Organic Beet Leaves and Stalk Juice Attenuates the Glutathione Peroxidase Increase Induced by High-Fat Meal in Dyslipidemic Patients: A Pilot Double-Blind, Randomized, Controlled Trial
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Beet Leaves and Stalks: Organic vs. Conventional
2.1.1. Sample Collection and Pre-Preparation
2.1.2. Nutritional Composition
2.1.3. Total Phenolics
2.2. Clinical Trial
2.2.1. Preparation of the Juice for the Clinical Trial
2.2.2. Recruitment
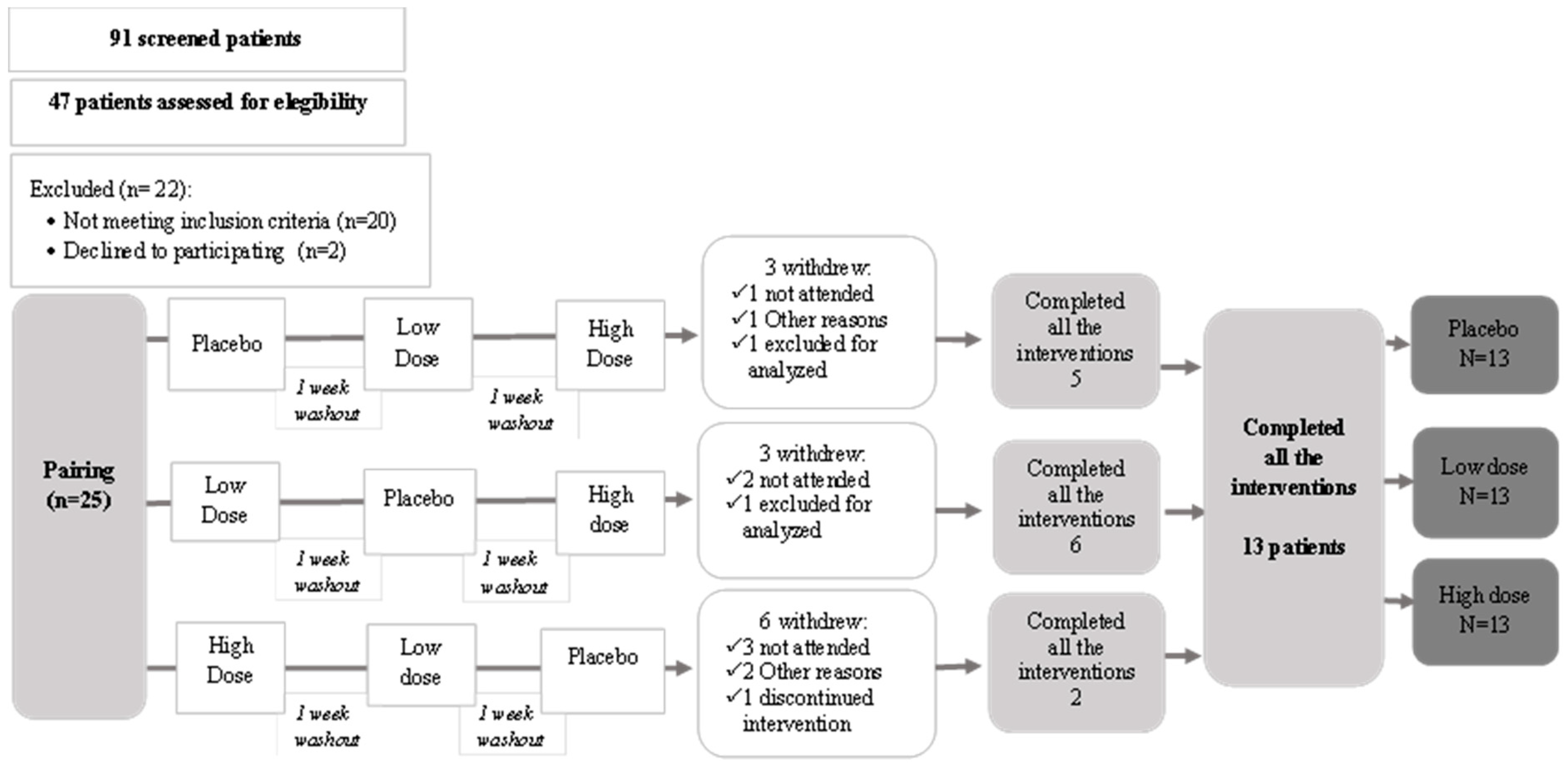
2.2.3. Study Design
2.2.4. Determination of the Phenolic Compound Concentration in Plasma
2.2.5. Determination of Malondialdehyde (MDA) Concentration in Plasma
2.2.6. Determination of Enzyme Activity (Primary Outcome)
2.2.7. Determination of Tumor Necrosis Factor α (TNF-α) Concentration
2.2.8. Statistical Analyses
3. Results
3.1. Beet Leaves and Stalks: Organic vs. Conventional
3.2. Characterization of Organic BLS Juice Phenolic Profile
3.3. Population Characterization
3.4. Induction of Hypertriglyceridemia and Lipid Peroxidation
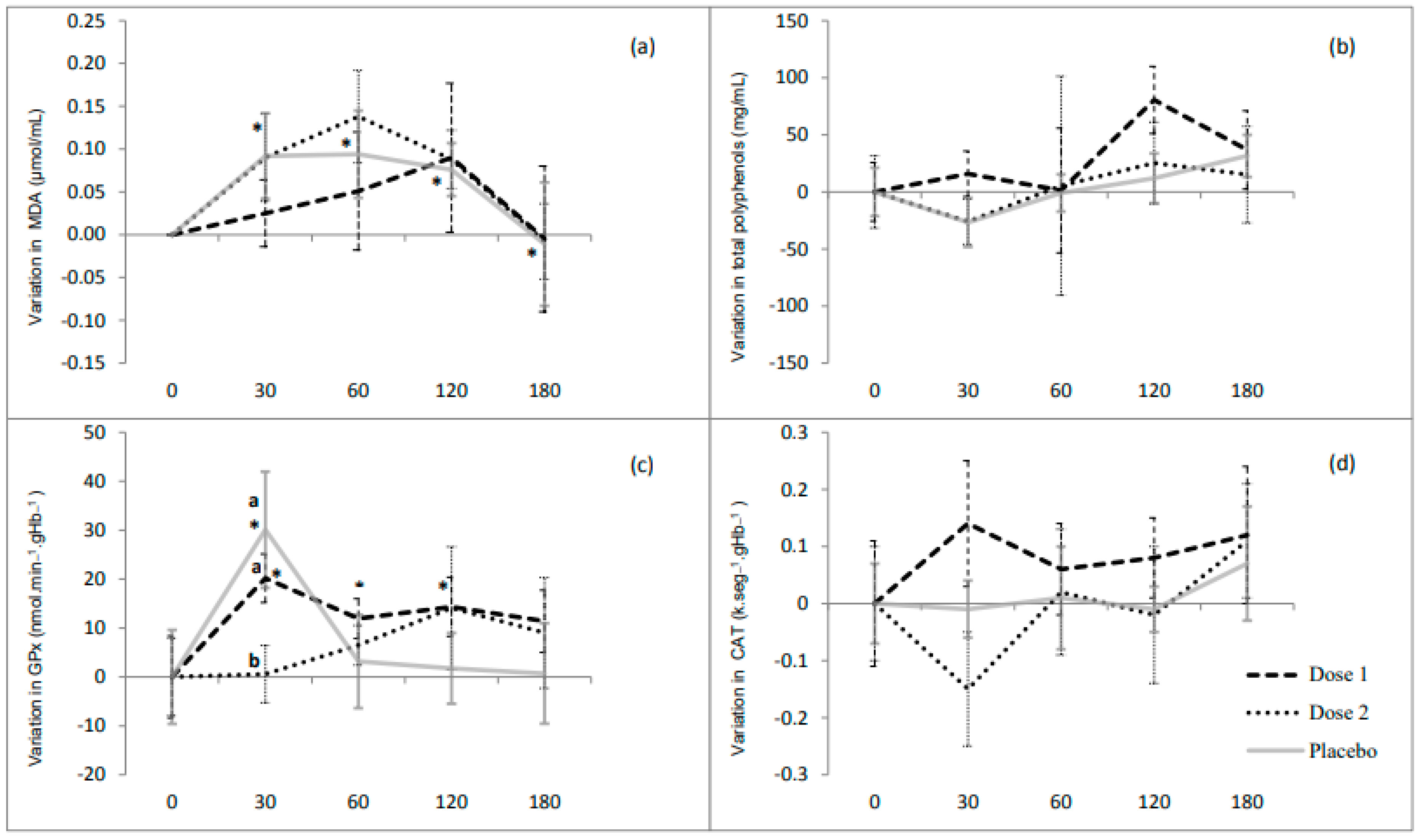
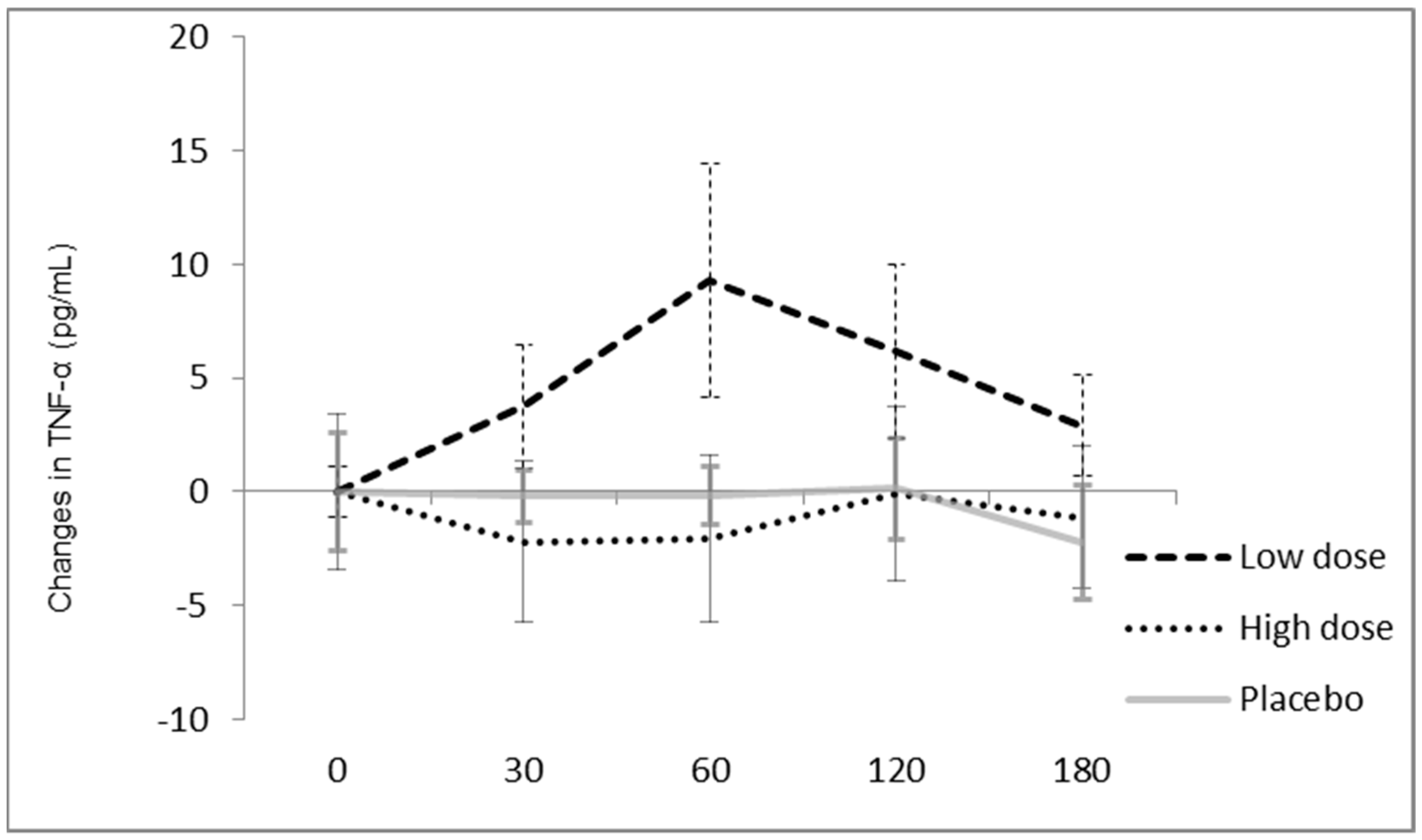
3.5. Effect of Beet Leaves and Stalk Juice on Total Polyphenols, MDA, TNFα and Antioxidant Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burton-Freeman, B. Postprandial metabolic events and fruit-derived phenolics: A review of the science. Br. J. Nutr. 2010, 104, S1–S14. [Google Scholar] [CrossRef] [PubMed]
- Lacorix, S.; Rosiers, C.; Tardif, J.C.; Nigam, A. The role of oxidative stress in postprandial endothelial dysfunction. Nutr. Res. Rev. 2012, 25, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Pang, J.; Romic, G.; Watts, G.F. Postprandial Hypertriglyceridemia and Cardiovascular Disease: Current and Future Therapies. Curr. Atheroscler. Rep. 2013, 15, 309. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S. Clinical Considerations and Mechanistic Determinants of Postprandial Lipemia in Older Adults. Adv. Nutr. Int. Rev. J. 2014, 5, 226–234. [Google Scholar] [CrossRef]
