Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement
Abstract
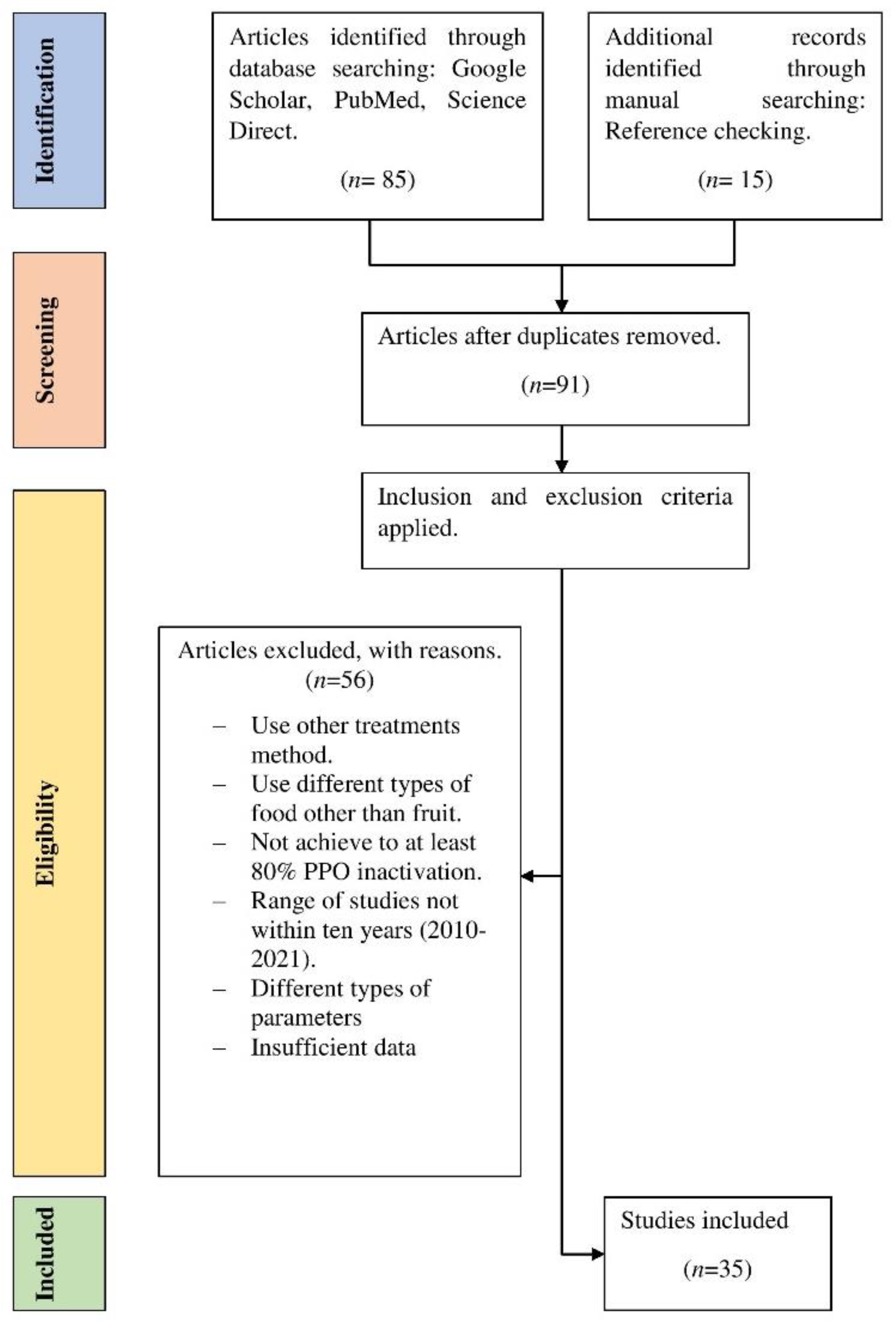
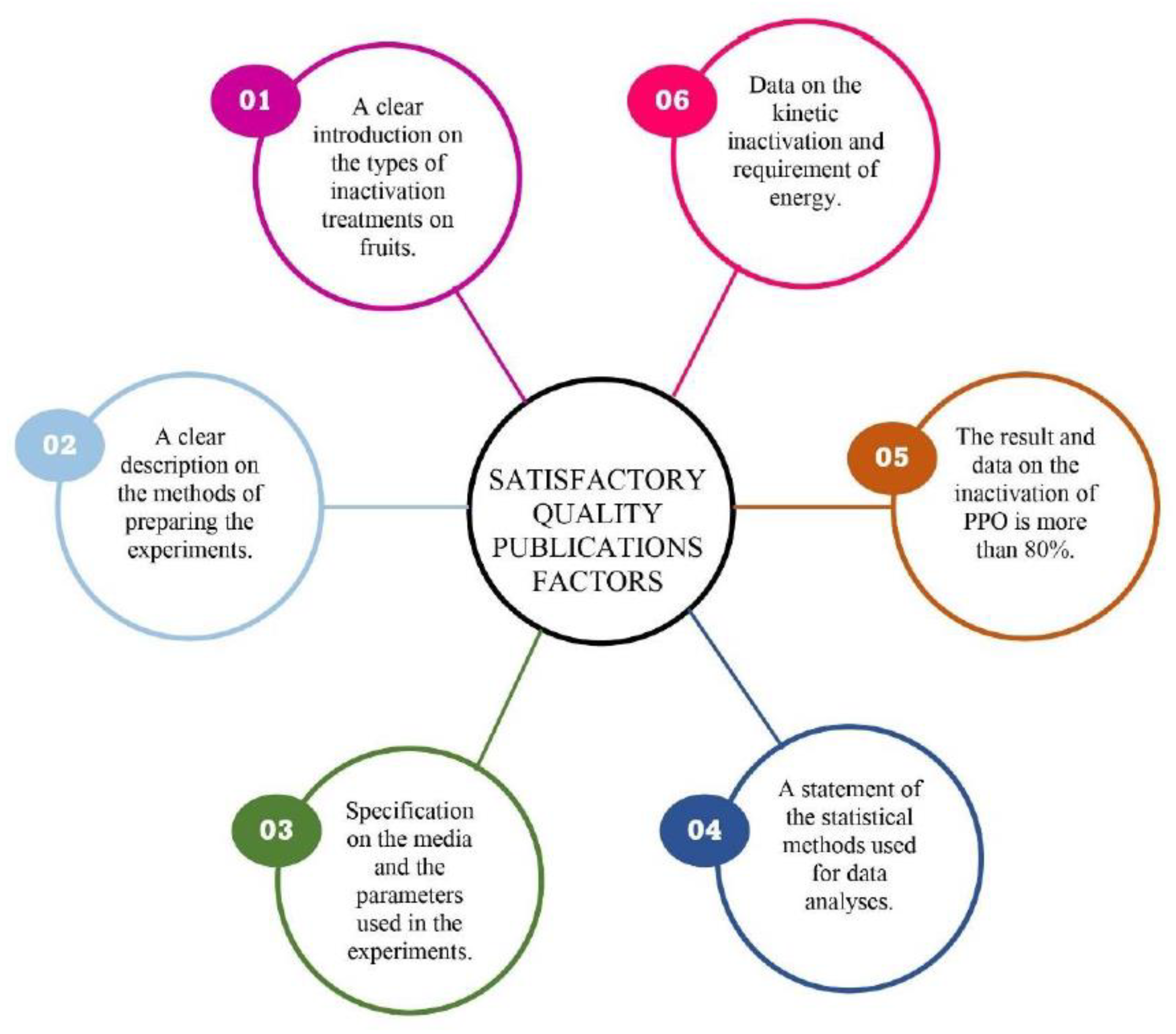
:1. Introduction
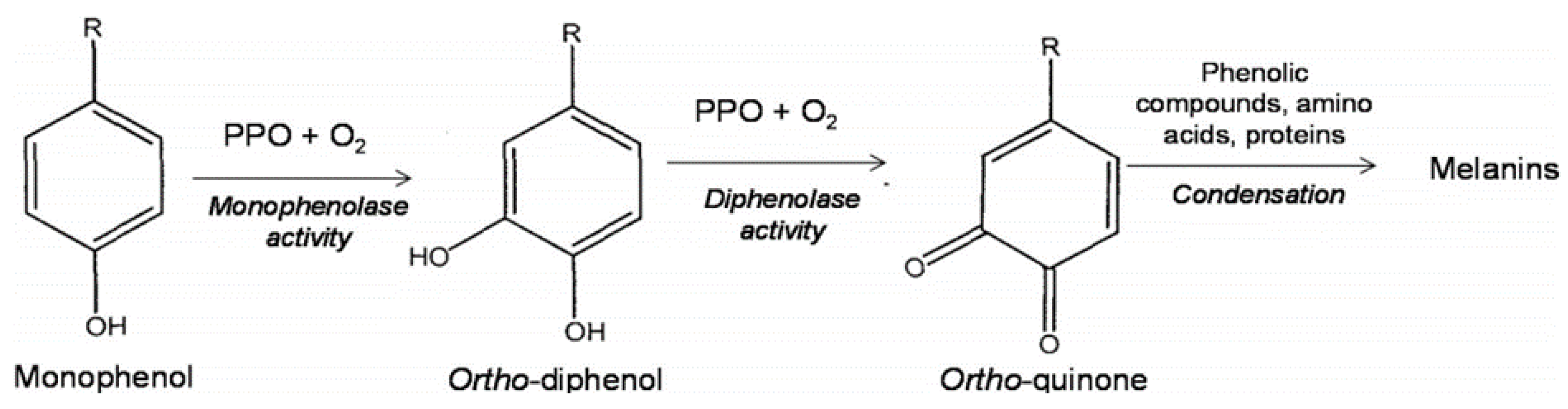
2. Enzymatic Browning: Polyphenol Oxidase
3. Thermal and Innovative Technologies for PPO Inactivation
3.1. Thermal Treatment
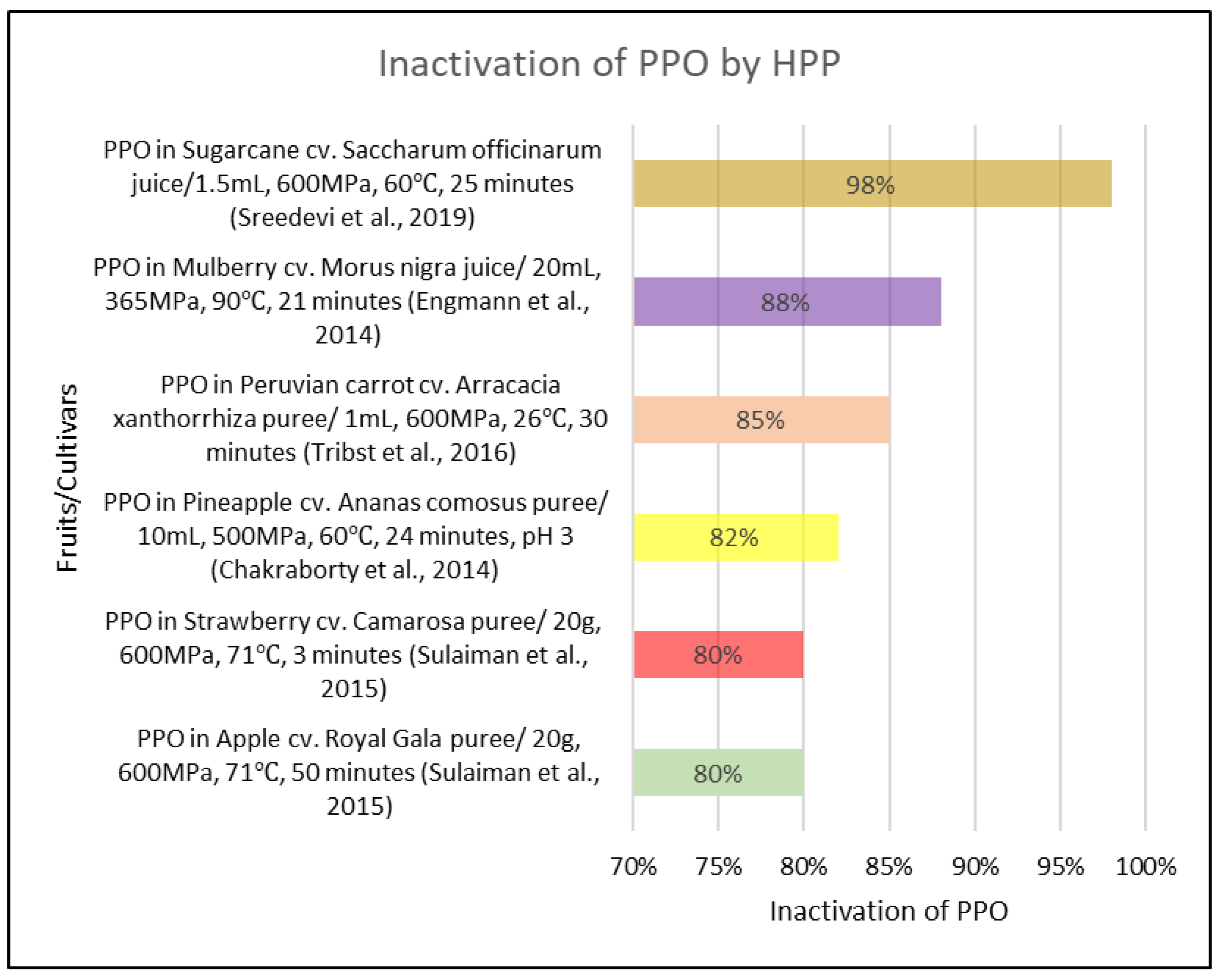
3.2. High Pressure Processing
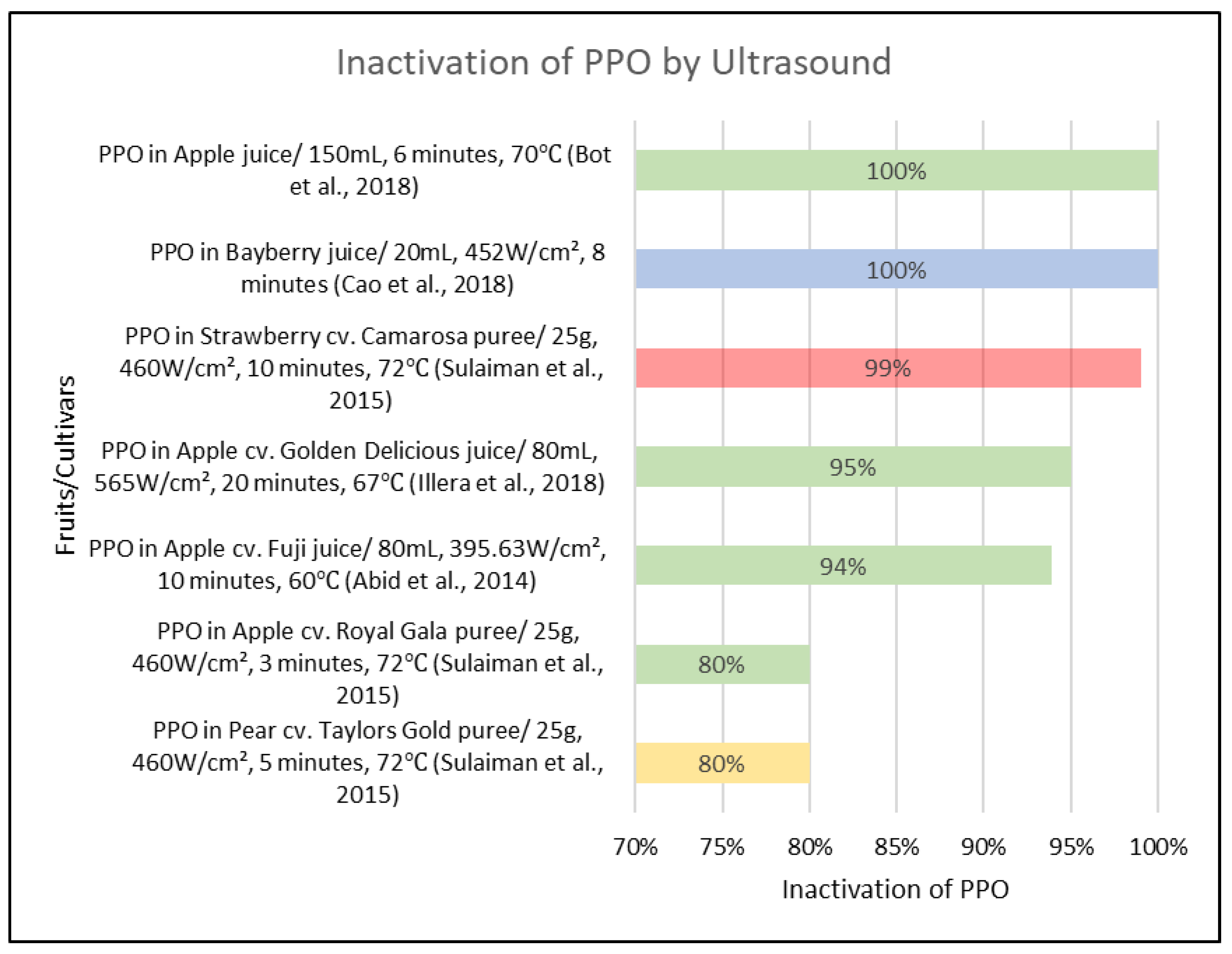
3.3. Ultrasound Processing
4. Kinetic Modelling of PPO Inactivation
4.1. Kinetic Inactivation Model
4.2. Thermal Inactivation Kinetics of PPO
4.3. High Pressure Inactivation Kinetics of PPO
4.4. Ultrasound Inactivation Kinetics of PPO
5. Energy Requirement Estimation
5.1. Energy Calculations
5.2. Thermal Processing
| Fruit/Cultivar | Mass (kg) | ∆T (°C) | T Final (°C) | Cp (kJ/kg.K) | Q = mcp∆T (kJ) | Specific Energy (kJ/kg) | Data Collected from |
|---|---|---|---|---|---|---|---|
| Kalipatti sapota (Manilkara zapota) pulp | 0.004 | 55 | 75 | 3.94 [66] | 0.87 | 217 | [36] |
| 65 | 85 | 1.02 | 256 | ||||
| Peach (Prunus persica cv. Jubileu) puree | 0.414 | 50 | 70 | 1.92 [67] | 39.8 | 96 | [37] |
| 70 | 90 | 55.7 | 134 | ||||
| Blueberry (Vacciniumcorym-Bosum) juice | 0.050 | 40 | 60 | 3.64 [67] | 7.28 | 146 | [38] |
| 60 | 80 | 10.9 | 218 | ||||
| Bayberry (Myrica) juice | 0.099 | 45 | 65 | 1.94 [67] | 8.64 | 87 | [39] |
| 55 | 75 | 10.6 | 107 | ||||
| Sugarcane (Saccharum officinarum) juice | 0.001 | 40 | 60 | 3.64 [68] | 0.21 | 146 | [35] |
| Pear (Pyrus communis cv. Taylor’s Gold) puree | 0.020 | 60 | 80 | 2.06 [67] | 2.47 | 124 | [28] |
| Apple (Malus domestica cv. Royal Gala) puree | 0.02 | 55 | 75 | 1.88 [69] | 2.07 | 103 | [28] |
| Strawberries (Fragaria ananassa cv. Camarosa) puree | 0.02 | 40 | 60 | 3.60 [70] | 2.88 | 144 | [28] |
5.3. High Pressure Processing
5.4. Ultrasound
6. Final Remarks and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, A.; Murtaza, A.; Hu, W.; Ahmad, I.; Ahmed, A.; Xu, X. Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioprod. Process. 2019, 117, 170–182. [Google Scholar] [CrossRef]
- Ferreira-Holderbaum, D.; Kon, T.; Kudo, T.; Pedro Guerra, M. HortScience: A publication of the American Society for Horticultural Science. HortScience 2010, 45, 1150–1154. [Google Scholar]
- Kaanane, A.; Labuza, T.P. The Maillard reaction in foods. Prog. Clin. Biol. Res. 1989, 304, 301–327. [Google Scholar] [PubMed]
- He, Q.; Luo, Y.; Chen, P. Elucidation of the mechanism of enzymatic browning inhibition by sodium chlorite. Food Chem. 2008, 110, 847–851. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 501–523. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Muhammad, Z.; Elkhedir, A.E.; Tao, M.; Xu, X. Inactivation, aggregation and conformational changes of polyphenol oxidase from quince (Cydonia oblonga Miller) juice subjected to thermal and high pressure carbon dioxide treatment. Molecules 2018, 23, 1743. [Google Scholar] [CrossRef] [Green Version]
