein and Amino Acid Analysis, Environmental Contaminants
2.6. Bioprospecting
2.6.1. Cytotoxic Activity against Human Cell Lines
2.6.2. Antibacterial Activity
2.6.3. Inhibition of Biofilm Formation
2.6.4. Antioxidative Activity
2.6.5. Anti-Inflammatory Activity
3. Results
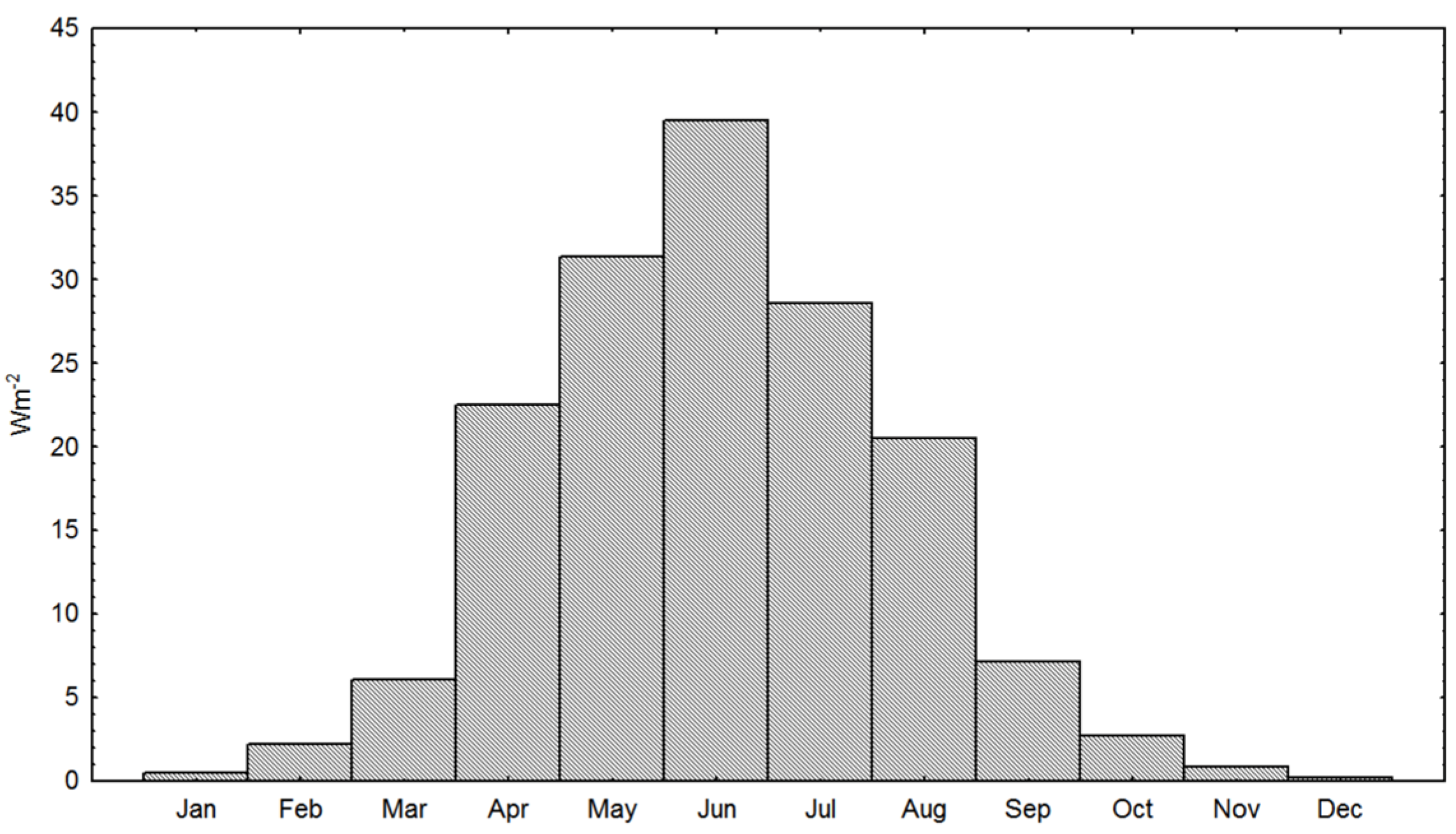
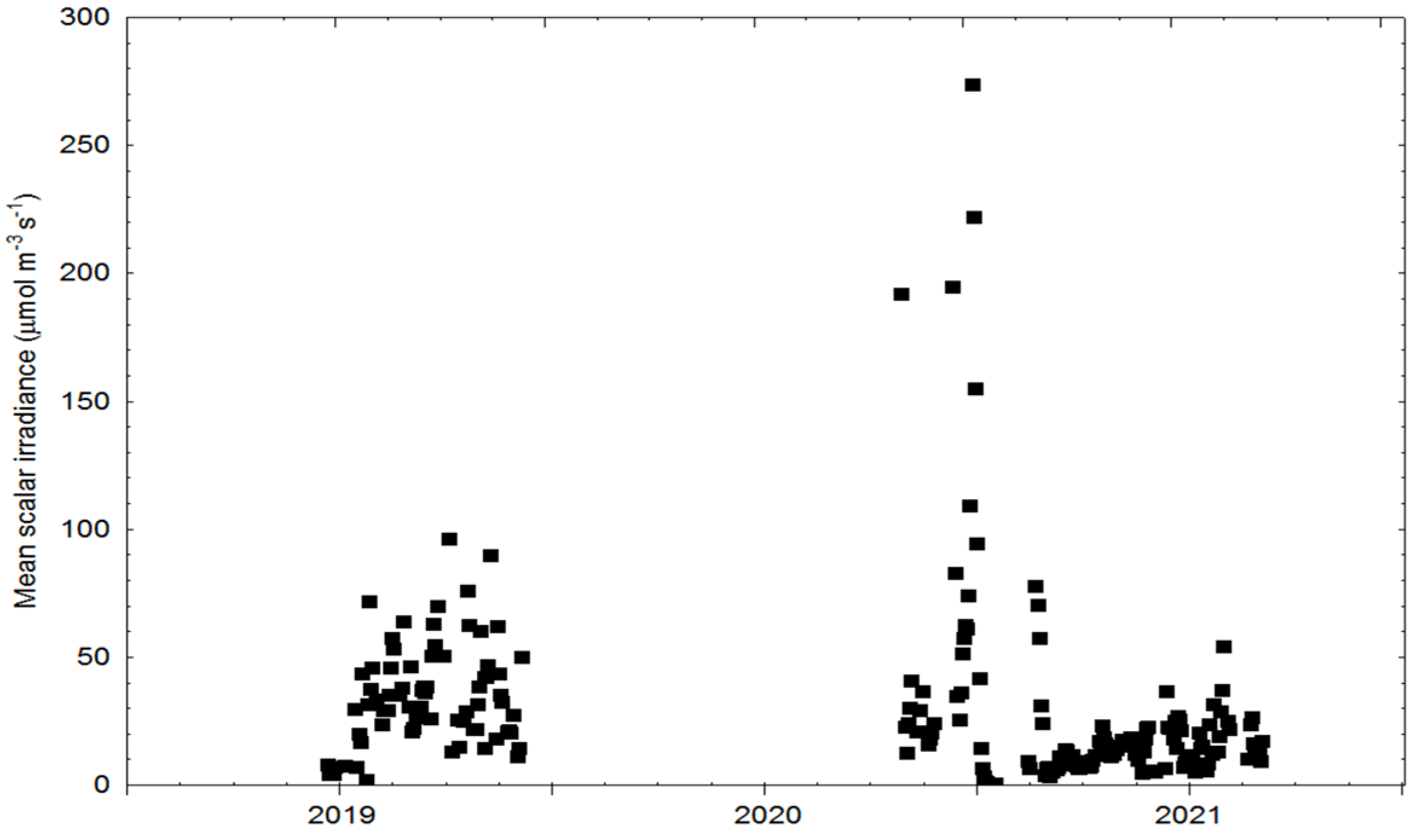
3.1. Physical Cultivation Environment
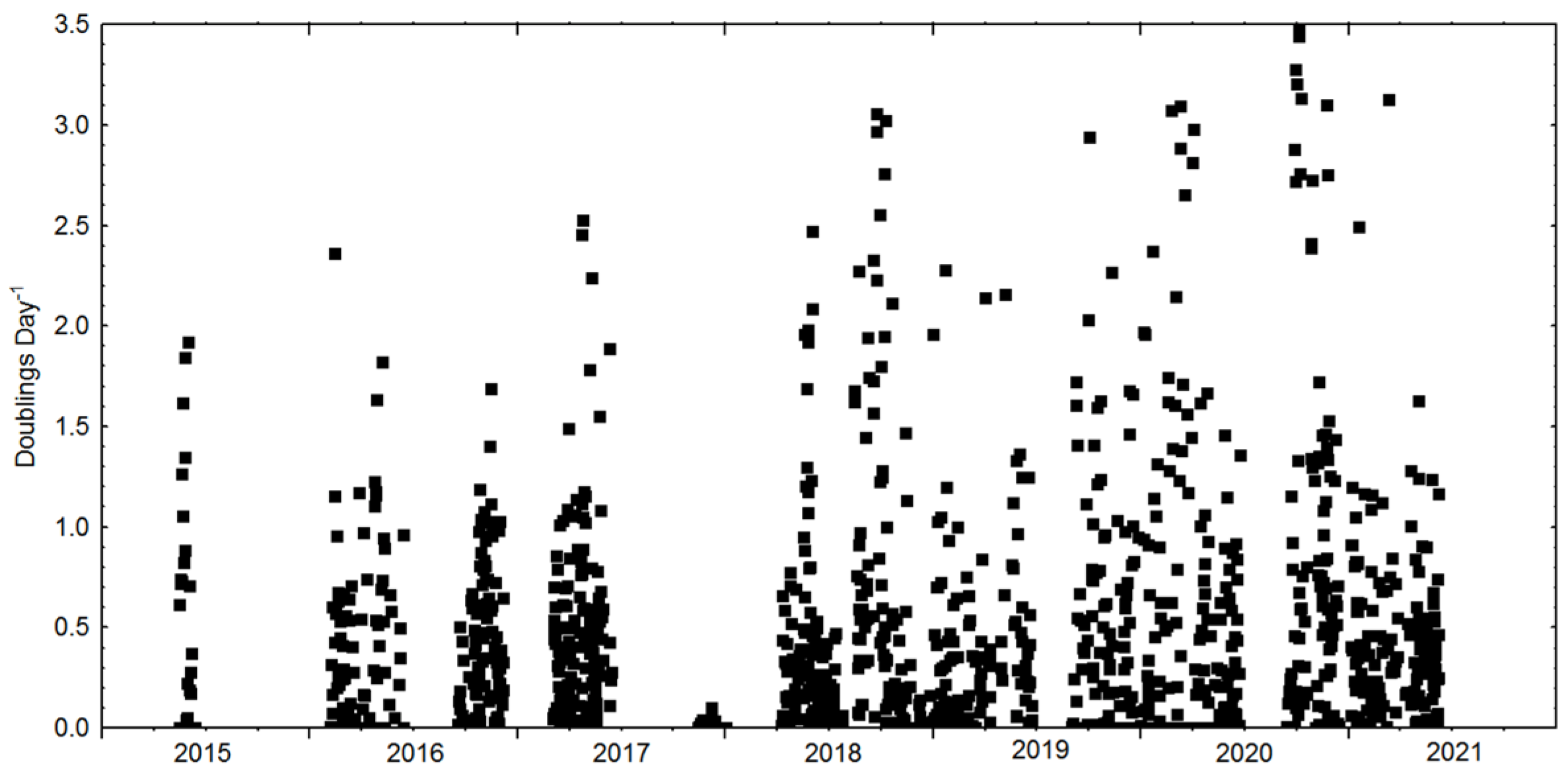
3.2. Cultivation: Growth Rates, Biomass Concentration and CO2 Sequestration
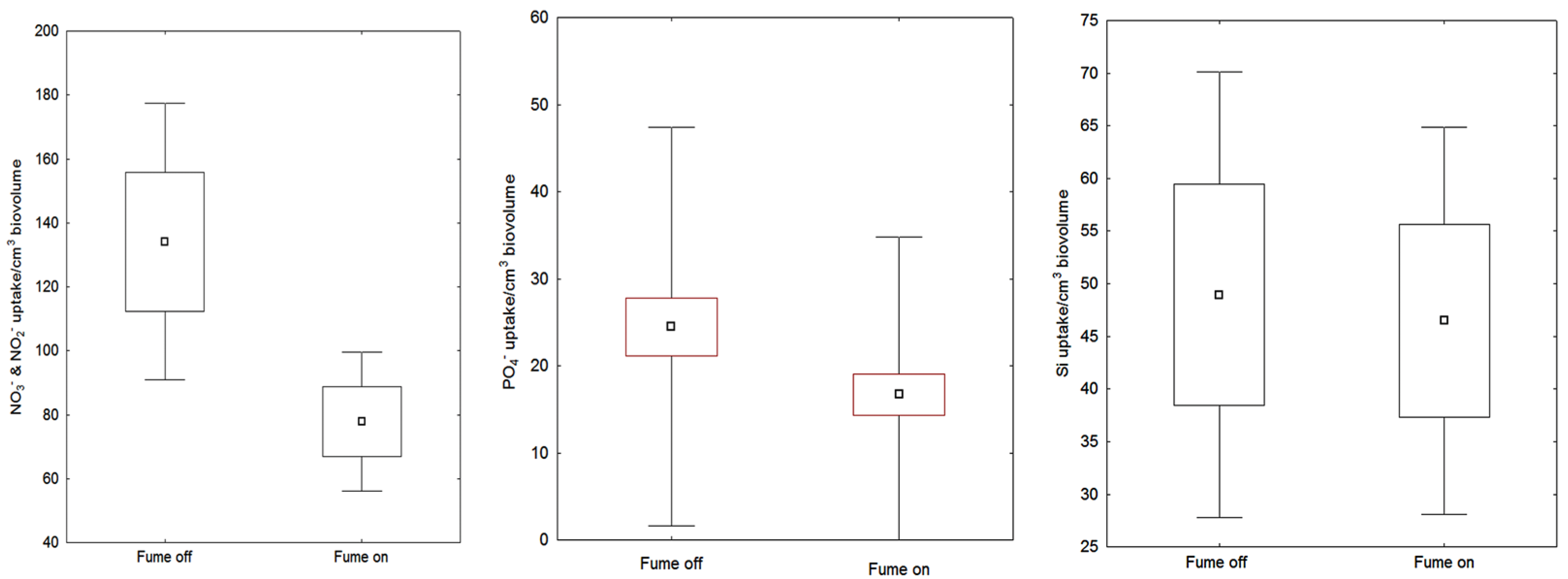
3.3. Chemical Content in Diatoms with and without Fume
3.4. Bioactivity in Diatom Biomass with and without Fume
3.4.1. Cytotoxic Activity against Human Cell Lines
3.4.2. Antibacterial Activity
3.4.3. Inhibition of Biofilm Formation
3.4.4. Antioxidative Activity
3.4.5. Anti-Inflammatory Activity
3.4.6. Anti-Diabetes Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.L.; Boyd, E.S.; Peters, J.W.; Posewitz, M.C. Engineering algae for biohydrogen and biofuel production. Curr. Opin. Biotechnol. 2009, 20, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Yadugiri, V. Milking diatoms–A new route to sustainable energy. Curr. Sci. 2009, 97, 748–750. Available online: https://www.jstor.org/stable/24112109 (accessed on 1 June 2021).
- Varfolomeev, S.; Wasserman, L. Microalgae as source of biofuel, food, fodder, and medicines. Appl. Biochem. Microbiol. 2011, 47, 789–807. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae–A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Lim, S.-L.; Chu, W.-L.; Phang, S.-M. Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour. Technol. 2010, 101, 7314–7322. [Google Scholar] [CrossRef]
- Ramachandra, T.; Mahapatra, D.M.; Gordon, R. Milking diatoms for sustainable energy: Biochemical engineering versus gasoline-secreting diatom solar panels. Ind. Eng. Chem. Res. 2009, 48, 8769–8788. [Google Scholar] [CrossRef]
- Beigbeder, J.-B.; Sanglier, M.; de Medeiros Dantas, J.M.; Lavoie, J.-M. CO2 capture and inorganic carbon assimilation of gaseous fermentation effluents using ParaChlorella kessleri microalgae. J. CO2 Util. 2021, 50, 101581. [Google Scholar] [CrossRef]
- Schiano di Visconte, G.; Spicer, A.; Chuck, C.J.; Allen, M.J. The microalgae biorefinery: A perspective on the current status and future opportunities using genetic modification. Appl. Sci. 2019, 9, 4793. [Google Scholar] [CrossRef] [Green Version]
- Bibi, F.; Jamal, A.; Huang, Z.; Urynowicz, M.; Ali, M.I. Advancement and role of abiotic stresses in microalgae biorefinery with a focus on lipid production. Fuel 2022, 316, 123192. [Google Scholar] [CrossRef]
- Mobin, S.M.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications–A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Pires, J.; Alvim-Ferraz, M.; Martins, F.; Simões, M. Carbon dioxide capture from flue gases using microalgae: Engineering aspects and biorefinery concept. Renew. Sustain. Energy Rev. 2012, 16, 3043–3053. [Google Scholar] [CrossRef]
- Seth, J.R.; Wangikar, P.P. Challenges and opportunities for microalgae-mediated CO2 capture and biorefinery. Biotechnol. Bioeng. 2015, 112, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—A review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Alam, M.A.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae cultivation technologies as an opportunity for bioenergetic system development—advantages and limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of microalgae biomass co-substrate on biogas production from the common agricultural biogas plants feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Beijierinck, M. Kulturversuche mit Zoochloren, Lichenenggonidien und anderen niederen Algen. Physis 1890, 48, 725–785. [Google Scholar]
- Warburg, O. Über die Geschwindigkeit der Kohlensäurezersetzung in lebenden Zellen. Biochem. Z. 1919, 100, 230–270. [Google Scholar] [CrossRef]
- Jorgensen, J.; Convit, J.; de Cabo Blanco, L.; Maiquetia, D. Cultivation of complexes of algae with other fresh-water microorganisms in the tropics. In Algal Culture—From Laboratory to Pilot Plant; Burlew, J.S., Ed.; Carnegie Institution of Washington Publication: Washington, DC, USA, 1953; p. 190. [Google Scholar]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Nethravathy, M.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent advances in microalgal bioactives for food, feed, and healthcare products: Commercial potential, market space, and sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef] [Green Version]
- García, J.L.; De Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, J.; Heimdal, B. Pelagic productivity and food chains in fjord systems. In Fjord Oceanography; Springer: New York, NY, USA, 1980; pp. 377–398. [Google Scholar]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.A.; Chong, M.N.; Foo, S.C.; Ilankoon, I. Analysis of direct and indirect quantification methods of CO2 fixation via microalgae cultivation in photobioreactors: A critical review. Renew. Sustain. Energy Rev. 2021, 137, 110579. [Google Scholar] [CrossRef]
- Ugwu, C.; Aoyagi, H.; Uchiyama, H. Photobioreactors for mass cultivation of algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef]