- Masuda, D.; Yamashita, S. Postprandial Hyperlipidemia and Remnant Lipoproteins. J. Atheroscler. Thromb. 2017, 24, 95–109. [Google Scholar] [CrossRef]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef]
- Silva, L.G.S.; Morelli, A.P.; Pavan, I.C.P.; Tavares, M.R.; Pestana, N.F.; Rostagno, M.A.; Simabuco, F.M.; Bezerra, R.M.N. Protective effects of beet (Beta vulgaris) leaves extract against oxidative stress in endothelial cells in vitro. Phytother. Res. 2020, 34, 1385–1396. [Google Scholar] [CrossRef]
- Lorizola, I.M.; Miyamoto, J.; Vieira, A.L.F.; Sumere, B.R.; Bezerra, R.M.N.; Torsoni, M.A.; Torsoni, A.S.; Rostagno, M.A.; Milanski, M.; Capitani, C.D. Beet (Beta vulgaris L.) stalk and leaf supplementation changes the glucose homeostasis and inflammatory markers in the liver of mice exposed to a high-fat diet. Food Chem. Mol. Sci. 2021, 2, 100018. [Google Scholar] [CrossRef]
- Nurdiana, S.; Goh, Y.M.; Ahmad, H.; Dom, S.M.; Azmi, N.S.; Zin, N.S.N.M.; Ebrahimi, M. Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Complement. Altern. Med. 2017, 17, 290. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Hallmanna, E.; Rozparab, E.; Słowianekc, M.; Leszczyńskac, J. The effect of organic and conventional farm management on the allergenic potency and bioactive compounds status of apricots (Prunus armeniaca L.). Food Chem. 2019, 279, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Wilches-Peres, D.; Hallmann, E.; Kazimierczak, R.; Rembiałkowska, E. Organic versus conventional beetroot. Bioactive compounds and antioxidant properties. LWT 2019, 116, 108552. [Google Scholar] [CrossRef]
- Burri, S.C.M.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pregnolato, W.; Pregnolato, N.P. Normas Analíticas do Instituto Adolf Lutz; Instituto Adolf Lutz: São Paulo, Brazil, 1985. [Google Scholar]