- How, M.S.; Jones, J.R.; Morgenstern, M.P.; Gray-Stuart, E.; Bronlund, J.E.; Saint-Eve, A.; Trelea, I.C.; Souchon, I. Modelling the role of oral processing on in vivo aroma release of white rice: Conceptual model and experimental validation. LWT 2021, 141, 110918, Corrigendum to LWT 2021, 144, 111391. [Google Scholar] [CrossRef]
- Sulaiman, A.; Silva, F.V.M. High pressure processing, thermal processing and freezing of ‘Camarosa’ strawberry for the inactivation of polyphenoloxidase and control of browning. Food Control 2013, 33, 424–428. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Yoon, M.M.L.; Farid, M.; Silva, F.V.M. Modeling the polyphenoloxidase inactivation kinetics in pear, apple and strawberry purees after High Pressure Processing. J. Food Eng. 2015, 147, 89–94. [Google Scholar] [CrossRef]
- Selvarajan, E.; Veena, R.; Manoj Kumar, N. Polyphenol oxidase, beyond enzyme browning. In Microbial Bioprospecting for Sustainable Development; Springer: Singapore, 2018; pp. 203–222. [Google Scholar]
- Xu, J.; Zhou, L.; Miao, J.; Yu, W.; Zou, L.; Zhou, W.; Liu, C.; Liu, W. Effect of Cinnamon Essential Oil Nanoemulsion Combined with Ascorbic Acid on Enzymatic Browning of Cloudy Apple Juice. Food Bioprocess Technol. 2020, 13, 860–870. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Kinetic modeling of polyphenoloxidase and peroxidase inactivation in pineapple (Ananas comosus L.) puree during high pressure and thermal treatments. Innov. Food Sci. Emerg. Technol. 2015, 27, 57–68. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Xiong, Z.; Zou, L.; Liu, J.; Zhong, J.; Chen, J. Effect of ultrasound combined with malic acid on the activity and conformation of mushroom (Agaricus bisporus) polyphenoloxidase. Enzym. Microb. Technol. 2016, 90, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Hamid, N.; Oey, I.; Kantono, K.; Farouk, M. The impact of high pressure processing on physicochemical properties and sensory characteristics of three different lamb meat cuts. Molecules 2020, 25, 2665. [Google Scholar] [CrossRef] [PubMed]
- Characteristics, C.; Cuts, L. Effect of high hydrostatic pressure processing on the chemical characteristics of different lamb cuts. Foods 2020, 9, 1444. [Google Scholar]
- Sulaiman, A.; Farid, M.; Silva, F.V.M. Strawberry puree processed by thermal, high pressure, or power ultrasound: Process energy requirements and quality modeling during storage. Food Sci. Technol. Int. 2017, 23, 293–309. [Google Scholar] [CrossRef]
- Sulaiman, A.; Farid, M.; Silva, F.V.M. Quality stability and sensory attributes of apple juice processed by thermosonication, pulsed electric field and thermal processing. Food Sci. Technol. Int. 2017, 23, 265–276. [Google Scholar] [CrossRef]
- Corzo-Martinez, M.; Corzo, N.; Villamiel, M.; del Castillo, M.D. Chapter 4: Browning reactions. In Browning Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 56–83. [Google Scholar]