- Blanken, W.; Cuaresma, M.; Wijffels, R.H.; Janssen, M. Cultivation of microalgae on artificial light comes at a cost. Algal Res. 2013, 2, 333–340. [Google Scholar] [CrossRef]
- Tamiya, H. Mass culture of algae. Annu. Rev. Plant Physiol. 1957, 8, 309–334. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine natural products from microalgae: An-omics overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, D.; Morao, A.; Johnson, J.K.; Shen, L. Life cycle assessment of heterotrophic algae omega-3. Algal Res. 2021, 60, 102494. [Google Scholar] [CrossRef]
- Mann, D.G. The species concept in diatoms. Phycologia 1999, 38, 437–495. [Google Scholar] [CrossRef] [Green Version]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Chepurnov, V.A.; Chaerle, P.; Roef, L.; Van Meirhaeghe, A.; Vanhoutte, K. Classical breeding in diatoms: Scientific background and practical perspectives. In The Diatom World; Springer: New York, NY, USA, 2011; pp. 167–194. [Google Scholar]
- Collins, L.; Alvarez, D.; Chauhan, A. Phycoremediation Coupled with Generation of Value-Added Products. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2014; pp. 341–387. [Google Scholar]
- Cushing, D.H. Plankton production and year-class strength in fish populations: An update of the match/mismatch hypothesis. Adv. Mar. Biol. 1990, 26, 249–293. [Google Scholar] [CrossRef]
- Degerlund, M.; Eilertsen, H.C. Main species characteristics of phytoplankton spring blooms in NE Atlantic and Arctic waters (68–80° N). Estuar. Coasts 2010, 33, 242–269. [Google Scholar] [CrossRef]
- Shimizu, Y. Microalgal metabolites: A new perspective. Annu. Rev. Microbiol. 1996, 50, 431–465. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1R–49R. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Artamonova, E.; Vasskog, T.; Eilertsen, H.C. Lipid content and fatty acid composition of Porosira glacialis and Attheya longicornis in response to carbon dioxide (CO2) aeration. PLoS ONE 2017, 12, e0177703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svenning, J.B.; Dalheim, L.; Eilertsen, H.C.; Vasskog, T. Temperature dependent growth rate, lipid content and fatty acid composition of the marine cold-water diatom Porosira glacialis. Algal Res. 2019, 37, 11–16. [Google Scholar] [CrossRef]
- Jónasdóttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goessling, J.W.; Su, Y.; Cartaxana, P.; Maibohm, C.; Rickelt, L.F.; Trampe, E.C.; Walby, S.L.; Wangpraseurt, D.; Wu, X.; Ellegaard, M. Structure-based optics of centric diatom frustules: Modulation of the in vivo light field for efficient diatom photosynthesis. New Phytol. 2018, 219, 122–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, L.-L.; Yu, D.; Zhang, J.; Liu, F.-f.; Wu, Y.-H.; Zhang, T.-Y.; Dao, G.-H.; Hu, H.-Y. The characteristics and influencing factors of the attached microalgae cultivation: A review. Renew. Sustain. Energy Rev. 2018, 94, 1110–1119. [Google Scholar] [CrossRef]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K. Microalgae Production Systems in Asia; Elsevier: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Venkataraman, L. Blue-green algae as biofertilizer. In CRC Handbook of Microalgal Mass Culture; CRC Press: Boca Raton, FL, USA, 1986; pp. 455–472. [Google Scholar]
- Lee, Y.-K. Commercial production of microalgae in the Asia-Pacific rim. J. Appl. Phycol. 1997, 9, 403–411. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Huang, J.; Hankamer, B.; Yarnold, J. Design scenarios of outdoor arrayed cylindrical photobioreactors for microalgae cultivation considering solar radiation and temperature. Algal Res. 2019, 41, 101515. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Chen, C.; Zhang, W.; Wu, P.; Sun, M.; Wu, H.; Wu, H.; Fu, P.; Fan, J. Transgenic eukaryotic microalgae as green factories: Providing new ideas for the production of biologically active substances. J. Appl. Phycol. 2021, 33, 705–728. [Google Scholar] [CrossRef]
- Geider, R.; Osborne, B. Light absorption by a marine diatom: Experimental observations and theoretical calculations of the package effect in a small Thalassiosira species. Mar. Biol. 1987, 96, 299–308. [Google Scholar] [CrossRef]
- Chisholm, S.W. Phytoplankton size. In Primary Productivity and Biogeochemical Cycles in the Sea; Springer: Cham, Switzerland, 1992; pp. 213–237. [Google Scholar]
- Agustí, S.; Enriquez, S.; Frost-Christensen, H.; Sand-Jensen, K.; Duarte, C. Light harvesting among photosynthetic organisms. Funct. Ecol. 1994, 8, 273–279. [Google Scholar] [CrossRef]
- Cermeno, P.; Estévez-Blanco, P.; Maranón, E.; Fernóndez, E. Maximum photosynthetic efficiency of size-fractionated phytoplankton assessed by 14C uptake and fast repetition rate fluorometry. Limnol. Oceanogr. 2005, 50, 1438–1446. [Google Scholar] [CrossRef] [Green Version]
- Maraóón, E.; Cermeóo, P.; Rodríguez, J.; Zubkov, M.V.; Harris, R.P. Scaling of phytoplankton photosynthesis and cell size in the ocean. Limnol. Oceanogr. 2007, 52, 2190–2198. [Google Scholar] [CrossRef]
- Martinez-Jeronimo, F.; Gutierrez-Valdivia, A. Fecundity, reproduction, and growth of Moina macrocopa fed different algae. Hydrobiologia 1991, 222, 49–55. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, D. Light scattering by spherical particles. JOSA 1947, 37, 475–480. [Google Scholar] [CrossRef]