- Kjeldahl, J.G.C.T. Neue Methodezur Bestimmung des Stickstoffs in organis chen Körpern. Fresenius Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists—AOAC. Official Methods of Analysis, 14th ed.; Arlington (VA): Washington, DC, USA, 1984; p. 1141. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Gomes, A.P.O.; Ferreira, M.A.; Camargo, J.M.; Araújo, M.D.O.; Mortoza, A.S.; Mota, J.F.; Coelho, A.S.G.; Capitani, C.D.; Coltro, W.K.T.; Botelho, P.B. Organic beet leaves and stalk juice attenuates HDL-C reduction induced by high-fat meal in dyslipidemic patients: A pilot randomized controlled trial. Nutrition 2019, 65, 68–73. [Google Scholar] [CrossRef]
- Xavier, H.T.; Izar, M.C.; Faria Neto, J.R.; Assad, M.H.; Rocha, V.Z.; Sposito, A.C.; Fonseca, F.A.; dos Santos, J.E.; Santos, R.D.; Bertolami, M.C.; et al. V Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq. Bras. Cardiol. 2013, 101, 1–22. [Google Scholar] [CrossRef]
- Adzic, M.; Niciforovic, A.; Vucic, V.; Neskovic-Konstantinovic, Z.; Spasic, S.D.; Jones, D.R. Systemic NF-κB activation in blood cells of breast cancer patients. Redox. Rep. 2006, 11, 39–44. [Google Scholar] [CrossRef]
- Heyward, V.H.; Stolyk, L.M. Avaliação da Composição Corporal Aplicada, 1st ed.; Manole: São Paulo, Brazil, 2000. [Google Scholar]
- Serafini, M.; Maiani, G.; Ferro-Luzzi, A. Alcohol-free red wine enhances plasma antioxidant capacity in humans. J. Nutr. 1998, 128, 1003–1007. [Google Scholar] [CrossRef]
- Antunes, M.V.; Lazzaretti, C.; Gamaro, G.D.; Linden, R. Preanalytical and validation studies for the determination of malondialdehyde in human plasma through high performance liquid chromatography after derivatization with 2,4-dinitrophenylhydrazine. Rev. Bras. Ciências Farm. 2008, 44, 279–287. [Google Scholar] [CrossRef][Green Version]
- Aebi, H. Catalase in vitro. In Methods in Enzymology, 1st ed.; Academic Press: New York, NY, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. Fundamentals of Biostatistics, 7th ed.; Broke/Colen: Boston, MA, USA, 2011. [Google Scholar]