- Laurila, E.; Kervinen, R.; Ahvenainen, R. The inhibition of enzymatic browning in minimally processed vegetables and fruits. Postharvest. News Inf. 1998, 9, 53–65. [Google Scholar]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Ryan, C.A. The systemin signaling pathway: Differential activation of plant defensive genes. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 2000, 1477, 112–121. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S. Polyphonel oxidases: Biochemical and molecular characterization, distribution, role and its control. Enzym. Eng. 2016, 5, 141–149. [Google Scholar]
- Queiroz, C.; Mendes Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase: Characteristics and mechanisms of browning control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Ruiz, P.A.; Tudela, J.; Varón, R.; García-Cánovas, F. Monophenolase and Diphenolase Reaction Mechanisms of Apple and Pear Polyphenol Oxidases. J. Agric. Food Chem. 1998, 46, 2968–2975. [Google Scholar] [CrossRef]
- Peñalver, M.J.; Fenoll, L.G.; Rodríguez-López, J.N.; García-Ruiz, P.A.; García-Molina, F.; Varón, R.; García-Cánovas, F.; Tudela, J. Reaction mechanism to explain the high kinetic autoactivation of tyrosinase. J. Mol. Catal. B Enzym. 2005, 33, 35–42. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhang, M.; Adhikari, B. The inactivation kinetics of polyphenol oxidase in mushroom (Agaricus bisporus) during thermal and thermosonic treatments. Ultrason. Sonochem. 2013, 20, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.M.; Sulaiman, A. Polyphenoloxidase in Fruit and Vegetables: Inactivation by Thermal and Non-Thermal Processes. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128140451. [Google Scholar]
- Sulaiman, A.; Soo, M.J.; Farid, M.; Silva, F.V.M. Thermosonication for polyphenoloxidase inactivation in fruits: Modeling the ultrasound and thermal kinetics in pear, apple and strawberry purees at different temperatures. J. Food Eng. 2015, 165, 133–140. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Effect of pH on Enzyme inactivation kinetics in high pressure processed pineapple (Ananas comosus L.) puree using response surface methodology. Food Bioprocess Technol. 2014, 7, 3629–3645. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.; Khan, M.A.; Zeng, X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason. Sonochem. 2014, 21, 984–990. [Google Scholar] [CrossRef]
- Terefe, N.S.; Delon, A.; Buckow, R.; Versteeg, C. Blueberry polyphenol oxidase: Characterization and the kinetics of thermal and high pressure activation and inactivation. Food Chem. 2015, 188, 193–200. [Google Scholar] [CrossRef]
- Myer, P.R.; Parker, K.R.; Kanach, A.T.; Zhu, T.; Morgan, M.T.; Applegate, B.M. The effect of a novel low temperature-short time (LTST) process to extend the shelf-life of fluid milk. Springerplus 2016, 5, 660. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chattopadhyay, P.K. Whirling bed blanching of potato cubes and its effects on product quality. J. Food Eng. 2007, 78, 52–60. [Google Scholar] [CrossRef]