- Baker, E.T.; Lavelle, J.W. The effect of particle size on the light attenuation coefficient of natural suspensions. J. Geophys. Res. Ocean. 1984, 89, 8197–8203. [Google Scholar] [CrossRef]
- Agustí, S. Light environment within dense algal populations: Cell size influences on self-shading. J. Plankton Res. 1991, 13, 863–871. [Google Scholar] [CrossRef]
- Nelson, N.B.; Prézelin, B.B.; Bidigare, R.R. Phytoplankton light absorption and the package effect in California coastal waters. Mar. Ecol. Prog. Ser. 1993, 94, 217–227. Available online: https://www.jstor.org/stable/24832708 (accessed on 1 June 2021). [CrossRef]
- Harris, G.P. Photosynthesis, productivity and growth. Adv. Limnol. 1978, 4, 10. [Google Scholar]
- Kirk, J.T.O. Optical properties of picoplankton suspensions. In Photosynthetic Picoplankton; Platt, T., Li, W.K.W., Eds.; Canadian Bulletin of Fisheries and Aquatic Sciences: Ottawa, ON, Canada, 1986; Volume 214, pp. 501–520. [Google Scholar]
- Lee, C.-G. Calculation of light penetration depth in photobioreactors. Biotechnol. Bioprocess Eng. 1999, 4, 78–81. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Pienkos, P.T.; Darzins, A. The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod. Biorefin. Innov. Sustain. Econ. 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef]
- Putt, R.; Singh, M.; Chinnasamy, S.; Das, K. An efficient system for carbonation of high-rate algae pond water to enhance CO2 mass transfer. Bioresour. Technol. 2011, 102, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; Sneddon, A.A.; Sprague, M.; Horgan, G.W.; Brouwer, I.A. The potential impact of compositional changes in farmed fish on its health-giving properties: Is it time to reconsider current dietary recommendations? Public Health Nutr. 2017, 20, 2042–2049. [Google Scholar] [CrossRef] [Green Version]
- Hansen, L. The Weak Sustainability of the Salmon Feed Transition in Norway—A Bioeconomic Case Study. Front. Mar. Sci. 2019, 6, 764. [Google Scholar] [CrossRef] [Green Version]
- Hamre, K.; Yúfera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.; Izquierdo, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquac. 2013, 5, S26–S58. [Google Scholar] [CrossRef] [Green Version]
- Midtbø, L.K.; Borkowska, A.G.; Bernhard, A.; Rønnevik, A.K.; Lock, E.-J.; Fitzgerald, M.L.; Torstensen, B.E.; Liaset, B.; Brattelid, T.; Pedersen, T.L. Intake of farmed Atlantic salmon fed soybean oil increases hepatic levels of arachidonic acid-derived oxylipins and ceramides in mice. J. Nutr. Biochem. 2015, 26, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargent, J.; Eilertsen, H.; Falk-Petersen, S.; Taasen, J. Carbon assimilation and lipid production in phytoplankton in northern Norwegian fjords. Mar. Biol. 1985, 85, 109–116. [Google Scholar] [CrossRef]
- Eilertsen, H.; Wyatt, T. Phytoplankton models and life history strategies. S. Afr. J. Mar. Sci. 2000, 22, 323–338. [Google Scholar] [CrossRef] [Green Version]
- Haslam, R.P.; Keys, A.J.; Andralojc, P.J.; Madgwick, P.J.; Inger, A.; Grimsrud, A.; Eilertsen, H.C.; Parry, M.A. Specificity of diatom Rubisco. In Plant Responses to Air Pollution and Global Change; Springer: Cham, Switzerland, 2005; pp. 157–164. [Google Scholar]
- Eilertsen, H.C.; Degerlund, M. Phytoplankton and light during the northern high-latitude winter. J. Plankton Res. 2010, 32, 899–912. [Google Scholar] [CrossRef]
- Huseby, S.; Degerlund, M.; Eriksen, G.K.; Ingebrigtsen, R.A.; Eilertsen, H.C.; Hansen, E. Chemical diversity as a function of temperature in six northern diatom species. Mar. Drugs 2013, 11, 4232–4245. [Google Scholar] [CrossRef] [Green Version]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Stevenson, J.C., Ed.; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine plankton diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplanktonmethodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Tomas, C.R. Identifying Marine Phytoplankton; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a determination: Improvements in methodology. Oikos 1978, 30, 438–447. Available online: https://www.jstor.org/stable/3543338 (accessed on 1 June 2021). [CrossRef]
- Platt, T.; Irwin, B. Caloric content of phytoplankton. Limnol. Oceanogr. 1973, 18, 306–310. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Eilertsen, H.C.; Holm-Hansen, O. Effects of high latitude UV radiation on phytoplankton and nekton modelled from field measurements by simple algorithms. Polar Res. 2000, 19, 173–182. [Google Scholar] [CrossRef]
- Jensen, I.; Mæhre, H.; Tømmerås, S.; Eilertsen, K.; Olsen, R.; Elvevoll, E. Farmed Atlantic salmon (Salmo salar L.) is a good source of long chain omega-3 fatty acids. Nutr. Bull. 2012, 37, 25–29. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Cequier-Sánchez, E.; Rodriguez, C.; Ravelo, A.G.; Zarate, R. Dichloromethane as a solvent for lipid extraction and assessment of lipid classes and fatty acids from samples of different natures. J. Agric. Food Chem. 2008, 56, 4297–4303. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. (Ed.) Preparation of ester derivatives of fatty acids for chromatographic analysis. In Advances in Lipid Methodology Two; Oily Press: Dundee, Scotland, 1993; pp. 69–111. [Google Scholar]