- Mditshwa, A.; Magwaza, L.; Tesfay, S.Z.; Mbili, N. Postharvest quality and composition of organically and conventionally produced fruits: A review. Sci. Hortic. 2017, 216, 148–159. [Google Scholar] [CrossRef]
- Huber, M.; Rembiałkowska, E.; Średnicka-Tober, D.; Bügel, S.; van de Vijver, L. Organic food and impact on human health: Assessing the status quo and prospects of research. NJAS-Wagening J. Life Sci. 2011, 58, 103–109. [Google Scholar] [CrossRef]
- Mie, A.; Andersen, H.R.; Gunnarsson, S.; Kahl, J.; Kesse-Guyot, E.; Rembiałkowska, E.; Quaglio, G.; Grandjean, P. Human health implications of organic food and organic agriculture: A comprehensive review. Environ. Health 2017, 16, 111. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 5th ed.; Artmed: Porto Alegre, Brazil, 2013. [Google Scholar]
- Worthington, V. Nutritional Quality of Organic Versus Conventional Fruits, Vegetables, and Grains. J. Altern. Complement. Med. 2001, 7, 161–173. [Google Scholar] [CrossRef]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. SpringerPlus 2012, 1, 26. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2014, 95, 1143–1156. [Google Scholar] [CrossRef]
- Storck, C.R.; Nunes, G.L.; De Oliveira, B.B.; Basso, C. Leaves, stalk, pell and seeds of vegetables: Nutritional composition, utilization and sensory analysis in food preparations. Ciência Rural 2013, 43, 537–543. [Google Scholar] [CrossRef]
- Peng, Y.; Gan, R.; Li, H.; Yang, M.; McClements, D.J.; Gao, R.; Sun, Q. Absorption, metabolism, and bioactivity of vitexin: Recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food Sci. Nutr. 2020, 61, 1049–1064. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Lorizola, I.M.; Furlan, C.P.B.; Portovedo, M.; Milanski, M.; Botelho, P.B.; Bezerra, R.M.N.; Sumere, B.R.; Rostagno, M.; Capitani, C.D. Beet Stalks and Leaves (Beta vulgaris L.) Protect Against High-Fat Diet-Induced Oxidative Damage in the Liver in Mice. Nutrients 2018, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Shah, N.A.; Warrington, J.A.; Anderson, N.N.; Park, S.W.; Brown, M.S.; Goldstein, J.L. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA 2003, 100, 12027–12032. [Google Scholar] [CrossRef]