- Sreedevi, P.; Jayachandran, L.E.; Rao, P.S. Kinetic modeling of high pressure induced inactivation of polyphenol oxidase in sugarcane juice (Saccharum officinarum). J. Sci. Food Agric. 2019, 99, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Vishwasrao, C.; Chakraborty, S.; Ananthanarayan, L. Partial purification, characterisation and thermal inactivation kinetics of peroxidase and polyphenol oxidase isolated from Kalipatti sapota (Manilkara zapota). J. Sci. Food Agric. 2017, 97, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.M.; Toralles, R.P.; Rombaldi, C.V. Thermal inactivation of polyphenoloxidase and peroxidase in Jubileu clingstone peach and yeast isolated from its spoiled puree. Food Sci. Technol. 2014, 34, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wang, Y.; Li, X.; Li, B.; Liu, S.; Chang, N.; Jie, D.; Ning, C.; Gao, H.; Meng, X. Combined effect of ultrasound, heat, and pressure on Escherichia coli O157:H7, polyphenol oxidase activity, and anthocyanins in blueberry (Vaccinium corymbosum) juice. Ultrason. Sonochem. 2017, 37, 251–259. [Google Scholar] [CrossRef]
- Cao, X.; Cai, C.; Wang, Y.; Zheng, X. The inactivation kinetics of polyphenol oxidase and peroxidase in bayberry juice during thermal and ultrasound treatments. Innov. Food Sci. Emerg. Technol. 2018, 45, 169–178. [Google Scholar] [CrossRef]
- Hogan, E.; Kelly, A.L.; Sun, D.W. High pressure processing of foods: An Overview. In Emerging Technologies for Food Processing; Academic Press: Cambridge, MA, USA, 2005; pp. 3–32. [Google Scholar]
- Seyderhelm, I.; Boguslawski, S.; Michaelis, G.; Knorr, D. Pressure induced inactivation of selected food enzymes. J. Food Sci. 1996, 61, 308–310. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Innov. Food Sci. Emerg. Technol. 2015, 27, 48–56. [Google Scholar] [CrossRef]
- Tribst, A.A.L.; Leite Júnior, B.R.D.C.; De Oliveira, M.M.; Cristianini, M. High pressure processing of cocoyam, Peruvian carrot and sweet potato: Effect on oxidative enzymes and impact in the tuber color. Innov. Food Sci. Emerg. Technol. 2016, 34, 302–309. [Google Scholar] [CrossRef]
- Cano, M.P.; Hernandez, A.; De Ancos, B. High pressure and temperature effects on enzyme inactivation in strawberry and orange products. J. Food Sci. 1997, 62, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Heinisch, O.; Kowalski, E.; Goossens, K.; Frank, J.; Heremans, K.; Ludwig, H.; Tauscher, B. Pressure effects on the stability of lipoxygenase: Fourier transform-infrared spectroscopy (FT-IR) and enzyme activity studies. Z. Lebensm. Unters. Forsch. 1995, 201, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.P.; Srinivasa Rao, P. Process optimization for high pressure processing of black tiger shrimp (Penaeus monodon) using response surface methodology. Food Sci. Technol. Int. 2017, 23, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Chin, N.L. Application of Thermosonication Treatment in Processing and Production of High Quality and Safe-to-Drink Fruit Juices. Agric. Agric. Sci. Procedia 2014, 2, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Engmann, F.N.; Ma, Y.; Zhang, H.; Yu, L.; Deng, N. The application of response surface methodology in studying the effect of heat and high hydrostatic pressure on anthocyanins, polyphenol oxidase, and peroxidase of mulberry (Morus nigra) juice. J. Sci. Food Agric. 2014, 94, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.; Yildirim, H.B.; Tekin, Z.H. Handbook of Ultrasonics and Sonochemistry; Springer: New York, NY, USA, 2016. [Google Scholar]