- Abreu, S.; Solgadi, A.; Chaminade, P. Optimization of normal phase chromatographic conditions for lipid analysis and comparison of associated detection techniques. J. Chromatogr. 2017, 1514, 54–71. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Chromatographic determination of amino acids by the use of automatic recording equipment. Methods Enzymol. 1963, 6, 819–831. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Mæhre, H.; Hamre, K.; Elvevoll, E. Nutrient evaluation of rotifers and zooplankton: Feed for marine fish larvae. Aquacult. Nutr. 2013, 19, 301–311. [Google Scholar] [CrossRef]
- WHO. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Rome, Italy, 2003; Volume 916. [Google Scholar]
- Nash, S.B.; Poulsen, A.; Kawaguchi, S.; Vetter, W.; Schlabach, M. Persistent organohalogen contaminant burdens in Antarctic krill (Euphausia superba) from the eastern Antarctic sector: A baseline study. Sci. Total Environ. 2008, 407, 304–314. [Google Scholar] [CrossRef]
- Osvik, R.D.; Andersen, J.H.; Eilertsen, H.C.; Geneviere, A.-M.; Hansen, E.H. Bioactivity of a Marine Diatom (Porosira glacialis [Grunow] Jörgensen 1905) Cultivated with and Without Factory Smoke CO2. Ind. Biotechnol. 2021, 17, 38–48. [Google Scholar] [CrossRef]
- Olsen, E.K.; Hansen, E.; Isaksson, J.; Andersen, J.H. Cellular antioxidant effect of four bromophenols from the red algae, Vertebrata lanosa. Mar. Drugs 2013, 11, 2769–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, K.F.; Hansen, E.; Østerud, B.; Eilertsen, K.-E.; Bayer, A.; Engqvist, M.; Leszczak, K.; Jørgensen, T.Ø.; Andersen, J.H. Antioxidant and anti-inflammatory activities of Barettin. Mar. Drugs 2013, 11, 2655–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimenes, A.; Thue-Hansen, V. The reduction of global radiation in south-eastern Norway during the last 50 years. Theor. Appl. Climatol. 2006, 85, 37–40. [Google Scholar] [CrossRef]
- Cornet, J.; Dussap, C.; Gros, J. Conversion of radiant light energy in photobioreactors. AIChE J. 1994, 40, 1055–1066. [Google Scholar] [CrossRef]
- Zhang, X. Microalgae Removal of CO2 from Flue Gas; IEA Clean Coal Centre: Paris, France, 2015. [Google Scholar]
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Alameh, K.; Moheimani, N.R. Light management technologies for increasing algal photobioreactor efficiency. Algal Res. 2019, 39, 101433. [Google Scholar] [CrossRef]
- Zurano, A.S.; Cárdenas, J.G.; Serrano, C.G.; Amaral, M.M.; Acién-Fernández, F.; Sevilla, J.F.; Grima, E.M. Year-long assessment of a pilot-scale thin-layer reactor for microalgae wastewater treatment. Variation in the microalgae-bacteria consortium and the impact of environmental conditions. Algal Res. 2020, 50, 101983. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Fernández-del Olmo, P.; Guzmán, J.; Fernández-Sevilla, J.; Fernández, F.A. Evaluation of photosynthetic light integration by microalgae in a pilot-scale raceway reactor. Bioresour. Technol. 2019, 280, 404–411. [Google Scholar] [CrossRef]
- Olseth, J.A.; Skartveit, A. Solar radiation climate of Norway. Solar Energy 1986, 37, 423–428. [Google Scholar] [CrossRef]
- Bell, A.M.; von der Au, M.; Regnery, J.; Schmid, M.; Meermann, B.; Reifferscheid, G.; Ternes, T.; Buchinger, S. Does galvanic cathodic protection by aluminum anodes impact marine organisms? Environ. Sci. Eur. 2020, 32, 157. [Google Scholar] [CrossRef]
- Šetlík, I.; Šust, V.; Málek, I. Dual purpose open circulation units for large scale culture of algae in temperate zones. I. Basic design considerations and scheme of a pilot plant. Algol. Stud./Archiv für Hydrobiol. 1970, 1, 111–164. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A.; Lichtenberg, E.; Stahl, B.; Vonshak, A. Quantitative assessment of the major limitations on productivity of Spirulina platensis in open raceways. J. Appl. Phycol. 1990, 2, 195–206. [Google Scholar] [CrossRef]
- Huntley, M.E.; Redalje, D.G. CO2 mitigation and renewable oil from photosynthetic microbes: A new appraisal. Mitig. Adapt. Strateg. Glob. Change 2007, 12, 573–608. [Google Scholar] [CrossRef]
- Pushparaj, B.; Pelosi, E.; Tredici, M.R.; Pinzani, E.; Materassi, R. As integrated culture system for outdoor production of microalgae and cyanobacteria. J. Appl. Phycol. 1997, 9, 113–119. [Google Scholar] [CrossRef]
- Moreno, J.; Vargas, M.Á.; Rodrıguez, H.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of a nitrogen-fixing marine cyanobacterium, Anabaena sp. ATCC 33047. Biomol. Eng. 2003, 20, 191–197. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J. Appl. Phycol. 2006, 18, 811–826. [Google Scholar] [CrossRef]
- Rubio, F.C.; Fernández, F.A.; Pérez, J.S.; Camacho, F.G.; Grima, E.M. Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnol. Bioeng. 1999, 62, 71–86. [Google Scholar] [CrossRef]