- Moon, Y.-A.; Liang, G.; Xie, X.; Frank-Kamenetsky, M.; Fitzgerald, K.; Koteliansky, V.; Brown, M.S.; Goldstein, J.L.; Horton, J.D. The Scap/SREBP Pathway Is Essential for Developing Diabetic Fatty Liver and Carbohydrate-Induced Hypertriglyceridemia in Animals. Cell Metab. 2012, 15, 240–246. [Google Scholar] [CrossRef]
- El Khoury, D.; Hwalla, N.; Frochot, V.; Lacorte, J.-M.; Chabert, M.; Kalopissis, A.D. Postprandial metabolic and hormonal responses of obese dyslipidemic subjects with metabolic syndrome to test meals, rich in carbohydrate, fat or protein. Atherosclerosis 2010, 210, 307–313. [Google Scholar] [CrossRef]
- Miglio, C.; Peluso, I.; Raguzzini, A.; Villaño, D.V.; Cesqui, E.; Catasta, G.; Toti, E.; Serafini, M. Antioxidant and inflammatory response following high-fat meal consumption in overweight subjects. Eur. J. Nutr. 2012, 52, 1107–1114. [Google Scholar] [CrossRef]
- Krüger, R.L.; Farinha, J.B.; Teixeira, B.C.; Reischak-Oliveira, A. Estresse oxidativo e a função endotelial: Efeitos do exercício físico associado à lipemia pós-prandial. J. Vasc. Bras. 2015, 14, 328–340. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv. Nutr. Int. Rev. J. 2017, 8, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry Modulates LDL Oxidation and Postprandial Lipemia in Response to High-Fat Meal in Overweight Hyperlipidemic Men and Women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Lee, K.W.; Kim, J.Y.; Kwon, O. A beverage of Asiatic plantain extracts alleviated postprandial oxidative stress in overweight hyperlipidemic subjects challenged with a high-fat meal: A preliminary study. Nutr. Res. 2013, 33, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Pereira, A.C.D.S.; Dionísio, A.P.; Wurlitzer, N.; Alves, R.E.; de Brito, E.S.; Silva, A.; Brasil, I.M.; Filho, J.M. Effect of antioxidant potential of tropical fruit juices on antioxidant enzyme profiles and lipid peroxidation in rats. Food Chem. 2014, 157, 179–185. [Google Scholar] [CrossRef]
