Featured Application
Increased Atopobium parvulum, Enterococcus faecalis, Fusobacterium nucleatum, and Porphyromonas gingivalis can be a high risk for the incidence of postoperative pneumonia.
Abstract
Postoperative pneumonia is a serious problem for patients and medical staff. In Japan, many hospitals introduced perioperative oral care management for the efficient use of medical resources. However, a high percentage of postoperative pneumonia still developed. Therefore, there is a need to identify the specific respiratory pathogens to predict the incidence of pneumonia The purpose of this study was to find out the candidate of bacterial species for the postoperative pneumonia. This study applied case-control study design for the patients who had a cancer operation with or without postoperative pneumonia. A total of 10 patients undergoing a cancer operation under general anesthesia participated in this study. The day before a cancer operation, preoperative oral care management was applied. Using the next generation sequence, oral microbiome of these patients was analyzed at the time of their first visit, the day before and after a cancer operation. Porphyromonas gingivalis and Fusobacterium nucleatum group can be a high risk at first visit. Atopobium parvulum and Enterococcus faecalis before a cancer operation can be a high risk. Poor oral hygiene increased the risk of incidence of postoperative pneumonia. Increased periodontal pathogens can be a high risk of the incidence of postoperative pneumonia. In addition, increased intestinal bacteria after oral care management can also be a high risk for the incidence of postoperative pneumonia.
1. Introduction
The oral microbiome contains over 700 species of bacteria in the oral cavity which comprises diverse anatomic structures [1]. Tooth surface as a hard tissue can be the scaffold for the biofilm formation. Oral biofilm contains not only commensal oral bacteria, but external pathogenic bacteria including opportunistic pathogens [2]. Periodontal pocket harbors pathogenic anaerobes for periodontal tissue [3]. These pathogens also impact on respiratory disease [4] by causing inflammatory response to the endotoxin of the periodontal pathogens. Poor oral hygiene causes the dysbiosis of oral microbiome. For dysbiosis, Porphyromonas gingivalis and Streptococcus mutans are emphasized because of their bacterial metabolism and virulence, community development, and bacteria–host interactions [5]. Oral microbiome is the main source of lung microbiome [6]. Aspiration of opportunistic respiratory pathogens can cause pneumonia [7]. Increased anaerobe including periodontal pathogens in the oral microbiome are suggested to be a risk for the multiple respiratory diseases. Among the common postoperative complications, incidence of postoperative pneumonia is the third in all surgical procedures [8]. It is a serious problem for patients and as well as medical staff. In addition, it increases the hospitalization days and workload of medical staff. Finally, it increases medical costs. The medical costs increased from USD12,000 to USD40,000 by the development of ventilator-associated pneumonia [9,10,11,12].
Dental intervention can alter the aspiration of oral and respiratory pathogens into the lungs and prevent inflammatory responses. A systematic review reported an average of 40% reduction in the incidence of nosocomial pneumonia by dental interventions [13]. Therefore, in Japan, many hospitals introduced perioperative oral care management for the efficient use of medical resources. However, even by the application of perioperative oral care management, a high percentage of postoperative pneumonia still developed. This may be because several times or short terms dental intervention may not alter the dysbiosis of oral microbiome completely. Therefore, there is a need to identify the specific respiratory pathogens to predict the incidence of pneumonia. The purpose of this study was to find out the bacterial species that can be the candidate of postoperative pneumonia by applying a case control study design.
2. Materials and Methods
2.1. Study Design
This study applied a case-control study design for the patients who had a cancer operation with or without postoperative pneumonia. Hospitalized patients at Kyusyu University Hospital from December 2020 to July 2021 who underwent a cancer operation and visited perioperative oral management center were recruited. The inclusion criterion was having undergone surgery under general anesthesia with epidural anesthesia All patients were extubated in the operating room. Edentulous patients were excluded. Among them, patients who had postoperative pneumonia were selected. Type of cancer was used for matching. Informed consent was obtained from all the patients. All the patients underwent chemotherapy after a cancer operation.