- Butz, P.; Tauscher, B. Emerging technologies: Chemical aspects. Food Res. Int. 2002, 35, 279–284. [Google Scholar] [CrossRef]
- López-Malo, A.; Palou, E.; Jiménez-Fernández, M.; Alzamora, S.M.; Guerrero, S. Multifactorial fungal inactivation combining thermosonication and antimicrobials. J. Food Eng. 2005, 67, 87–93. [Google Scholar] [CrossRef]
- Wu, J.; Gamage, T.V.; Vilkhu, K.S.; Simons, L.K.; Mawson, R. Effect of thermosonication on quality improvement of tomato juice. Innov. Food Sci. Emerg. Technol. 2008, 9, 186–195. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Patras, A.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C. Effect of thermosonication on bioactive compounds in watermelon juice. Food Res. Int. 2011, 44, 1168–1173. [Google Scholar] [CrossRef]
- Šimunek, M.; Jambrak, A.R.; Dobrović, S.; Herceg, Z.; Vukušić, T. Rheological properties of ultrasound treated apple, cranberry and blueberry juice and nectar. J. Food Sci. Technol. 2014, 51, 3577–3593. [Google Scholar] [CrossRef] [Green Version]
- Illera, A.E.; Sanz, M.T.; Benito-Román, O.; Varona, S.; Beltrán, S.; Melgosa, R.; Solaesa, A.G. Effect of thermosonication batch treatment on enzyme inactivation kinetics and other quality parameters of cloudy apple juice. Innov. Food Sci. Emerg. Technol. 2018, 47, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Bot, F.; Calligaris, S.; Cortella, G.; Plazzotta, S.; Nocera, F.; Anese, M. Study on high pressure homogenization and high power ultrasound effectiveness in inhibiting polyphenoloxidase activity in apple juice. J. Food Eng. 2018, 221, 70–76. [Google Scholar] [CrossRef]
- Jayachandran, L.E.; Chakraborty, S.; Rao, P.S. Inactivation Kinetics of the Most Baro-Resistant Enzyme in High Pressure Processed Litchi-Based Mixed Fruit Beverage. Food Bioprocess Technol. 2016, 9, 1135–1147. [Google Scholar] [CrossRef]
- Shinwari, K.J.; Rao, P.S. Enzyme inactivation and its kinetics in a reduced-calorie sapodilla (Manilkara zapota L.) jam processed by thermal-assisted high hydrostatic pressure. Food Bioprod. Process. 2021, 126, 305–316. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Terefe, N.S. The Inactivation Kinetics of Soluble and Membrane-Bound Polyphenol Oxidase in Pear during Thermal and High Pressure Processing. Food Bioprocess Technol. 2018, 11, 1039–1049. [Google Scholar] [CrossRef]
- Başlar, M.; Ertugay, M.F. The effect of ultrasound and photosonication treatment on polyphenoloxidase (PPO) activity, total phenolic component and colour of apple juice. Int. J. Food Sci. Technol. 2013, 48, 886–892. [Google Scholar] [CrossRef]