- Macedo, L.F.L.; Rogero, M.M.; Guimarães, J.P.; Granato, D.; Lobato, L.P.; Castro, I.A. Effect of red wines with different in vitro antioxidant activity on oxidative stress of high-fat diet rats. Food Chem. 2013, 137, 122–129. [Google Scholar] [CrossRef]
- Fustinoni-Reis, A.M.; Arruda, S.F.; Dourado, L.P.S.; Da Cunha, M.S.B.; Siqueira, E.M.A. Tucum-Do-Cerrado (Bactris setosa Mart.) Consumption Modulates Iron Homeostasis and Prevents Iron-Induced Oxidative Stress in the Rat Liver. Nutrients 2016, 8, 38. [Google Scholar] [CrossRef]
- Cunha, M.D.S.B.D.; Arruda, S.F. Tucum-do-Cerrado (Bactris setosa Mart.) May Promote Anti-Aging Effect by Upregulating SIRT1-Nrf2 Pathway and Attenuating Oxidative Stress and Inflammation. Nutrients 2017, 9, 1243. [Google Scholar] [CrossRef]

| Nutrients | Organic Leaf | Conventional Leaf | p |
|---|---|---|---|
| Moisture (%) | 91.09 ± 0.66 | 89.68 ± 0.46 | 0.038 |
| Ash (g/100 g) | 2.20 ± 0.04 | 2.23 ± 0.02 | 0.411 |
| CHO (g/100 g) | 1.85 ± 0.62 | 3.54 ± 0.40 | 0.016 |
| LIP (g/100 g) | 1.06 ± 0.00 | 0.98 ± 0.00 | 0.000 |
| PTN (g/100 g) | 3.80 ± 0.00 | 3.58 ± 0.12 | 0.035 |
| Total phenolics (mg/100 g) | 89.20 ± 9.78 | 95.75 ± 0.69 | 0.366 |
| Nutrients | Organic Stalks | Conventional Stalks | p |
|---|---|---|---|
| Moisture (%) | 94.22 ± 0.88 | 96.31 ± 0.24 | 0.017 |
| Ash (g/100 g) | 1.28 ± 0.42 | 0.92 ± 0.00 | 0.000 |
| CHO (g/100 g) | 3.18 ± 0.90 | 1.68 ± 0.24 | 0.049 |
| LIP (g/100 g) | 0.17 ± 0.00 | 0.31 ± 0.00 | 0.001 |
| PTN (g/100 g) | 1.14 ± 0.00 | 0.77 ± 0.02 | 0.000 |
| Total phenolics (mg/100 g) | 41.13 ± 0.55 | 27.84 ± 0.85 | 0.000 |
| Variables | Means (Standard Error) |
|---|---|
| Age (y) | 40.6 (2.3) |
| Body mass index (kg/m2) | 32.7 (1.5) |
| Waist circumference (cm) | 96.8 (3.1) |
| Body fat (%) | 46.2 (2.2) |
| Total cholesterol (mg/dl) | 211.0 (9.8) |
| HDL cholesterol (mg/dL) | 47.1 (3.3) |