A total of 10 patients participated in this study. The diagnosis was stomach cancer for four patients, esophageal cancer for four patients and pancreatic cancer for two patients.
2.2. Oral Examination and Preoperative Oral Care
One dentist (Y.I) conducted oral examination and preoperative oral care. Number of remaining teeth, O’Leary plaque control records at initial visit to perioperative oral management center and the day before cancer operation were recorded. Mel Sage PC pellets (SHOFU Inc., Kyoto, Japan) was used for staining dental plaque.
For preoperative oral care management, dental plaque was completely removed by Professional Mechanical Tooth Cleaning until no stained plaque was observed. Dental plaque was removed by the dentist (Y.I) by using contra-angle handpiece with polishing paste (MERSSAGE, SHOFU, Kyoto, Japan) until no stained plaque was observed. A sponge brush was used to clean up the oral mucosa. When dental calculous was detected, scaling was performed. This procedure was performed at the initial visit and the day before the cancer operation [14].
2.3. Sampling
Coat of the tongue was scrapped by mucosal brush (ERAC 541 S: LION., Tokyo, Japan) 5 times. The tongue coat attached to the mucosal brush was suspended in ice-cold phosphate-buffered saline and immediately stocked in the freezer (−20 °C) until the microbiome analysis. Sampling was performed at initial visit for perioperative oral management center, the day before a cancer operation and the day after a cancer operation.
2.4. Microbial DNA Extraction
Tongue surface samples suspended in PBS were collected by a centrifuge at 3000 rpm for 10 min. DNA extraction was performed by a Maxwell 16 LEV Blood DNA Kit (Promega KK, Tokyo, Japan) according to the manufacturer’s instructions. DNA concentration was measured by a NanoDrop ND-2000 (Thermo Fisher Scientific KK, Tokyo, Japan). Degradation of DNA was visually checked by electrophoresis on 1% agarose gel. Degradation of DNA and contamination of RNA were checked by a Qubit dsDNA BR Assay Kit (Thermo Fisher Scientific KK, Tokyo, Japan). Samples meeting the following criteria were used for further sequence analysis: conc > 20 ng/μL, volume ≥ 20 μL, A260/280 ≥ 1.8 and A260/230 > 1.5. In this study, all samples met the criteria.
2.5. Microbial Community Analysis
Extracted DNA was analyzed in a laboratory (Chun Lab, Seoul, Korea). Polymerase chain reaction PCR amplification was performed using primers specific to the V3–V4 region pyrosequencing tags of the 16S rRNA gene in the extracted bacterial DNA. Taxonomic classification of each read was assigned based on a search of the EzBioCloud 16S database [15,16], which contains the 16S rRNA genes of type strains that have valid published names and representative species-level phylotypes of both cultured and uncultured entries in the GenBank database, with complete hierarchical taxonomic classification from the phylum to species level [17].
2.6. Diagnosis of Pneumonia
Pneumonia was diagnosed by standard criteria: fever (body temperature of ≥37.5 °C), high serum C-reactive protein levels and an infiltration shadow on chest computed tomography [18].
2.7. Statistical Analysis
Descriptive statistics, ROC analysis and decision analysis were carried out by SPSS Statistics ver 27.0 (IBM, Tokyo, Japan). Microbiome analysis was conducted by Bioconductor on free software R ver 4.03. The package used in this study were microbiome, phyloseq, vegan, ape, knitr and Rtsne [19,20].
For the ordination analysis, tSNE was used. t-SNE is a tool to visualize high-dimensional data. It converts similarities between data points to joint probabilities and tries to minimize the Kullback–Leibler divergence between the joint probabilities of the low-dimensional embedding and the high-dimensional data. It is highly recommended to use another dimensionality reduction method to reduce the number of dimensions to a reasonable amount if the number of features is very high. This method suppresses some noise. It plots the similar data points on the same map as close as possible. Therefore, tSNE is often used for microbiome analysis as a popular new ordination technique [21,22,23,24].