- Molina, E.; Fernández, J.; Acién, F.; Chisti, Y. Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- Ugwu, C.; Ogbonna, J.; Tanaka, H. Improvement of mass transfer characteristics and productivities of inclined tubular photobioreactors by installation of internal static mixers. Appl. Microbiol. Biotechnol. 2002, 58, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, P. Dilution of solar radiation through “culture” lamination in photobioreactor rows facing south–north: A way to improve the efficiency of light utilization by cyanobacteria (Arthrospira platensis). Biotechnol. Bioeng. 2003, 81, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- García-Malea, M.; Acién, F.; Fernández, J.; Cerón, M.; Molina, E. Continuous production of green cells of Haematococcus pluvialis: Modeling of the irradiance effect. Enzym. Microb. Technol. 2006, 38, 981–989. [Google Scholar] [CrossRef]
- Cheng-Wu, Z.; Zmora, O.; Kopel, R.; Richmond, A. An industrial-size flat plate glass reactor for mass production of Nannochloropsis sp. (Eustigmatophyceae). Aquaculture 2001, 195, 35–49. [Google Scholar] [CrossRef]
- Converti, A.; Lodi, A.; Del Borghi, A.; Solisio, C. Cultivation of Spirulina platensis in a combined airlift-tubular reactor system. Biochem. Eng. J. 2006, 32, 13–18. [Google Scholar] [CrossRef]
- Carlozzi, P. Hydrodynamic aspects and Arthrospira growth in two outdoor tubular undulating row photobioreactors. Appl. Microbiol. Biotechnol. 2000, 54, 14–22. [Google Scholar] [CrossRef]
- Zittelli, G.C.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture 2006, 261, 932–943. [Google Scholar] [CrossRef]
- Sato, T.; Usui, S.; Tsuchiya, Y.; Kondo, Y. Invention of outdoor closed type photobioreactor for microalgae. Energy Convers. Manag. 2006, 47, 791–799. [Google Scholar] [CrossRef]
- Doucha, J.; Straka, F.; Lívanský, K. Utilization of flue gas for cultivation of microalgae (Chlorella sp.) in an outdoor open thin-layer photobioreactor. J. Appl. Phycol. 2005, 17, 403–412. [Google Scholar] [CrossRef]
- Bani, A.; Fernandez, F.G.A.; D’Imporzano, G.; Parati, K.; Adani, F. Influence of photobioreactor set-up on the survival of microalgae inoculum. Bioresour. Technol. 2021, 320, 124408. [Google Scholar] [CrossRef] [PubMed]
- Paladino, O.; Neviani, M. Scale-up of photo-bioreactors for microalgae cultivation by π-theorem. Biochem. Eng. J. 2020, 153, 107398. [Google Scholar] [CrossRef]
- Jung, S.-H.; McHardy, C.; Rauh, C.; Jahn, A.; Luzi, G.; Delgado, A.; Buchholz, R.; Lindenberger, C. A new approach for calculating microalgae culture growth based on an inhibitory effect of the surrounding biomass. Bioprocess Biosyst. Eng. 2021, 44, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Mirón, A.; García Camacho, F.; Contreras Gómez, A.; Grima, E.M.; Chisti, Y. Bubble-column and airlift photobioreactors for algal culture. AIChE J. 2000, 46, 1872–1887. [Google Scholar] [CrossRef]
- Tennessen, D.J.; Bula, R.J.; Sharkey, T.D. Efficiency of photosynthesis in continuous and pulsed light emitting diode irradiation. Photosynth. Res. 1995, 44, 261–269. [Google Scholar] [CrossRef]
- Eilertsen, H.C.; Taasen, J. Investigations on the plankton community of Balsfjorden, northern Norway. The phytoplankton 1976–1978. Environmental factors, dynamics of growth, and primary production. Sarsia 1984, 69, 1–15. [Google Scholar] [CrossRef]
- Eppley, R.W. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972, 70, 1063–1085. [Google Scholar]
- Gilstad, M.; Sakshaug, E. Growth rates of ten diatom species from the Barents Sea at different irradiances and day lengths. Marine ecology progress series. Oldendorf 1990, 64, 169–173. Available online: https://www.jstor.org/stable/24844602 (accessed on 1 June 2021). [CrossRef]
- Rhee, G.-Y. Effects of environmental factors and their interactions on phytoplankton growth. Adv. Microb. Ecol. 1982, 6, 33–74. [Google Scholar] [CrossRef]
- Geider, R.; MacIntyre, H.; Kana, T. Dynamic model of phytoplankton growth and acclimation: Responses of the balanced growth rate and the chlorophyll a: Carbon ratio to light, nutrient-limitation and temperature. Mar. Ecol. Prog. Ser. 1997, 148, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Madani, N.S.H.; Mehrgan, M.S.; Shekarabi, S.P.H.; Pourang, N. Regulatory effect of gibberellic acid (GA3) on the biomass productivity and some metabolites of a marine microalga, Isochrysis galbana. J. Appl. Phycol. 2021, 33, 255–262. [Google Scholar] [CrossRef]
- Eilertsen, H.C.; Sandberg, S.; Tøllefsen, H. Photoperiodic control of diatom spore growth: A theory to explain the onset of phytoplankton blooms. Marine ecology progress series. Oldendorf 1995, 116, 303–307. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour. Technol. 2013, 129, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, J.C.; Yada, H.; Tanaka, H. Light supply coefficient: A new engineering parameter for photobioreactor design. J. Ferment. Bioeng. 1995, 80, 369–376. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Brzychczyk, B.; Hebda, T.; Fitas, J.; Giełżecki, J. The follow-up photobioreactor illumination system for the cultivation of photosynthetic microorganisms. Energies 2020, 13, 1143. [Google Scholar] [CrossRef] [Green Version]
- Costache, T.; Fernández, F.G.A.; Morales, M.; Fernández-Sevilla, J.; Stamatin, I.; Molina, E. Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 7627–7637. [Google Scholar] [CrossRef]