- Peleg, M.; Cole, M.B. Critical Reviews in Food Science and Nutrition Reinterpretation of Microbial Survival Curves. Crit. Rev. Food Sci. Nutr. 2007, 38, 353–380. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Rapeanu, G.; Van Loey, A.; Smout, C.; Hendrickx, M. Biochemical characterization and process stability of polyphenoloxidase extracted from Victoria grape (Vitis vinifera ssp. Sativa). Food Chem. 2006, 94, 253–261. [Google Scholar] [CrossRef]
- Sibi, M.P.; Mastracchio, A.; Hong, J.B.; Ashton, K.; Macmillan, D.W.C.; Hong, J.B.; Macmillan, D.W.C.; Macmillan, D.W.C.; Hasegawa, M.; Winkler, J.R.; et al. Temperature-induced hydrophobic-hydrophilic transition observed by water adsorption. Science 2008, 322, 80–83. [Google Scholar]
- Cengel, Y.A.; Boles, M. Energy Transfer by Heat, Work, and Mass. In Thermodynamics: An Engineering Approach; McGrawhill: New York, NY, USA, 2015; pp. 124–150. [Google Scholar]
- Athmaselvi, K.A.; Jenney, P.; Pavithra, C.; Roy, I. Physical and biochemical properties of selected tropical fruits. Int. Agrophys. 2014, 28, 383–388. [Google Scholar] [CrossRef] [Green Version]
- American Society of Heating, Refrigeration and A-Conditioning Engineers. Thermal properties of foods. In 2006 ASHRAE Handbook: Refrigeration; American Society of Heating, Refrigeration and Air-Conditioning Engineers: Atlanta, GA, USA, 2006; pp. 1–31. [Google Scholar]
- Astolfi-Filho, Z.; Minim, L.A.; Telis-Romero, J.; Minim, V.P.R.; Telis, V.R.N. Thermophysical properties of industrial sugar cane juices for the production of bioethanol. J. Chem. Eng. Data 2010, 55, 1200–1203. [Google Scholar] [CrossRef]
- Muthukumarappan, K.; Marella, C.; Sunkesula, V. Chapter 15—Food Freezing Technology. In Handbook of Farm, Dairy and Food Machinery Engineering, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 389–415. [Google Scholar]
- Budžaki, S.; Šeruga, B. Specific heat of strawberry and raspberry puree. J. Food Process. Preserv. 2014, 38, 2240–2245. [Google Scholar] [CrossRef]
- Kell, G.S. Density, Thermal Expansivity, and Compressibility of Liquid Water from 0° to 150 °C: Correlations and Tables for Atmospheric Pressure and Saturation Reviewed and Expressed on 1968 Temperature Scale. J. Chem. Eng. Data 1975, 20, 97–105. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
| Models | Equations |
|---|---|
| First-order kinetic model | where A is the enzyme activity of sample at different treatment times (t), A0 is the initial enzyme activity of the raw unprocessed sample, A/A0 is the residual activity of the enzyme, k is the first-order inactivation rate constant at the operating conditions (min−1), and t is the treatment time (min). The decimal reduction time (D: the time required to reduce enzyme activity by 90%) can be calculated from the inactivation rate constant (D = 2.303/k). The negative reciprocal slope of the regression line of log D as a function of T was used to calculate z, which is defined as the temperature increase required to reduce the D value by 90%. Furthermore, the temperature dependence of the PPO inactivation can be described using the linearized version of the Arrhenius equation as follows: where temperatures T2 and T1 correspond to decimal reduction times D2 and D1 or constants k2 and k1, respectively. Ea is the activation energy (kJ/mol), and R is the universal gas constant (8.31 J/(mol·K)). Using linear regression analysis, the activation energy was calculated from the slope of the ln (k) versus 1/T plot. |