3. Results
3.1. Clincal Parameters of the Patients Who Paticipated in This Study
In this study, a total of 10 patients were analyzed. Five patients with postoperative pneumonia as a case and five patients without postoperative pneumonia as a control participated. Clinical data of these patients were shown in Table S1. No statically significant difference was observed except for O’Leary plaque control record.
3.2. Sequence and Adiversity
From 30 tongue coat samples obtained from the 10 subjects, 895,680 reads (minimum, 4273; maximum, 49,714) passed quality control. Sequences were clustered to 22 phyla, 51 classes, 87 orders, 137 families, 298 genera and 682 species. All 682 species are visualized using a heatmap in Supplementary Materials Figure S1. Indexes concerning α diversity are shown in Table S2. The rarefaction curve is presented in Figure S2.
3.3. Changes in Oral Microbiome Composition at Phylum and Genous Level
The changes in oral microbiome compositions at baseline, before and after a cancer operation at the phylum and genius level were shown in Figure 1. For phylum levels of oral microbiome, Firmicutes was decreased in all cases after a cancer operation when compared with baseline.
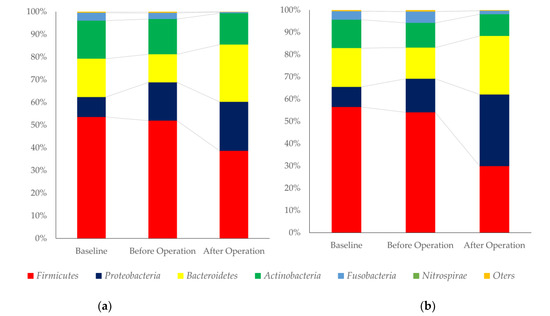
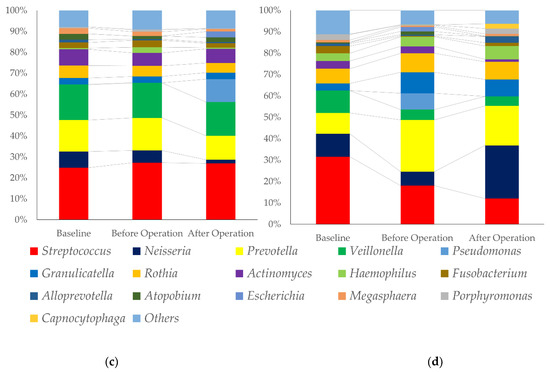
Figure 1.
Composition of oral microbiome of the patients with or without fever after a cancer operation. (a): Phylum level changes in oral microbiome, patients with pneumonia after a cancer operation, (b): Phylum level changes in oral microbiome, control, (c): Genius level changes in oral microbiome, patients with pneumonia after a cancer operation, and (d): Genius level changes in oral microbiome, control.
Proteobacteria and Bacteroides were increased after operation. For genius level, Streptococcus was decreased, and Neisseria was increased in the control groups. Pseudomonas and Fusobacterium were higher in patients with a fever after operation.
3.4. Prediction of Incience of Pneumonia by Oral Bacterial-Specific Species
3.4.1. ROC Analysis for the Prediction of Incidence of Pneumonia
To find out the specific species which can predict the incidence of pneumonia after a cancer operation, ROC analysis was performed separately by oral microbiome of baseline and before operation. The species that had more than 0.75 AUR were selected. In these species, periodontal pathogens (Porphyromonas gingivalis, Tannerella forsythia, and Fusobacterium nucleatum) were included. The results were shown in Table 1.

Table 1.
Sensitivity and specificity of species levels of oral bacteria for the incidence of fever after operation; (a) Baseline; (b) Before cancer operation.