- Endres, C.H.; Roth, A.; Brück, T.B. Modeling microalgae productivity in industrial-scale vertical flat panel photobioreactors. Environ. Sci. Technol. 2018, 52, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Chanquia, S.N.; Vernet, G.; Kara, S. Photobioreactors for cultivation and synthesis: Specifications, challenges, and perspectives. Eng. Life Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Nagase, H.; Yoshihara, K.-i.; Eguchi, K.; Okamoto, Y.; Murasaki, S.; Yamashita, R.; Hirata, K.; Miyamoto, K. Uptake pathway and continuous removal of nitric oxide from flue gas using microalgae. Biochem. Eng. J. 2001, 7, 241–246. [Google Scholar] [CrossRef]
- Kethineni, C.; Choragudi, S.; Kokkiligadda, S.; Jaswanthi, N.; Ronda, S.R. Development of sequential processes for multiple product recovery from microalgae. Ind. Biotechnol. 2018, 14, 95–106. [Google Scholar] [CrossRef]
- Losh, J.L.; Young, J.N.; Morel, F.M. Rubisco is a small fraction of total protein in marine phytoplankton. New Phytol. 2013, 198, 52–58. [Google Scholar] [CrossRef]
- Valegård, K.; Andralojc, P.J.; Haslam, R.P.; Pearce, F.G.; Eriksen, G.K.; Madgwick, P.J.; Kristoffersen, A.K.; van Lun, M.; Klein, U.; Eilertsen, H.C. Structural and functional analyses of Rubisco from arctic diatom species reveal unusual posttranslational modifications. J. Biol. Chem. 2018, 293, 13033–13043. [Google Scholar] [CrossRef] [Green Version]
- Svenning, J.B.; Dalheim, L.; Vasskog, T.; Matricon, L.; Vang, B.; Olsen, R.L. Lipid yield from the diatom Porosira glacialis is determined by solvent choice and number of extractions, independent of cell disruption. Sci. Rep. 2020, 10, 22229. [Google Scholar] [CrossRef]
- Dalheim, L.; Svenning, J.B.; Eilertsen, H.C.; Vasskog, T.; Olsen, R.L. Stability of lipids during wet storage of the marine diatom Porosira glacialis under semi-preserved conditions at 4 and 20 degrees C. J. Appl. Phycol. 2020, 11, 385–395. [Google Scholar] [CrossRef]
- Fields, F.J.; Kociolek, J.P. An evolutionary perspective on selecting high-lipid-content diatoms (Bacillariophyta). J. Appl. Phycol. 2015, 27, 2209–2220. [Google Scholar] [CrossRef]
- Widjaja, A.; Chien, C.-C.; Ju, Y.-H. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2009, 40, 13–20. [Google Scholar] [CrossRef]
- Artamonova, E.Y.; Svenning, J.B.; Vasskog, T.; Hansen, E.; Eilertsen, H.C. Analysis of phospholipids and neutral lipids in three common northern cold water diatoms: Coscinodiscus concinnus, Porosira glacialis, and Chaetoceros socialis, by ultra-high performance liquid chromatography-mass spectrometry. J. Appl. Phycol. 2017, 29, 1241–1249. [Google Scholar] [CrossRef]
- Dalheim, L.; Svenning, J.B.; Olsen, R.L. In vitro intestinal digestion of lipids from the marine diatom Porosira glacialis compared to commercial LC n-3 PUFA products. PLoS ONE 2021, 16, e0252125. [Google Scholar] [CrossRef]
- Sayanova, O.; Mimouni, V.; Ulmann, L.; Morant-Manceau, A.; Pasquet, V.; Schoefs, B.; Napier, J.A. Modulation of lipid biosynthesis by stress in diatoms. Philos. Trans. R. Soc. B-Biol. Sci. 2017, 372, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Sustainable aquaculture and animal feed from microalgae–Nutritive value and techno-functional components. Renew. Sustain. Energy Rev. 2021, 150, 111549. [Google Scholar] [CrossRef]
- Becker, W. 18 microalgae in human and animal nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2004; Volume 312. [Google Scholar]
- Giæver, I.H. Diatom Protein and Amino Acids—A Mass Cultivated Northern Diatom and Its Potential for Utilization as Protein Source in Fish Feed; UiT—The Arctic University of Norway: Tromsø, Norway, 2020. [Google Scholar]
- FAO. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization, FAO: Geneva, Switzerland, 2007; Volume 935. [Google Scholar]
- Hackethal, C.; Kopp, J.F.; Sarvan, I.; Schwerdtle, T.; Lindtner, O. Total arsenic and water-soluble arsenic species in foods of the first German total diet study (BfR MEAL Study). Food Chem. 2021, 346, 128913. [Google Scholar] [CrossRef]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef]
- Jinadasa, B.; Jayasinghe, G.; Pohl, P.; Fowler, S.W. Mitigating the impact of mercury contaminants in fish and other seafood—A review. Mar. Pollut. Bull. 2021, 171, 112710. [Google Scholar] [CrossRef]
- EC. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed; European Comission: Brussels, Belgium, 2002; OJ L 140. [Google Scholar]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E.H. Lysophosphatidylcholines and chlorophyll-derived molecules from the diatom Cylindrotheca closterium with anti-inflammatory activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef] [Green Version]
- Eilertsen, H.C.; Elvevoll, E.; Giæver, I.H.; Svenning, J.B.; Dalheim, L.; Svalheim, R.A.; Vang, B.; Siikavuopio, S.; Dragøy, R.; Ingebrigtsen, R.A. Inclusion of photoautotrophic cultivated diatom biomass in salmon feed can deter lice. PLoS ONE 2021, 16, e0255370. [Google Scholar] [CrossRef]

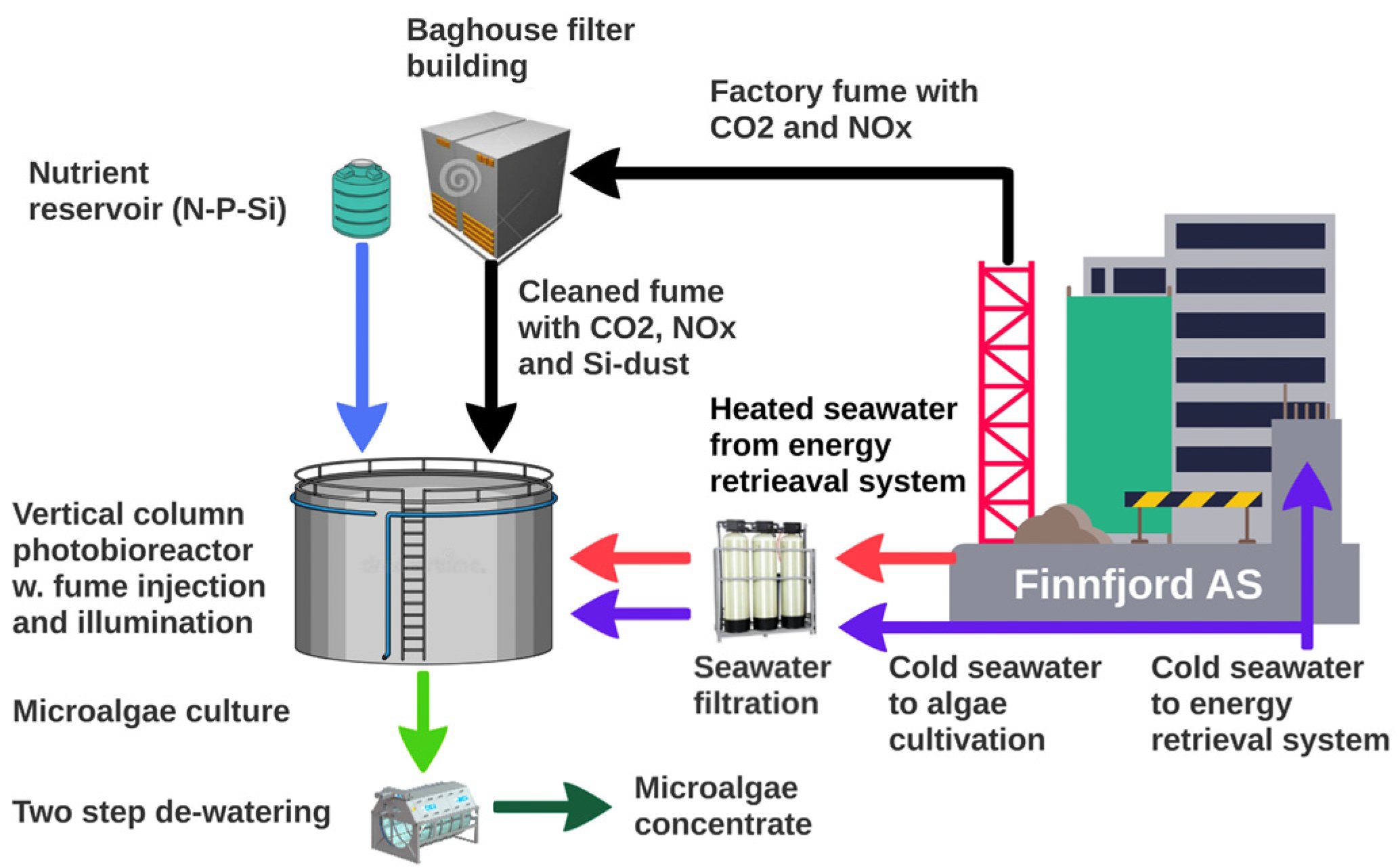
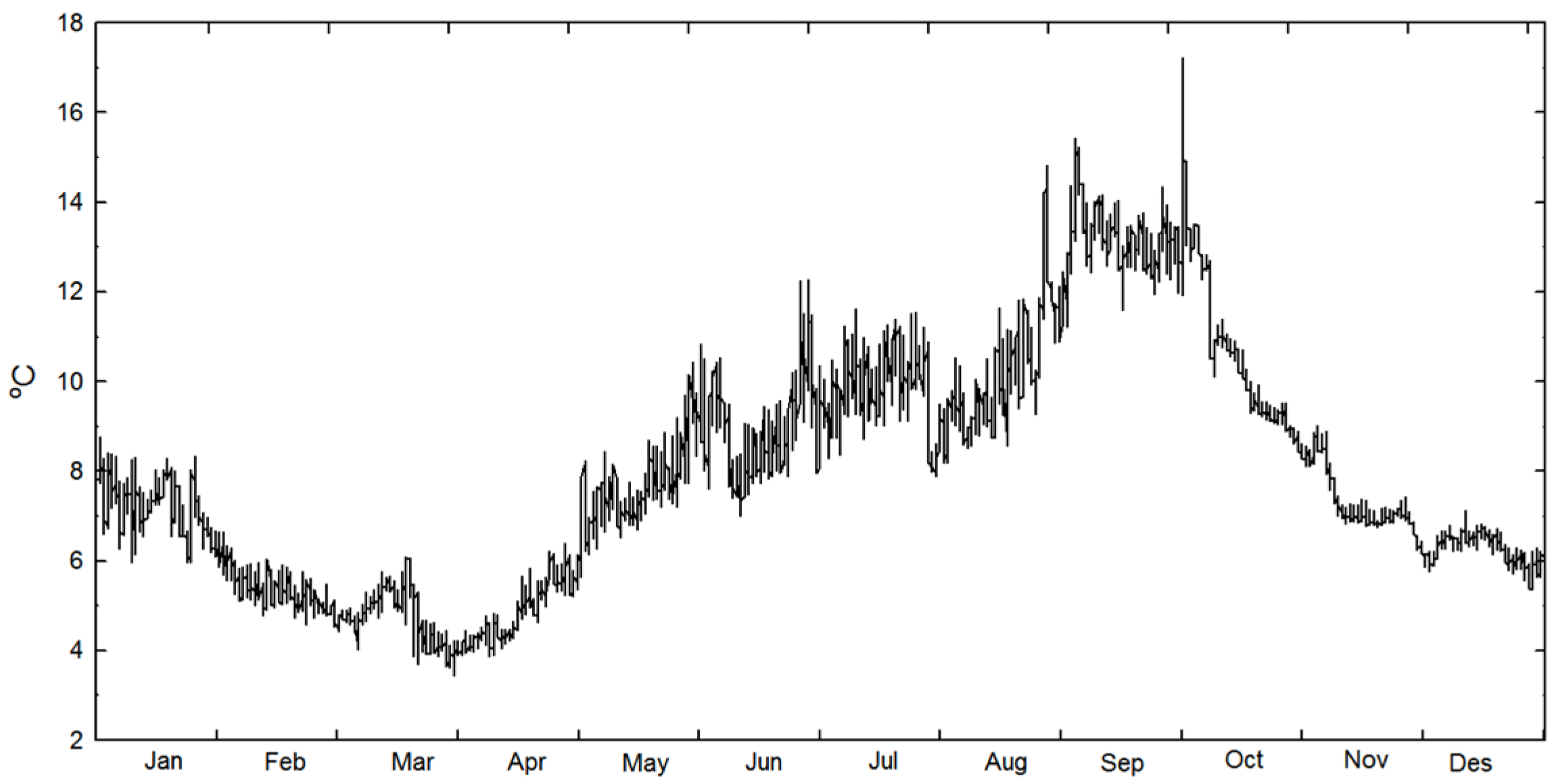
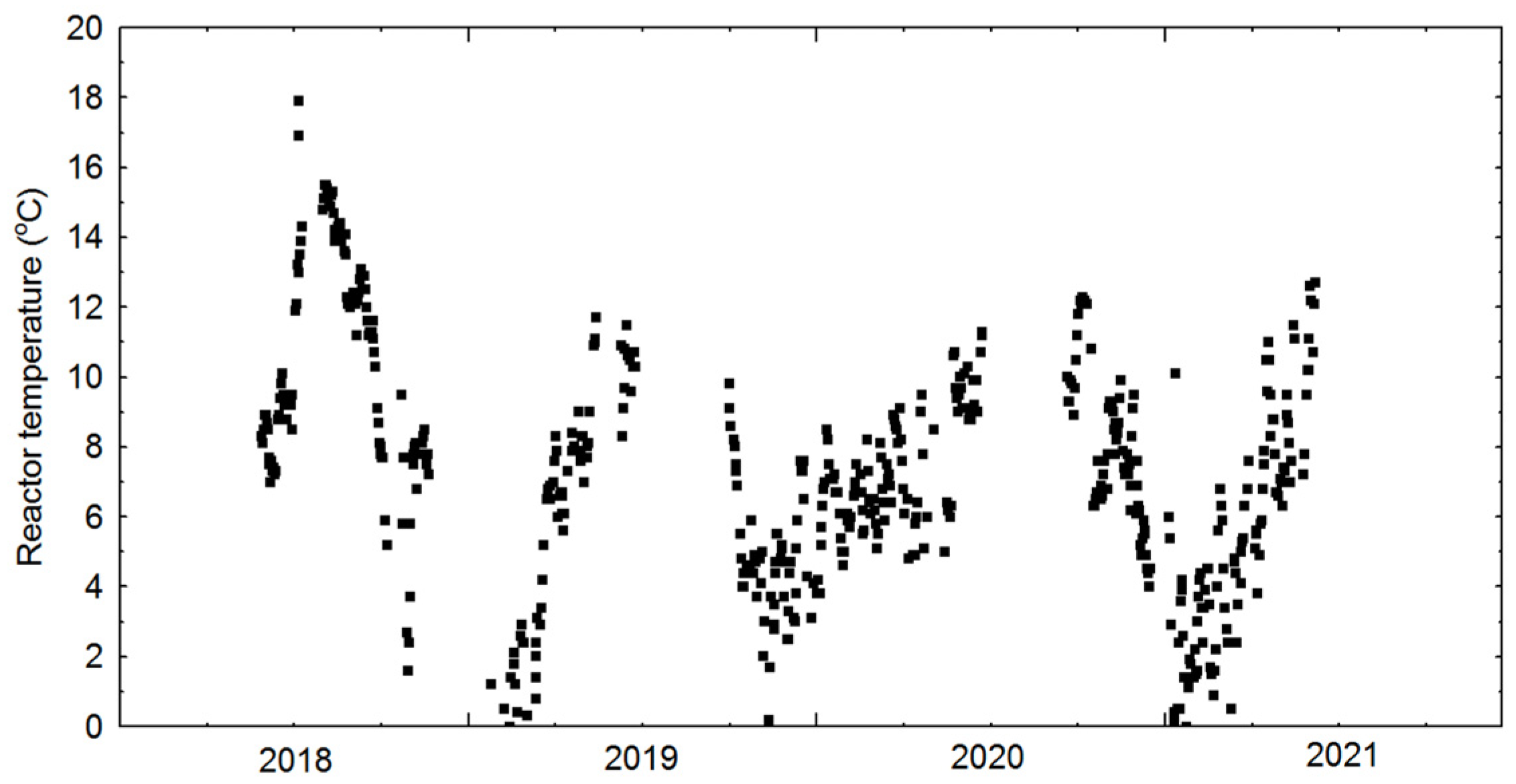
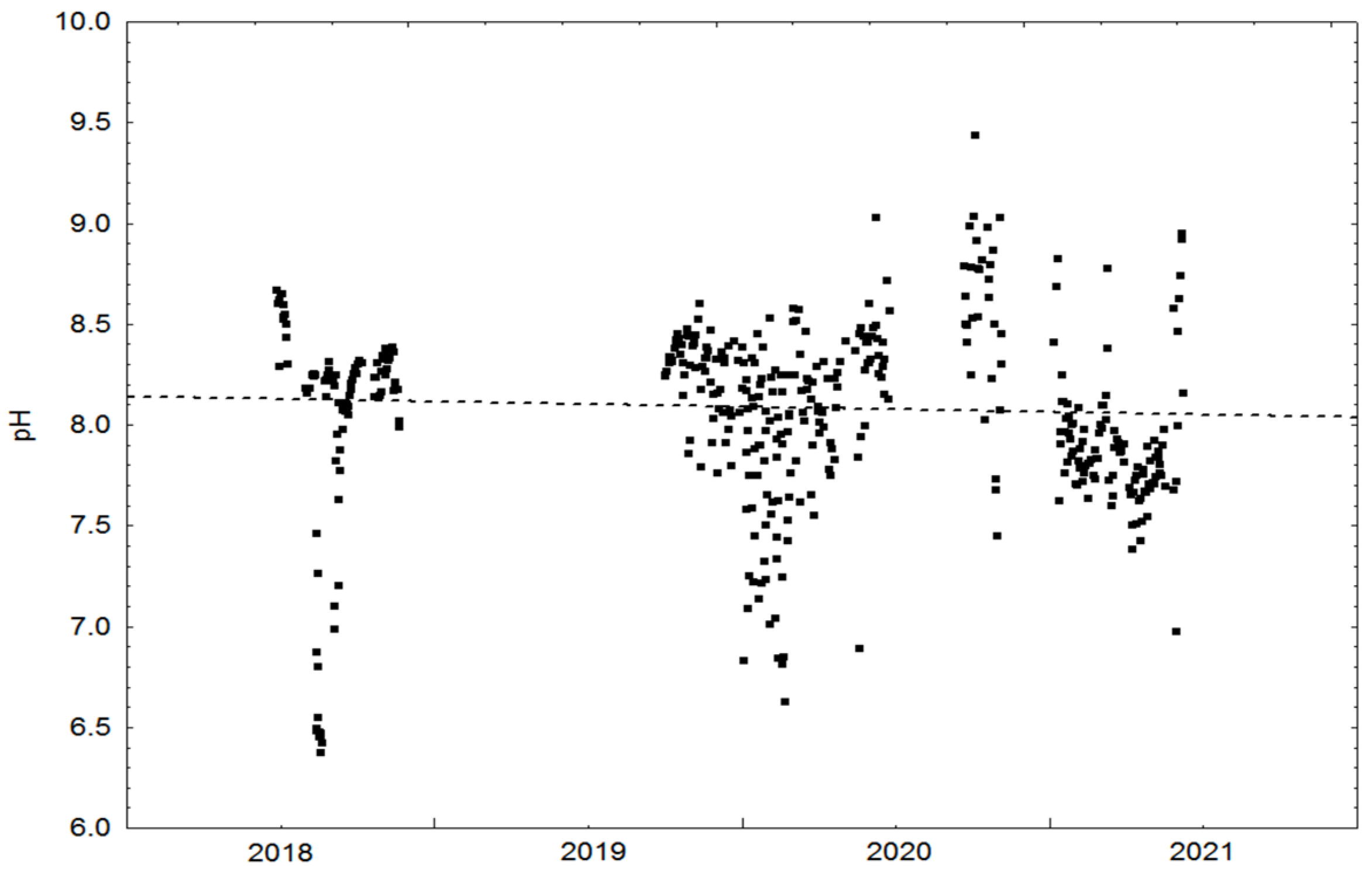


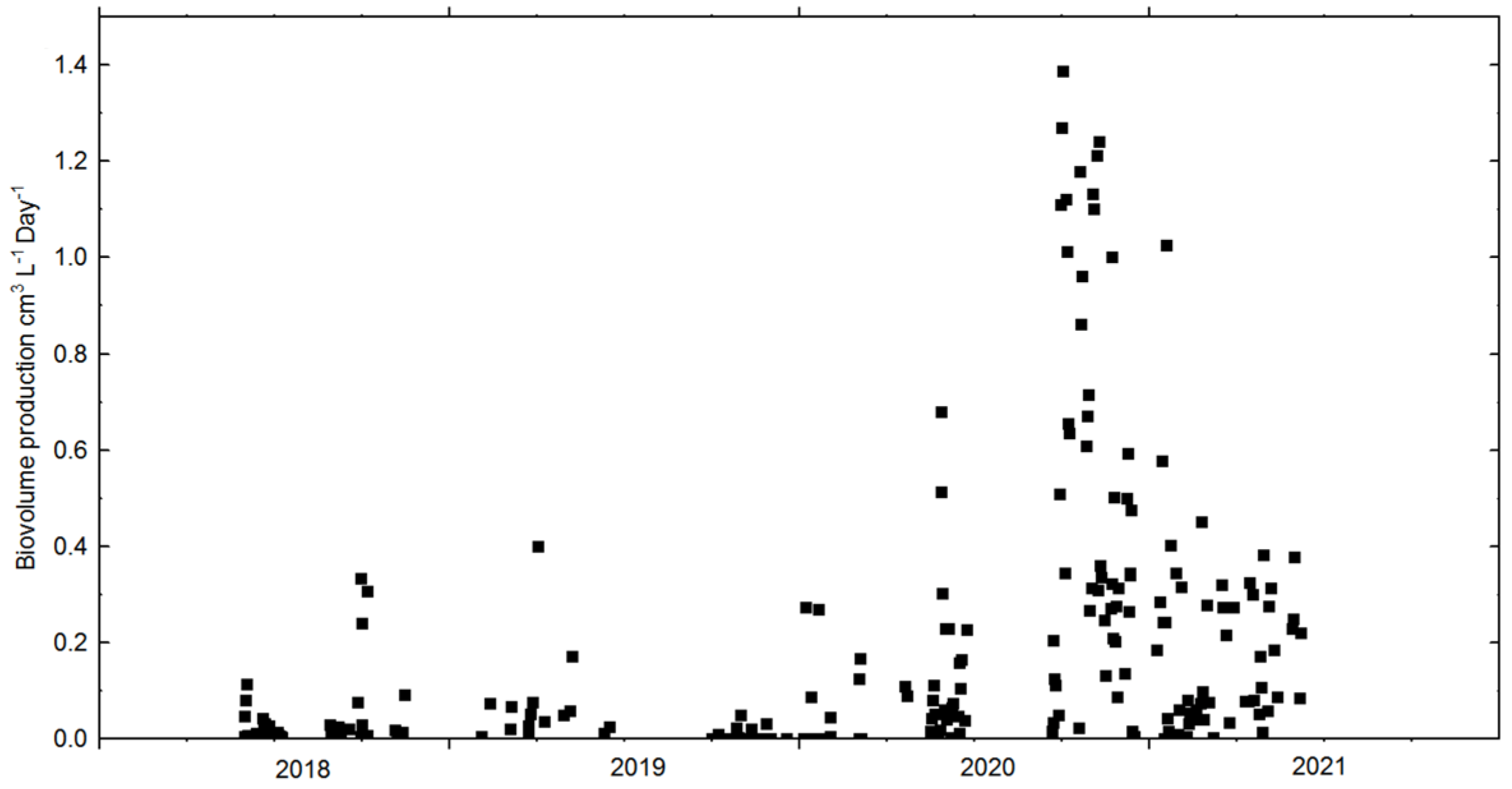

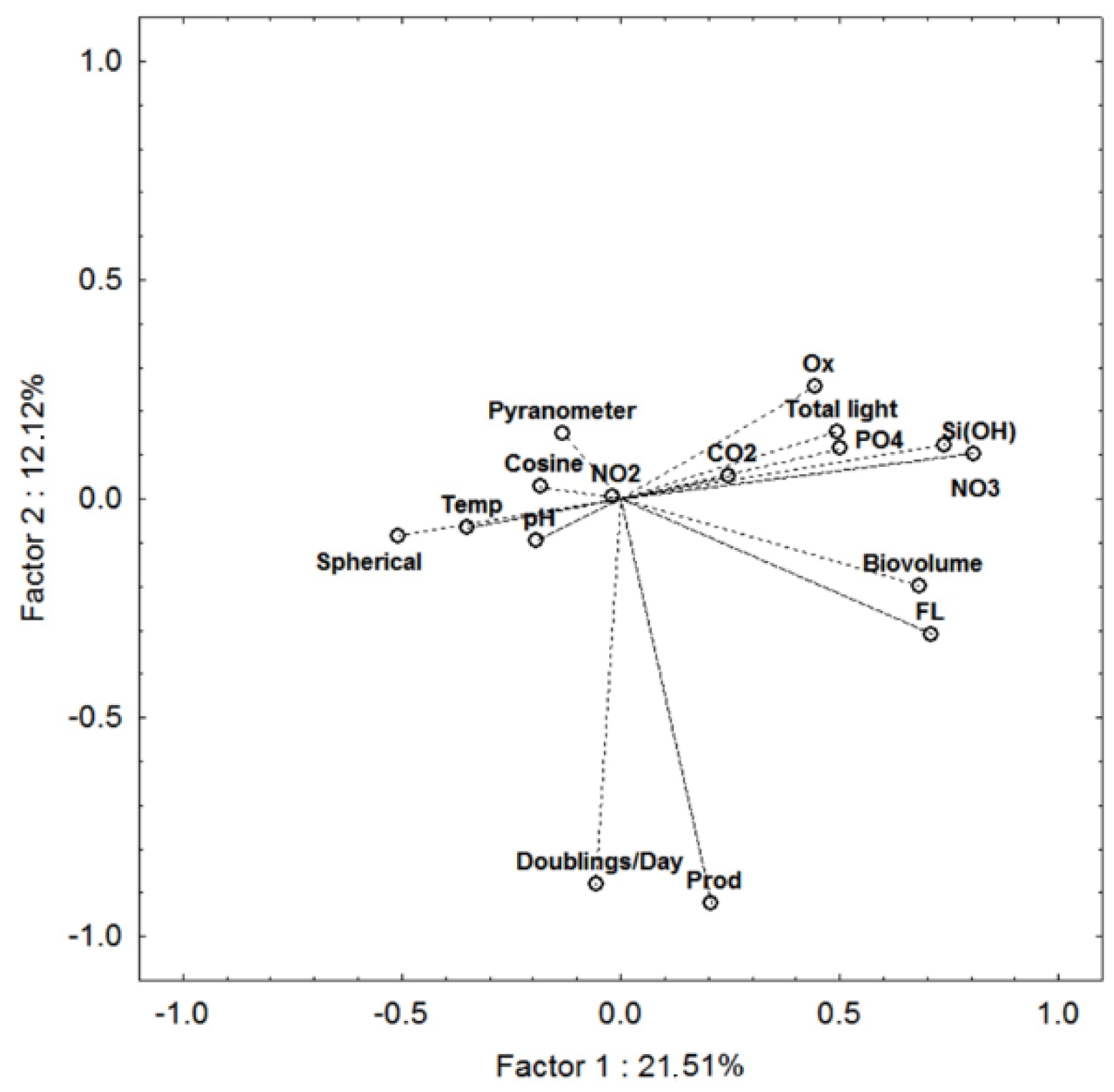
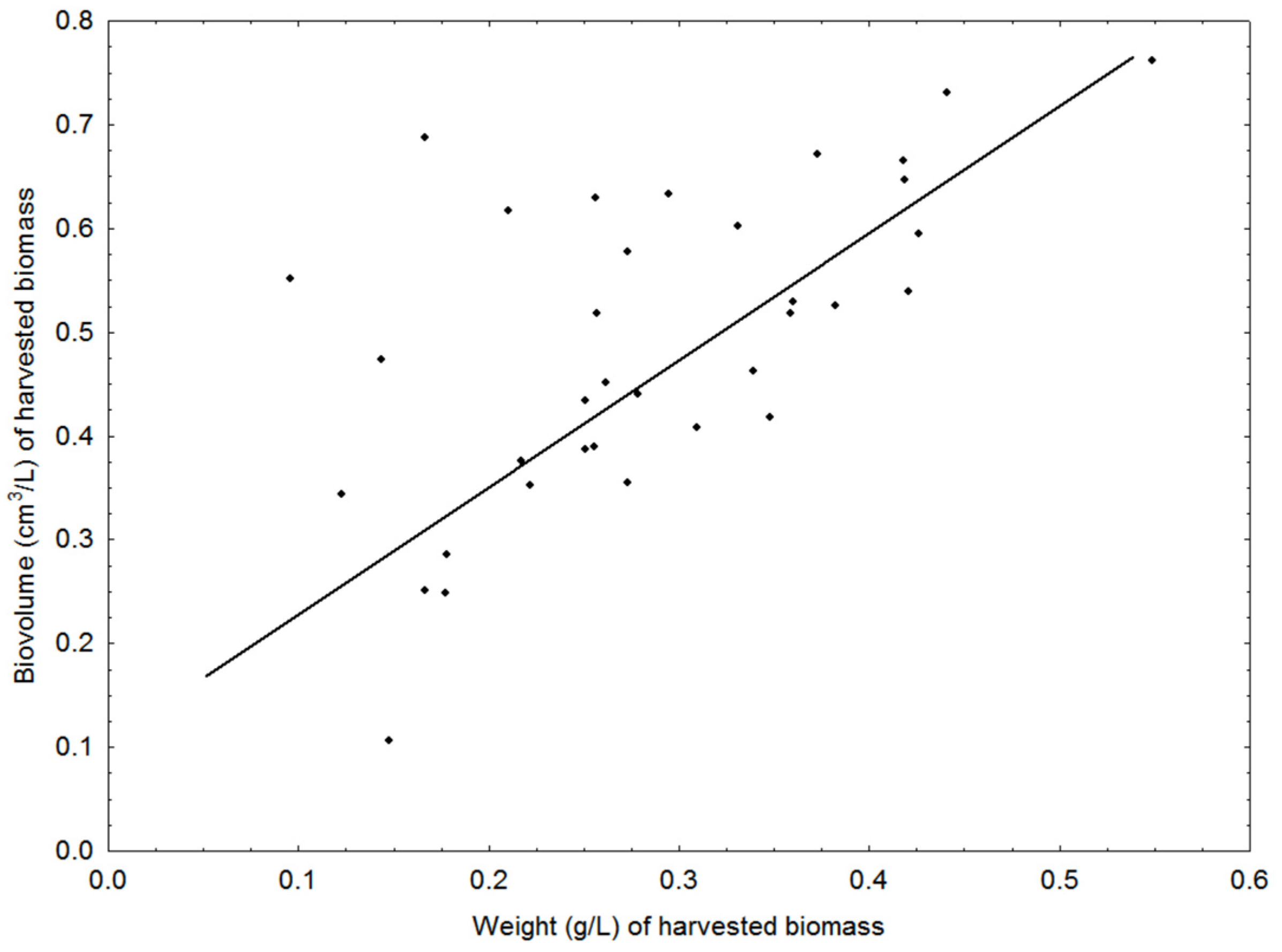

| Species | Diameter (μm) | Area (μm2) | Volume (μm3) | Area: Volume | Species: Chlorella Volume |
|---|---|---|---|---|---|
| Chlorella sp. | 5 | 79 | 65 | 1.22 | 1 |