| Biphasic model | For non-linear data, the biphasic model can be used, where AS and AL are activities of the stable and the labile fractions, respectively, and kS and kL are the inactivation rate constants of stable and labile fractions, respectively. |
| Weibull model | Weibull model is also suitable for non-linear, where b and n are scale and shape factors, respectively [61]. |
| Fruit/Cultivar | Temperature (°C) | k (min−1) | D-Value (min) | z-Value (°C) | Ea, (kJ/mol) | Reference |
|---|---|---|---|---|---|---|
| Kalipatti sapota (Manilkara zapota) pulp | 75 | 0.288 | 8 | 15.2 °C | 158 | [36] |
| Bayberry (Myrica) juice | 65 75 | 0.0888 0.370 | 26 6 | 13.2 °C | 152 | [39] |
| Pear (Pyrus communis cv. Taylor’s Gold) puree | 75 80 | 0.019 0.214 | 121 11 | 6.0 °C | 375 | [28] |
| Apple (Malus domestica cv. Royal Gala) puree | 75 | 0.103 | 22 | 17.0 °C | 134 | [28] |
| Strawberries (Fragaria ananassa cv. Camarosa) puree | 60 | 0.144 | 16 | 14.0 °C | 147 | [28] |
| (a) | ||||||||||
| Fruit/Cultivar | Parameters | ks (min−1) | Ea(s) (kJ/mol) | kL (min−1) | Ea(L), (kJ/mol) | DS-Value (min) | zS-Value (°C) | DL-Value (min) | zL-Value (°C) | Reference |
| Apple (Malus domestica cv. Royal Gala) puree | 600 MPa 57 °C | 0.0121 | 31 | 0.0266 | 54 | 190 | 69 | 86 | 40 | [9] |
| 600 MPa 71 °C | 0.0184 | 0.0613 | 124 | 38 | ||||||
| Strawberry (Fragaria ananassa cv. Camarosa) puree | 600 MPa 57 °C | 0.0182 | 99 | 0.213 | 57 | 127 | 22 | 11 | 38 | [9] |
| 600 MPa 71 °C | 0.0805 | 0.514 | 29 | 4.5 | ||||||
| (b) | ||||||||||
| Fruit/Cultivar | Parameters | k (min−1) | D-Value (min) | z-Value (°C) | Ea, (kJ/mol) | Reference | ||||
| Lychee (Litchi chinensis Sonn) pulp | 400 MPa 50 °C | 0.0256 | 90 | 65 | 40 | [57] | ||||
| Sapodilla (Manilkara zapota) Jam | 600 MPa 65 °C | 0.0136 | 169 | 108 | 18 | [58] | ||||
| Pear (Pyrus communis cv. Packham) juice | 600 MPa 60 °C | 0.0830 | 28 | 31 | 64 | [59] | ||||
| Fruit/Cultivar | Parameters | k (min−1) | D-Value (min) | z-Value (°C) | Ea, (kJ/mol) | References |
|---|---|---|---|---|---|---|
| Apple (Malus domestica cv. Golden Delicious) juice | 565 W/cm2 64 °C | 0.036 | 28 | 18 | 123 | [55] |
| 565 W/cm2 67 °C | 0.056 | 18 | ||||
| Pear (Pyrus communis cv. Taylor’s Gold) puree | 460 W/cm2 1.3 W/g 72 °C | 0.356 | 7 | 50 | 40 | [28] |
| Apple (Malus domestica cv. Royal Gala) puree | 460 W/cm2 1.3 W/g 72 °C | 0.540 | 4 | 39 | 52 | [28] |
| Strawberries (Fragaria ananassa cv. Camarosa) puree | 460 W/cm2 1.3 W/g 72 °C | 0.483 | 5 | 80 | 25 | [28] |
| Apple (Malus domestica cv. Golden Delicious) juice | 60 °C | 0.178 | 13 | 19 | 105 | [60] |