3.4.2. Decision Analysis for the Prediction of Incidence of Pneumonia
By using selected species, decision analysis was carried out to find out the rules to predict the fever after operation. The results were shown in Figure 2.

Figure 2.
Decision tree for the prediction of incidence of pneumonia by specific species; (a): Baseline; (b): Before cancer operation. “+”: incidence of pneumonia, “-“: without incidence of pneumonia.
To predict the incidence of pneumonia after operation, Porphyromonas gingivalis and Fusobacterium nucleatum group at baseline can be a high risk, Atopobium parvulum and Enterococcus faecalis before a cancer operation can be a high risk.
3.5. Ordonation Analysis of the Oral Pathogenic Species for the Pnumonia
To find out the correlations of the species listed in Table 1, tSNE analysis was carried out. The results were graphically illustrated in Figure 3. In the baseline microbiome, pathogenic bacteria for pneumonia were closely located; however, before operation, pathogenic bacteria for pneumonia were separately located. The correlation heatmaps of the pathogen for pneumonia against other oral microbiomes are shown in Figure S6.
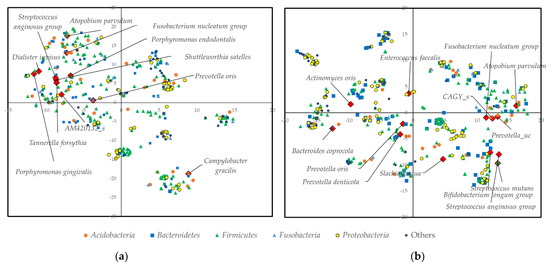
Figure 3.
tSNE analysis of the oral microbiome; (a) Baseline; (b) Before cancer operation.
4. Discussion
In this study, we investigated the pathogenic bacteria for the postoperative pneumonia after cancer surgery under general anesthesia. By decision analysis, pathogens that can be predict the incidence postoperative pneumonia were presented.
Figure 1 shows the changes in the proportion of oral microbiome. At the phylum level, Bacteroides and Proteobacteria were major components of Gram-negative bacteria and Firmicutes and Actinobacteria were major components of Gram-positive bacteria. The proportion of Gram-positive bacteria were decreased, and Gram-negative bacteria were increased. However, a clear difference between the two groups was not observed. The changes may be derived from the effect of lung dysbiosis by mechanical ventilation. A previous report had shown that mechanical ventilation was associated with changes in the respiratory microbiome [25]. The component of respiratory microbiome was different between patients with or without ventilation-associated pneumonia [26]. A “microbial shift” occurred in dental plaque, with colonization by potential VAP pathogens and reverted back to having a predominantly normal oral microbiota after extubation [27,28]. At the genus level, Streptococci and Veillonella were decreased, and Neisseria was increased after cancer operation in the control group. Streptococci and Veillonella congregate and promote the formation of early biofilm [29,30,31]. Neisseria increase at the stage of dental biofilm re-development [32].
The plaque control record was lower in the control group. The amount of biofilm before a cancer operation may reflect on the results. Neisseria and Granulicatella were decreased in pneumonia group after a cancer operation. Most of the species belong to these two genera are commensal bacteria of oral microbiome [32,33,34]. MALDI-TOF mass spectrometry made it possible to identify the pathogens in biofilm [35].
At the initial visit, Porphyromonas gingivalis and Fusobacterium nucleatum were a high risk for the incidence of postoperative pneumonia. These species were periodontal pathogens. Effect of the Porphyromonas gingivalis on the pneumonia has been intensively studied. Human respiratory epithelial cell lines induced proinflammatory cytokines [36]. Gingipains, which is known as proteolytic enzymes produced by Porphyromonas gingivalis manipulate innate immune responses and induce TNF, IL-6, IL-17 and C-reactive protein [37]. Outer membrane vesicles produced by induced cell death in lung epithelial cells [38]. However, when comparing infectious pneumonia and noninfectious pulmonary disease, the proportion of periodontopathic bacterial DNA did not differ between the two groups [39]. Periodontal pathogens may play an indicator for the prediction of postoperative pneumonia. Further study is necessary to detect periodontal pathogens directly from the inflated respiratory tissue. As the O’Leary plaque control records at both initial visit and after preoperative oral care management, remaining dental plaque may contain increased pathogens. For cases included in our study we did not take into consideration the possible associated pathology of respiratory allergies and asthma that can modify the commensal flora at the level of the aerodigestive tract and increase the risk of postoperative pneumonia [40]. In addition, we could set up the medical history completely. Diabetes is a risk for postoperative pneumonia [41]. It is one of the limitations of this study.