| LDL cholesterol (mg/dL) | 126.5 (8.8) |
| VLDL cholesterol (mg/dL) | 37.4 (2.9) |
| Triacylglycerol (mg/dL) | 187.0 (14.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, A.C.; Gomes, A.P.O.; Rodrigues, L.C.; Cunha, R.d.S.; Serra, T.M.; Schincaglia, R.M.; Silva, M.A.C.; Horst, M.A.; Rostagno, M.A.; Magalhães, K.G.; et al. Organic Beet Leaves and Stalk Juice Attenuates the Glutathione Peroxidase Increase Induced by High-Fat Meal in Dyslipidemic Patients: A Pilot Double-Blind, Randomized, Controlled Trial. Appl. Sci. 2022, 12, 1973. https://doi.org/10.3390/app12041973
de Oliveira AC, Gomes APO, Rodrigues LC, Cunha RdS, Serra TM, Schincaglia RM, Silva MAC, Horst MA, Rostagno MA, Magalhães KG, et al. Organic Beet Leaves and Stalk Juice Attenuates the Glutathione Peroxidase Increase Induced by High-Fat Meal in Dyslipidemic Patients: A Pilot Double-Blind, Randomized, Controlled Trial. Applied Sciences. 2022; 12(4):1973. https://doi.org/10.3390/app12041973
Chicago/Turabian Stylede Oliveira, Amanda Cristine, Anna Paula Oliveira Gomes, Lorena Charife Rodrigues, Raisa da Silva Cunha, Thaís Martins Serra, Raquel Machado Schincaglia, Marina Alves Coelho Silva, Maria Aderuza Horst, Maurício Ariel Rostagno, Kelly Grace Magalhães, and et al. 2022. "Organic Beet Leaves and Stalk Juice Attenuates the Glutathione Peroxidase Increase Induced by High-Fat Meal in Dyslipidemic Patients: A Pilot Double-Blind, Randomized, Controlled Trial" Applied Sciences 12, no. 4: 1973. https://doi.org/10.3390/app12041973
APA Stylede Oliveira, A. C., Gomes, A. P. O., Rodrigues, L. C., Cunha, R. d. S., Serra, T. M., Schincaglia, R. M., Silva, M. A. C., Horst, M. A., Rostagno, M. A., Magalhães, K. G., Cunha, L. C., & Botelho, P. B. (2022). Organic Beet Leaves and Stalk Juice Attenuates the Glutathione Peroxidase Increase Induced by High-Fat Meal in Dyslipidemic Patients: A Pilot Double-Blind, Randomized, Controlled Trial. Applied Sciences, 12(4), 1973. https://doi.org/10.3390/app12041973