| Fruit/Cultivar | Conditions | * Mass (kg) | ∆T 1 (°C) | Cp (kJ/kg·K) | Q = mcp∆T (kJ) | α 2 (10−4, K−1) | β 2 (10−10 pa−1) | Compression Work, W (kJ) | Total Energy, Q + W (kJ) | Specific Energy (kJ/kg) | Data Collected from |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugarcane juice (Saccharum officinarum) juice | 600 MPa 25 min 60 °C | 1 | 22 | 3.64 [68] | 80 | 5.2307 | 4.4496 | 149 | 229 | 229 | [35] |
| Pineapple (Ananas comosus L.) puree | 500 MPa 24 min 60 °C | 1 | 25 | 3.68 [69] | 92 | 5.2307 | 4.4496 | 103 | 195 | 195 | [29] |
| Strawberry (Fragaria ananassa cv. Senga Sengana) puree | 500 MPa 15 min 50 °C | 0.75 | 15 | 3.60 [70] | 41 | 4.5759 | 4.4173 | 79 | 120 | 159 | [42] |
| Mulberry (Morus nigra) juice | 250 MPa 10 min 85 °C | 3.25 | 58 | 1.94 [67] | 366 | 6.6886 | 4.6748 | 86 | 452 | 139 | [48] |
| 365 MPa 17.5 min 90 °C | 59 | 372 | 6.9623 | 4.7429 | 187 | 559 | 172 | ||||
| Apple (Malus domestica cv. Royal Gala) puree | 600 MPa 70 min 57 °C | 1 | 19 | 1.88 [69] | 36 | 5.0401 | 4.4362 | 149 | 185 | 185 | [9] |
| 600 MPa 50 min 71 °C | 33 | 62 | 5.8960 | 4.5246 | 150 | 212 | 212 | ||||
| Strawberry (Fragaria ananassa cv. Camarosa) puree | 600 MPa 15 min 57 °C | 1 | 19 | 3.60 [70] | 68 | 5.0401 | 4.4362 | 149 | 217 | 217 | [9] |
| 600 MPa 3 min 71 °C | 33 | 119 | 5.8960 | 4.5246 | 150 | 269 | 269 |
| Fruit/Cultivar | Conditions | Mass (kg) | Sound Intensity (W/cm2) | Area (cm2) | E = Sound Intensity × A × t, (kJ) | Specific Energy (kJ/kg) | Data Collected from |
|---|---|---|---|---|---|---|---|
| Apple (Malus domestica cv.Golden Delicious) juice | 20 min 67 °C | 0.083 | 565 | 1.327 | 900 | 10843 | [55] |
| Apple (Malus domestica cv. Fuji) Juice | 10 min 60 °C | 0.083 | 396 | 1.327 | 315 | 3795 | [30] |
| Bayberry (Myrica) Juice | 8 min 60 °C | 0.198 | 271 | 1.327 | 173 | 874 | [39] |
| 8 min 70 °C | 452 | 1.327 | 288 | 1455 | |||
| Pear (Pyrus communis cv. Taylor’s Gold) Puree | 10 min 72 °C | 0.025 | 460 | 0.07068 | 19.5 | 780 | [28] |
| Apple (Malus domestica cv. Royal Gala) puree | 10 min 72 °C | 0.025 | 460 | 0.07068 | 19.5 | 780 | [28] |
| Strawberry (Fragaria ananassa cv. Camarosa) puree | 10 min 72 °C | 0.025 | 460 | 0.07068 | 19.5 | 780 | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.M.; Sulaiman, A. Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Appl. Sci. 2022, 12, 1864. https://doi.org/10.3390/app12041864
Zawawi NAF, Hazmi NAM, How MS, Kantono K, Silva FVM, Sulaiman A. Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Applied Sciences. 2022; 12(4):1864. https://doi.org/10.3390/app12041864
Chicago/Turabian StyleZawawi, Nur Aribah Fatini, Nurul Ashikin Md. Hazmi, Muhammad Syahmeer How, Kevin Kantono, Filipa V. M. Silva, and Alifdalino Sulaiman. 2022. "Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement" Applied Sciences 12, no. 4: 1864. https://doi.org/10.3390/app12041864
APA StyleZawawi, N. A. F., Hazmi, N. A. M., How, M. S., Kantono, K., Silva, F. V. M., & Sulaiman, A. (2022). Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Applied Sciences, 12(4), 1864. https://doi.org/10.3390/app12041864