A previous report had shown that Fusobacterium nucleatum, which is periodontal commensal and pathogen, can occasionally cause remote infections [42]. Human bronchial and pharyngeal epithelial cells induce proinflammatory cytokine production by exposure to an increased number of Fusobacterium nucleatum [43]. Colonization of oropharynx or lower respiratory tract led to the risk of ventilator-associated pneumonia [44]. In addition, it was significantly increased in COVID-19 patients [45]. Therefore, increased Fusobacterium nucleatum in oral microbiome may be the pathogen responsible for postoperative pneumonia at both initial visit and after preoperative oral care management.
After oral care management, Atopobium parvulum and Enterococcus faecalis were a high risk for the incidence of postoperative pneumonia. These pathogens were enterobacteria. Atopobium parvulum and Fusobacterium nucleatum are suggested to be associated with the tumorigenesis and stage of colorectal cancers. A transition to a lung microbiome enriched with gut flora is found in patients with acute respiratory distress syndrome with an increased inflammatory response [26,46].
Continuous elevated Fusobacterium nucleatum in human gut microbiome is associated with the stage from intramucosal carcinoma to more advanced. To control these species, antimicrobials or probiotics should be applied [47,48]. At the stage of multiple polypoid adenomas and/or intramucosal carcinomas, increased co-occurred Atopobium parvulum and Actinomyces odontolyticus were observed [49]. In addition, Atopobium parvulum was commonly detected from oral cavities of older adults [24]. Even though the patients who participated in this study did not have colorectal cancers, the patients had cancers associated with the digestive system. Increased enterobacteria can be a high risk for the incidence of postoperative pneumonia.
The high similarity between the microbiomes of dental plaque, non-directed bronchial lavages and endotracheal tube biofilms in mechanically ventilated patients [50]. In this study, we could not determine the causative agents of pneumonia. Sampling from bronchial lavage or aspirates is necessary to determine the causative agents. However, pathogenic bacteria in oral microbiome can be a risk for postoperative pneumonia after cancer surgery.
5. Conclusions
Poor oral hygiene increased the risk of incidence of postoperative pneumonia. Increased periodontal pathogens can be a high risk for the incidence of postoperative pneumonia. In addition, increased intestinal bacteria after oral care management can also be a high risk for the incidence of postoperative pneumonia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12062920/s1, Figure S1: Heatmap of the oral microbiome from 30 samples obtained by 10 patients; Figure S2: Rarefaction curve; Figure S3: Taxa prevalence; Figure S4: Canonical correspondence analysis (CCA); Figure S5: Network plot; Figure S6: Correlation heatmap of the pathogen for pneumonia; Table S1: Summary of the clinical data of the patients who participated in this study; Table S2: Indexes of αdiversity. File S1: All the data analyzed in this study.
Author Contributions
Conceptualization, Y.N. and Y.I.; methodology, Y.N. and Y.I.; software, Y.N.; validation, Y.S., A.O., Y.Y. and K.S.; formal analysis, Y.N. and Y.S.; investigation, Y.I., Y.S., A.O., Y.Y. and K.S.; resources, Y.I. and N.W.; data curation, A.O., Y.Y. and K.S.; writing—original draft preparation, Y.N.; writing—review and editing, N.H.; visualization, Y.N. and Y.S.; supervision, N.W. and N.H.; project administration, Y.N., Y.I., N.W. and N.H.; funding acquisition, Y.N., K.S. and N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the JSPS KAKENHI (grant numbers 20K10303 and 20K18815) and the SECOM Science and Technology Foundation.