| Attheya longicornis | 6 | 188 | 198 | 0.95 | 3 |
| Porosira glacialis 1 | 40 | 6280 | 43,960 | 0.14 | 676 |
| Porosira glacialis 2 | 45 | 9538 | 71,505 | 0.13 | 1100 |
| Coscinodiscus radiatus 1 | 180 | 152,604 | 2,289,060 | 0.07 | 35,216 |
| Coscinodiscus radiatus 2 | 220 | 227,964 | 8,366,666 | 0.03 | 128,718 |
| Function | Type | Unit |
|---|---|---|
| CO2 logging, underwater and atmospheric temperatures | CO2-infared (NIDR) detector (Franatech HR, Lüneburg, Germany), coupled to temperature sensor (4-wire platinum temperature 1000) | mg L−1 and % saturation °C |
| CO2, NO2, SO2 in factory fume | Kane Quintox 9206 flue gas analysers, Hertfordshire, UK | mg L−1 and % saturation, ppm |
| pH logging | Endress-Hauser sensor Orbsint CPS11D and 4-channel transmitter Liquiline CM444 | |
| Turbidity logging | Endress-Hauser sensors Turbimax CUS51D-HCC1A4 and 4-channel transmitter Liquiline | NTU |
| Conductivity, salinity logging | Endress-Hauser digital sensor Indumax CLS50D w. 4-channel transmitter Liquiline CM444 | μs cm−1 %o |
| Temperature | Endress-Hauser sensor iTHERM ModuLine TM131 w. 4-channel transmitter Liquiline CM444 | °C |
| pH manual measurement | WTWMulti 360 m withWTWSenTix 940 IDS probe (Xylem Analytics, Weilheim, Germany) | pH |
| Inorganic plant nutrient measurements (NO3−, NO2−, PO4−, Si(OH)4) | Auto analyser (Seal Analytical, Wisconsin, USA). Also every second day in lab at Finnfjord AS from June 2018 with Merck kits: 1.09713.0002; 1.14408.001; 1.14848.0001; 1.01813.0001, adapted to be quantified in plate reader (Molecular devices Filter Max F5) | μmol L−1 |
| Turbulence | NORTEK (Norway) Vectrino velocimeter x/y/z | cm s−1 |
| O2 | WTWMulti 360 w. CellOx 325 sensor (Xylem Analytics, Germany) | Mg L−1 |
| Irradiance logging | LI-COR LI-193 (UK) Spherical quantum, LI-192 (cosine) underwater and LI-200/R Pyranometer sensors with LI-1500 Light sensor logger | μmol quanta m−2 s−1 W m−2 |
| Irradiance and light scans manually atmosphere and sub-surface | Biospherical (USA) QSL-100 instrument, Trios, Rases-Acc-UV Hyperspectral; Irradiance sensor (280–570 nm) LI-COR (UK) LI-180 Spectrometer | μmol quanta m−2 s−1 mW cm−2 nm−1 |
| Chla fluorescence manually in vivo/in vitro | Turner Designs TD-700 (Turner Designs, San Jose, UK) | FL mg Chla L−1 |
| Compressing and move factory fume | 2015- March 2018: Biltema (Norway) OL 20-24; 2018: Nash Vectra XL-80 and XL-35 liquid ring vacuum pumps (Gardner Denver Nash, Quincy, IL, USA) | bar L min−1 |
| Illumination | 2015–2018: sub-surface white LEDs made by authors; 2016: White sub-surface 500W lens cluster LEDs; 2018–2019: 1000W blue and white units from Wuhan ZJKC Technology Co., Ltd., Wuhan, China; 2018–present: Aquagroup 400 W blue, 100 W Aurora white/green; Biomarine 2.5 kW blue (all Norway); Signify 680 W, 440 W (Netherlands); Biltema 46-3174 50W (Norway) | μmol quanta m−2 s−1 mW cm−2 nm−1 % efficiency |
| Water filtration | 2015–2018: Eaton filter cartridges; 2018–2021: AZUD type 203/4VX, self cleaning system, 2 Eaton 5 cyl. Filter cartidges and Ultra aqua UV sterilization unit (Spain) | L |
| De-watering | WRW 5 × 200 mL table centrifuge (UK), plankton nets, Veolia drum filter (Norway), Algae Centrifuge—Solid Bowl (USA), Manual Purge 25 kg–ATD-25 | Kg h−1 |
| Reactors | 2015–2020: 150, 250 L Plexi columns; 2015–2021: 6 m−3 stainless steel (DxH = 1.9 × 2.5 m); 2016–2021: 6 m−3 glass fiber reactor (2 × 2.4 m), 14 m−3 (2 × 3 m) stainless steel; 2018: 300 m−3 glass fiber (Figure 1) | m−3 |
| 0.1 m Depth | 1.2 m Depth | |||||
|---|---|---|---|---|---|---|
| Horizontally | Vertically | Vertically * | Horizontally | Vertically | Vertically * | |
| Absolute Value | −/+ Up/Down | Absolute Value | −/+ Up/Down | |||
| Mean | 0.09905 | 0.055289 | −0.033 | 0.9563 | 0.01714 | −0.00651 |
| Min | 0 | 0 | −0.3081 | 0 | 0 | −0.2079 |
| Max | 1.2802 | 0.3123 | 0.3123 | 1.069 | 0.2272 | 0.2272 |
| Biomass Specific k | Cell Size (μm Diameter) | Biovolume (cm3 L−1) | Cell Concentration (No. L−1) | Scalar Irradiance μmol m−2 s−1 | |
|---|---|---|---|---|---|
| 0 m | 0.6 m | ||||
| 6.5 | 42 | 1 | 18,000,000 | 100 | 2.024 |
| 12.3 | 24 | 1 | 90,000,000 | 100 | 0.062 |
| 3.1 | 42 | 0.5 | 11,000,000 | 100 | 15.56 |
| 5.6 | 24 | 0.5 | 40,000,000 | 100 | 3.47 |
| Variable | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Temperature | −0.350603 | −0.065362 | −0.305317 | −0.702564 |
| pH | −0.191139 | −0.095166 | −0.421719 | −0.390890 |
| CO2 | 0.246988 | 0.051189 | 0.019916 | −0.231155 |
| Fl | 0.709964 | −0.308751 | −0.142231 | 0.108382 |
| Doubl./Day | −0.055976 | −0.880949 | 0.077926 | −0.222984 |
| Oxygen | 0.444822 | 0.257117 | −0.137969 | −0.131388 |
| NO3 | 0.740952 | 0.124719 | 0.058572 | −0.321568 |
| NO2 | −0.017447 | 0.004179 | −0.062530 | 0.330749 |
| PO4 | 0.502714 | 0.115031 | 0.507148 | −0.279079 |
| SiOH | 0.808084 | 0.103553 | 0.048914 | −0.161543 |
| Cosine | −0.184288 | 0.027598 | 0.711832 | −0.237633 |
| Pyranometer | −0.132123 | 0.148798 | −0.348898 | −0.186659 |
| Spherical | −0.507825 | −0.084130 | 0.481529 | −0.076914 |
| Biovolume | 0.681223 | −0.197680 | −0.073498 | 0.335251 |
| Production | 0.207280 | −0.924152 | 0.017919 | −0.017458 |
| Total light | 0.494959 | 0.153890 | 0.022518 | −0.226947 |
| Mean | +/−S.D. | n | Unit | |
|---|---|---|---|---|
| CO2 uptake | 26.45 | 8.85 | - | % |
| Uptake NO3− + NO2− in diatoms * | 110.54 | 146.9 | 117 | μmol cm3 |
| Uptake PO4− in diatoms * | 20.15 | 19.37 | 127 | μmol cm3 |
| Uptake SiO2− in diatoms * | 47.70 | 67.46 | 96 | μmol cm3 |
| Light utilization efficiency | 21.66 | 22.71 | 84 | % (W vs. W) |
| Temperature in reactor | 6.64 | 5.07 | 852 | °C |
| pH | 8.089 | 0.41 | 679 | |
| Biovolume with cells < 30 μm ** | 0.189 | 0.151 | 152 | cm3 |
| Biovolume with cells > 30 μm ** | 0.622 | 0.205 | 102 | cm3 |
| Production with cells < 30 μm ** | 0.219 | 0.327 | 102 | cm3 L−1 Day−1 |
| Production with cells > 30 μm ** | 0.260 | 0.139 | 102 | cm3 L−1 Day−1 |
| With flue gas | 9.9 ± 0.11 |
| Without flue gas | 10.3 ± 0.73 |
| With Flue Gas | Without Flue Gas | |
|---|---|---|
| C14:0 | 9.6 ± 0.16 | 6.8 ± 0.29 |
| C16:0 | 5.7 ± 0.01 | 6.4 ± 0.05 |
| C16:1 n-7 | 8.4 ± 0.04 | 9.9 ± 0.06 |
| C16:3n-4 | 13.9 ± 0.06 | 12.7 ± 0.03 |
| C18:0 | 0.3 ± 0.02 | 0.1 ± 0.13 |
| C16:4n-1 | 15.6 ± 0.06 | 21.6 ± 0.04 |
| C18:2 n-6 | 0.3 ± 0.00 | 0.0 ± 0.00 |
| C20:0 | 0.1 ± 0.13 | 0.3 ± 0.01 |
| C18:4 n-3 | 9.4 ± 0.05 | 4.5 ± 0.01 |
| C22:1 n-11 | 0.3 ± 0.01 | 0.0 ± 0.00 |
| C20:5 n-3 | 32.6 ± 0.19 | 32.8 ± 0.27 |
| C22:6 n-3 | 3.7 ± 0.04 | 4.7 ± 0.06 |
| ∑SFA | 15.7 ± 0.17 | 13.7 ± 0.33 |
| ∑MUFA | 8.6 ± 0.05 | 9.9 ± 0.06 |
| ∑PUFA | 75.7 ± 0.17 | 76.4 ± 0.36 |
| ∑Omega-3 | 45.8 ± 0.26 | 42.1 ± 0.33 |
| With Fume | Without Fume | |
|---|---|---|
| EEA | 122.4 | 110.4 |
| Threonine | 15.2 ± 0.3 | 13.5 ± 0.0 |
| Valine | 16.6 ± 0.2 | 14.5 ± 0.3 |
| Methionine | 8.4 ± 0.3 | 7.4 ± 0.5 |
| Isoleucine | 14.5 ± 0.3 | 12.8 ± 0.2 |
| Leucine | 25.6 ± 0.7 | 23.7 ± 0.2 |
| Phenylalanine | 16.8 ± 0.1 | 16.0 ± 0.4 |
| Lysine | 19.1 ± 0.4 | 16.8 ± 0.5 |
| Histidine | 6.2 ± 0.5 | 5.7 ± 0.1 |
| NEAA | ||
| * Aspartic acid | 32.3± 0.5 | 33.9 ± 0.2 |
| Serine | 15.5 ± 0.0 | 13.9 ± 0.1 |
| * Glutamic acid | 46.9 ± 0.5 | 44.1 ± 0.8 |
| Proline | 24.0 ± 0.6 | 19.7 ± 0.4 |
| Glycine | 18.7 ± 0.7 | 17.7 ± 1.3 |
| Alanine | 38.8 ± 0.5 | 26.1 ± 0.4 |
| Cysteine | 2.4 ± 0.2 | 2.3 ± 0.1 |
| Tyrosine | 12.4 ± 0.4 | 9.9 ± 0.1 |
| Arginine | 21.8 ± 0.4 | 19.9 ± 1.5 |
| P-Serine | 4.0 ± 0.0 | 2.4 ± 0.0 |
| TAA | 339.2 | 300.3 |
| %EAA | 36 | 37 |
| Tot protein | 286.3 ± 4.6 | 254.7 ± 4.5 |
| CS | >1.0 | >1.0 |
| pg TE/g ww | With Fume UB Incl LOD | With Fume LB Excl LOD | Without Fume UB Incl LOD | Without Fume LB Excl LOD |
|---|---|---|---|---|
| Sum PCDD/PCDF * | 0.049 | 0.025 | 0.036 | 0.013 |
| Sum no-PCB ** | 0.007 | 0.007 | 0.003 | 0.003 |
| Sum mo-PCB *** | 0.0002 | 0.0001 | 8.9 × 10−5 | 5.3 × 10−5 |
| Total sum TE | 0.057 | 0.033 | 0.040 | 0.016 |
| ng/g ww | With Fume | Without Fume |
|---|---|---|
| Sum ICES-6 PCB * | 0.07 | 0.02 |
| PeCB | 0.012 | 0.047 |
| HCB | 0.026 | 0.041 |
| α-HCH | 0.005 | 0.006 |
| β-HCH | 0.003 | 0.002 |
| γ-HCH | 0.010 | 0.007 |
| op′-DDD | 0.001 | <0.001 |
| pp-DDD | 0.003 | <0.001 |
| PFUnDA | 0.008 | 0.016 |
| mg/kg dw | With Fume | Without Fume |
| Mercury (Hg) | 0.005 | 0.002 |
| Lead (Pb) | 1.32 | 0.16 |
| Cadmium (Cd) | 0.02 | 0.01 |
| Arsenic (As) | 2.58 | 1.72 |
| Selenium (Se) | 0.67 | 0.27 |
| Reactor Type | Production Range | Mean Production | Mean Growth Rate | Mean Volume Reactor |
|---|---|---|---|---|
| g L−1 day−1 | Doublings day−1 | L | ||
| Plate | 0.2–3.8 | 2.4 | 1.2 | 6500 |
| Tubular | 0.05–2.7 | 1.2 | 0.6 | 2800 |
| Column | 0.06–0.4 | 0.2 | 0.2 | 550 |
| Pond | 0.1–0.35 | 0.6 | 0.6 | 50,000 |
| Other | 0.05–0.5 | 0.08 | 0.08 | 100 |
| Finnfjord * | 0.18–0.42 | 0.22 | 0.47 | 300,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eilertsen, H.C.; Eriksen, G.K.; Bergum, J.-S.; Strømholt, J.; Elvevoll, E.; Eilertsen, K.-E.; Heimstad, E.S.; Giæver, I.H.; Israelsen, L.; Svenning, J.B.; et al. Mass Cultivation of Microalgae: I. Experiences with Vertical Column Airlift Photobioreactors, Diatoms and CO2 Sequestration. Appl. Sci. 2022, 12, 3082. https://doi.org/10.3390/app12063082
Eilertsen HC, Eriksen GK, Bergum J-S, Strømholt J, Elvevoll E, Eilertsen K-E, Heimstad ES, Giæver IH, Israelsen L, Svenning JB, et al. Mass Cultivation of Microalgae: I. Experiences with Vertical Column Airlift Photobioreactors, Diatoms and CO2 Sequestration. Applied Sciences. 2022; 12(6):3082. https://doi.org/10.3390/app12063082
Chicago/Turabian StyleEilertsen, Hans Chr., Gunilla K. Eriksen, John-Steinar Bergum, Jo Strømholt, Edel Elvevoll, Karl-Erik Eilertsen, Eldbjørg Sofie Heimstad, Ingeborg Hulda Giæver, Linn Israelsen, Jon Brage Svenning, and et al. 2022. "Mass Cultivation of Microalgae: I. Experiences with Vertical Column Airlift Photobioreactors, Diatoms and CO2 Sequestration" Applied Sciences 12, no. 6: 3082. https://doi.org/10.3390/app12063082
APA StyleEilertsen, H. C., Eriksen, G. K., Bergum, J.-S., Strømholt, J., Elvevoll, E., Eilertsen, K.-E., Heimstad, E. S., Giæver, I. H., Israelsen, L., Svenning, J. B., Dalheim, L., Osvik, R., Hansen, E., Ingebrigtsen, R. A., Aspen, T., & Wintervoll, G.-H. (2022). Mass Cultivation of Microalgae: I. Experiences with Vertical Column Airlift Photobioreactors, Diatoms and CO2 Sequestration. Applied Sciences, 12(6), 3082. https://doi.org/10.3390/app12063082