Institutional Review Board Statement
Approval for this study was obtained from the Institutional Review Board of Kyushu University Hospital (Approval Number: 2020473).
Informed Consent Statement
Written informed consent was obtained from all the patients included in the study.
Data Availability Statement
All the data analyzed in this study are presented in File S1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Könönen, E.; Müller, H.P. Microbiology of aggressive periodontitis. Periodontol 2000 2014, 65, 46–78. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Khadka, S.; Khan, S.; King, A.; Goldberg, L.R.; Crocombe, L.; Bettiol, S. Poor oral hygiene, oral microorganisms and aspiration pneumonia risk in older people in residential aged care: A systematic review. Age Ageing 2021, 50, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Shen, D.; Liu, C.; Ding, Y. Protein Tyrosine and Serine/Threonine phosphorylation in oral bacterial dysbiosis and bacteria-host interaction. Front. Cell. Infect. Microbiol. 2022, 11, 814659. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.J.; Scannapieco, F.A.; Sethi, S. Oral-lung microbiome interactions in lung diseases. Periodontol 2000 2020, 83, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontol 2000 2016, 72, 153–175. [Google Scholar] [CrossRef]
- Kazaure, H.S.; Martin, M.; Yoon, J.K.; Wren, S.M. Long-term results of a postoperative pneumonia prevention program for the inpatient surgical ward. JAMA Surg. 2014, 149, 914–918. [Google Scholar] [CrossRef] [Green Version]
- Chastre, J.; Fagon, J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef]
- Elwishahy, A.; Antia, K.; Bhusari, S.; Ilechukwu, N.C.; Horstick, O.; Winkler, V. Porphyromonas gingivalis as a risk factor to Alzheimer’s Disease: A Systematic Review. J. Alzheimers Dis. Rep. 2021, 5, 721–732. [Google Scholar] [CrossRef]
- Rello, J.; Ollendorf, D.A.; Oster, G.; Vera-Llonch, M.; Bellm, L.; Redman, R.; Kollef, M. Epidemiology and outcomes of ventilatorassociated pneumonia in a large US database. Chest 2002, 122, 2115–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, D.K.; Shukla, S.J.; Olsen, M.A.; Kollef, M.H.; Hollenbeak, C.S.; Cohen, M.M.; Fraser, V.J. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit. Care Med. 2003, 31, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Bush, R.B.; Paju, S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann. Periodontol. 2003, 8, 54–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inai, Y.; Nomura, Y.; Takarada, T.; Hanada, N.; Wada, N. Risk factors for postoperative pneumonia according to examination findings before surgery under general anesthesia. Clin. Oral Investig. 2020, 24, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Otsuka, R.; Hasegawa, R.; Hanada, N. Oral microbiome of children living in an isolated area in Myanmar. Int. J. Environ. Res. Public Health 2020, 17, 4033. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Kuroiwa, Y.; Nakamura, K.; Ito, H.; Kanemitsu, Y.; Masuda, N.; Tsubosa, Y.; Satoh, T.; Yokomizo, A.; Fukuda, H.; et al. Extended Clavien–Dindo classification of surgical com plications: Japan Clinical Oncology Group postoperative complications criteria. Surg. Today 2016, 46, 668–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimpo, Y.; Nomura, Y.; Sekiya, T.; Arai, C.; Okada, A.; Sogabe, K.; Hanada, N.; Tomonari, H. Effects of the dental caries preventive procedure on the white spot lesions during orthodontic treatment-An open label randomized controlled trial. J. Clin. Med. 2022, 11, 854. [Google Scholar] [CrossRef]
- Nomura, Y.; Kakuta, K.; Kaneko, N.; Nohno, K.; Yoshihara, A.; Hanada, N. The oral microbiome of healthy Japanese people at the age of 90. Appl. Sci. 2020, 10, 6450. [Google Scholar] [CrossRef]
- Xu, X.; Xie, Z.; Yang, Z.; Li, D.; Xu, X. t-SNE Based Classification Approach to Compositional Microbiome Data. Front. Genet. 2020, 11, 620143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shen, X.; Jiang, X.; Wei, K.; He, T.; Ma, Y.; Liu, J.; Hu, X. Nonlinear expression and visualization of nonmetric relationships in genetic diseases and microbiome data. BMC Bioinform. 2018, 19, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghannam, R.B.; Techtmann, S.M. Machine learning applications in microbial ecology, human microbiome studies, and environmental monitoring. Comput. Struct. Biotechnol. J. 2021, 19, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Kakuta, E.; Okada, A.; Otsuka, R.; Shimada, M.; Tomizawa, Y.; Taguchi, C.; Arikawa, K.; Daikoku, H.; Sato, T.; et al. Oral microbiome in four female centenarians. Appl. Sci. 2020, 10, 5312. [Google Scholar] [CrossRef]
- Zakharkina, T.; Martin-Loeches, I.; Matamoros, S.; Povoa, P.; Torres, A.; Kastelijn, J.B.; Hofstra, J.J.; de Wever, B.; de Jong, M.; Schultz, M.J.; et al. The dynamics of the pulmonary microbiome during mechanical ventilation in the intensive care unit and the association with occurrence of pneumonia. Thorax 2017, 72, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromentin, M.; Ricard, J.D.; Roux, D. Respiratory microbiome in mechanically ventilated patients: A narrative review. Intensive Care Med. 2021, 47, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Sands, K.M.; Twigg, J.A.; Lewis, M.A.O.; Wise, M.P.; Marchesi, J.R.; Smith, A.; Wilson, M.J.; Williams, D.W. Microbial profiling of dental plaque from mechanically ventilated patients. J. Med. Microbiol. 2016, 65, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Sands, K.M.; Wilson, M.J.; Lewis, M.A.O.; Wise, M.P.; Palmer, N.; Hayes, A.J.; Barnes, R.A.; Williams, D.W. Respiratory pathogen colonization of dental plaque, the lower airways, and endotracheal tube biofilms during mechanical ventilation. J. Crit. Care 2017, 37, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, N.I.; Palmer, R.J., Jr.; Cisar, J.O.; Kolenbrander, P.E. Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque. J. Bacteriol. 2008, 190, 8145–8154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashima, I.; Nakazawa, F. The influence of oral Veillonella species on biofilms formed by Streptococcus species. Anaerobe 2014, 28, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mashima, I.; Nakazawa, F. The interaction between Streptococcus spp. and Veillonella tobetsuensis in the early stages of oral biofilm formation. J. Bacteriol. 2015, 197, 2104–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzel, N.G.; Teles, F.R.; Teles, R.P.; Song, X.Q.; Torresyap, G.; Socransky, S.S.; Haffajee, A.D.J. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J. Clin. Periodontol. 2011, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cargill, J.S.; Scott, K.S.; Gascoyne-Binzi, D.; Sandoe, J.A.T. Granulicatella infection: Diagnosis and management. Med. Microbiol. 2012, 61, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeican, I.I.; Barbu Tudoran, L.; Florea, A.; Flonta, M.; Trombitas, V.; Apostol, A.; Dumitru, M.; Aluaș, M.; Junie, L.M.; Albu, S. Chronic Rhinosinusitis: MALDI-TOF Mass Spectrometry Microbiological Diagnosis and Electron Microscopy Analysis; Experience of the 2nd Otorhinolaryngology Clinic of Cluj-Napoca, Romania. J. Clin. Med. 2020, 9, 3973. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Yokoe, S.; Ogata, Y.; Sato, S.; Imai, K. Exposure to Porphyromonas gingivalis induces production of proinflammatory cytokine via TLR2 from human respiratory epithelial cells. J. Clin. Med. 2020, 9, 3433. [Google Scholar] [CrossRef] [PubMed]
- Benedyk, M.; Mydel, P.M.; Delaleu, N.; Płaza, K.; Gawron, K.; Milewska, A.; Maresz, K.; Koziel, J.; Pyrc, K.; Potempa, J. Gingipains: Critical factors in the development of aspiration pneumonia caused by Porphyromonas gingivalis. J. Innate Immun. 2016, 8, 185–198. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shiotsu, N.; Uchida-Fukuhara, Y.; Guo, J.; Weng, Y.; Ikegame, M.; Wang, Z.; Ono, K.; Kamioka, H.; Torii, Y.; et al. Outer membrane vesicles derived from Porphyromonas gingivalis induced cell death with disruption of tight junctions in human lung epithelial cells. Arch. Oral Biol. 2020, 118, 104841. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Yanagihara, K.; Harada, Y.; Yamada, K.; Migiyama, Y.; Morinaga, Y.; Izumikawa, K.; Kohno, S. Quantitative detection of periodontopathic bacteria in lower respiratory tract specimens by real-time PCR. J. Infect. Chemother. 2017, 23, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Costache, A.; Berghi, O.N.; Cergan, R.; Dumitru, M.; Neagos, A.; Popa, L.G.; Giurcaneanu, C.; Vrinceanu, D. Respiratory allergies: Salicaceae sensitization (Review). Exp. Ther. Med. 2021, 21, 609. [Google Scholar] [CrossRef]
- Ma, C.M.; Liu, Q.; Li, M.L.; Ji, M.J.; Zhang, J.D.; Zhang, B.H.; Yin, F.Z. The Effects of type 2 diabetes and postoperative pneumonia on the mortality in inpatients with surgery. Diabetes Metab. Syndr. Obes. 2019, 12, 2507–2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmeister, B.C.; Ducasse, C.K.; González, L.M.; Quilodrán, S.C.; Joyas, M.A. Pulmonary and thoracic infection by Fusobacterium nucleatum. Andes. Pediatr. 2021, 92, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hayata, M.; Watanabe, N.; Tamura, M.; Kamio, N.; Tanaka, H.; Nodomi, K.; Miya, C.; Nakayama, E.; Ueda, K.; Ogata, Y.; et al. The Periodontopathic bacterium Fusobacterium nucleatum induced proinflammatory cytokine production by human respiratory epithelial cell lines and in the lower respiratory organs in mice. Cell Physiol. Biochem. 2019, 53, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho Baptista, I.M.; Martinho, F.C.; Nascimento, G.G.; da Rocha Santos, C.E.; Prado, R.F.D.; Valera, M.C. Colonization of oropharynx and lower respiratory tract in critical patients: Risk of ventilator-associated pneumonia. Arch. Oral Biol. 2018, 85, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, L.; Zhang, C.; Lyu, J.; Yan, C.; Cao, R.; Pan, M.; Li, Y. Beware of pharyngeal Fusobacterium nucleatum in COVID-19. BMC Microbiol. 2021, 21, 277. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hountras, P.; Wunderink, R.G. The microbiome in mechanically ventilated patients. Curr. Opin. Infect. Dis. 2017, 30, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic alternative to chlorhexidine in periodontal therapy: Evaluation of clinical and microbiological parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in non-surgical periodontal therapy: Clinical and microbiological aspects in a 6-month follow-up domiciliary protocol for oral hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.J.; Wise, M.P.; Smith, A.; Marchesi, J.R.; Riggio, M.P.; Lewis, M.A.O.; Williams, D.W. Community analysis of dental plaque and endotracheal tube biofilms from mechanically ventilated patients. J. Crit. Care 2017, 39, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).