Thermal Stability of Ionic Liquids: Effect of Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
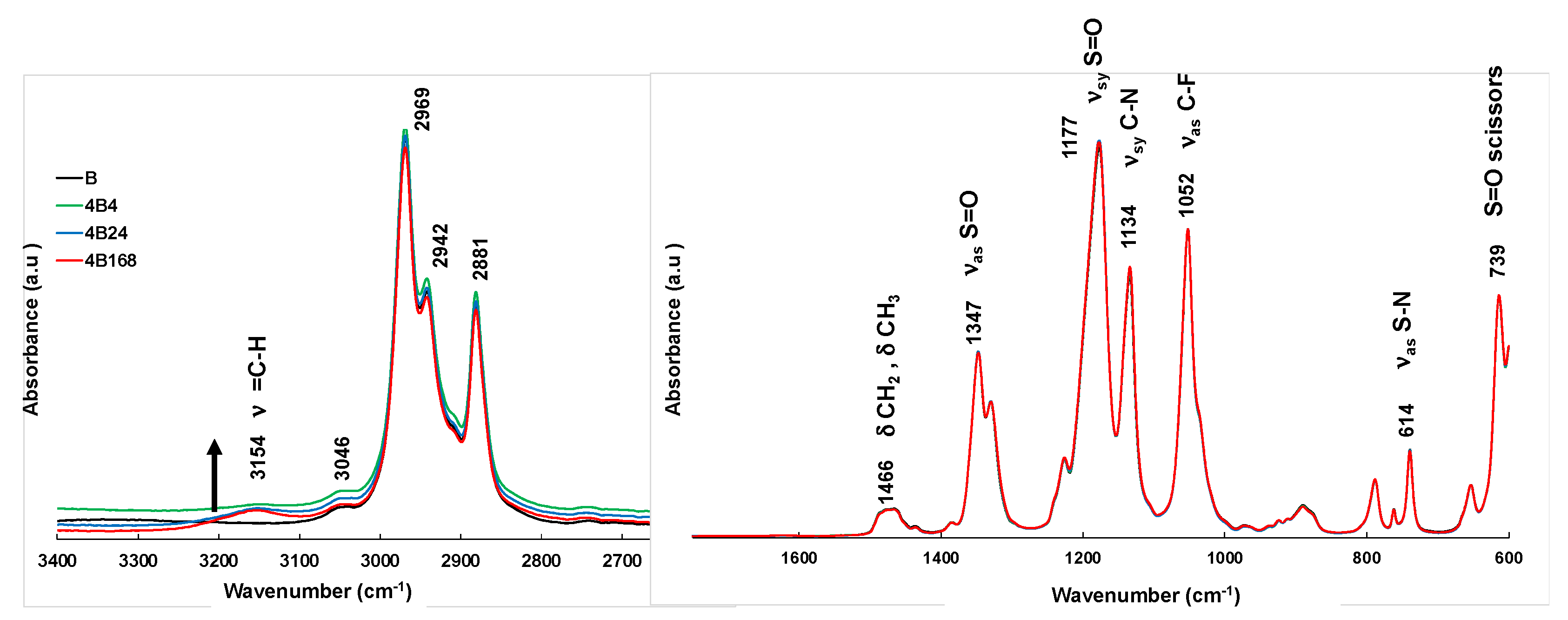
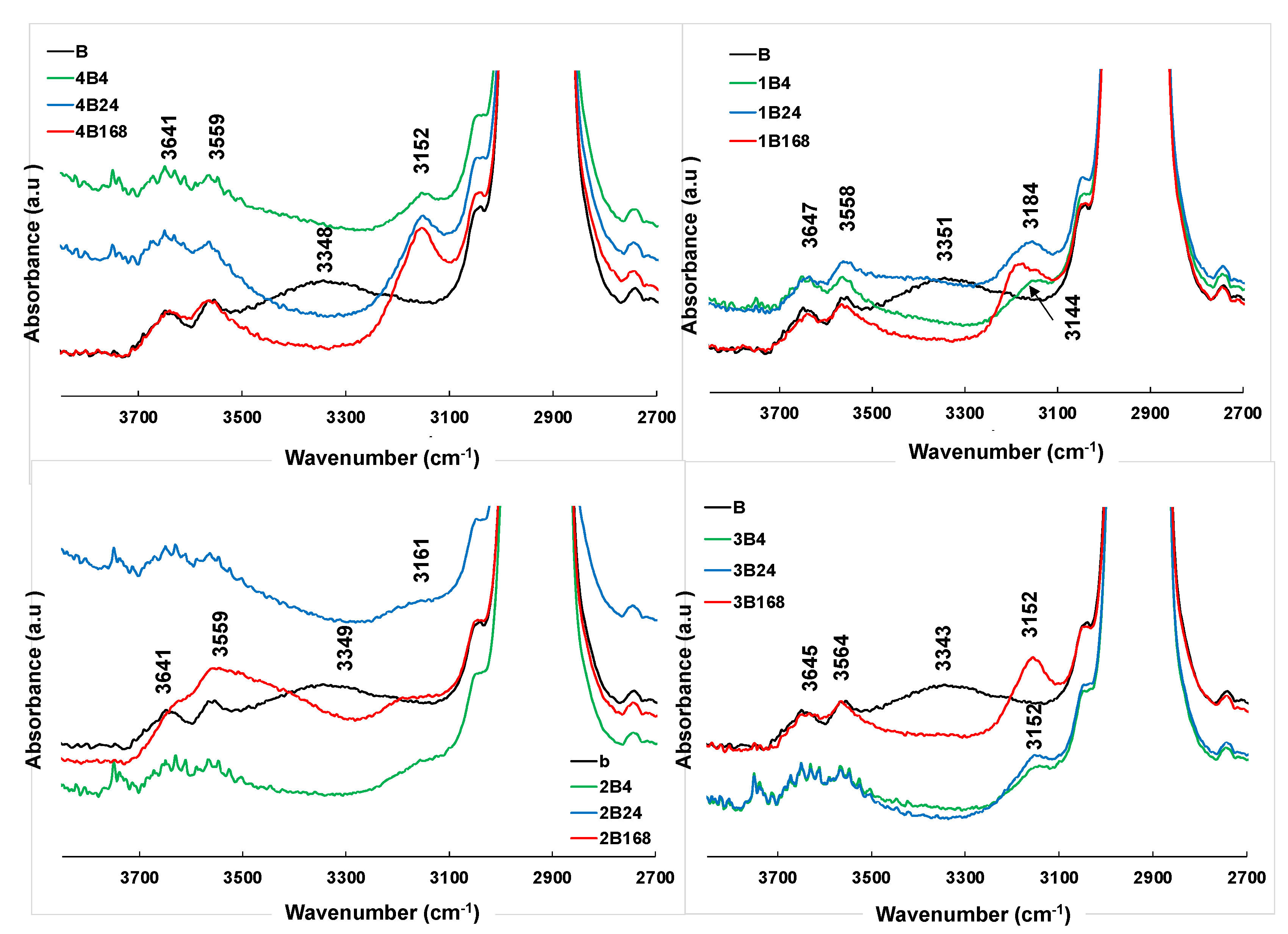
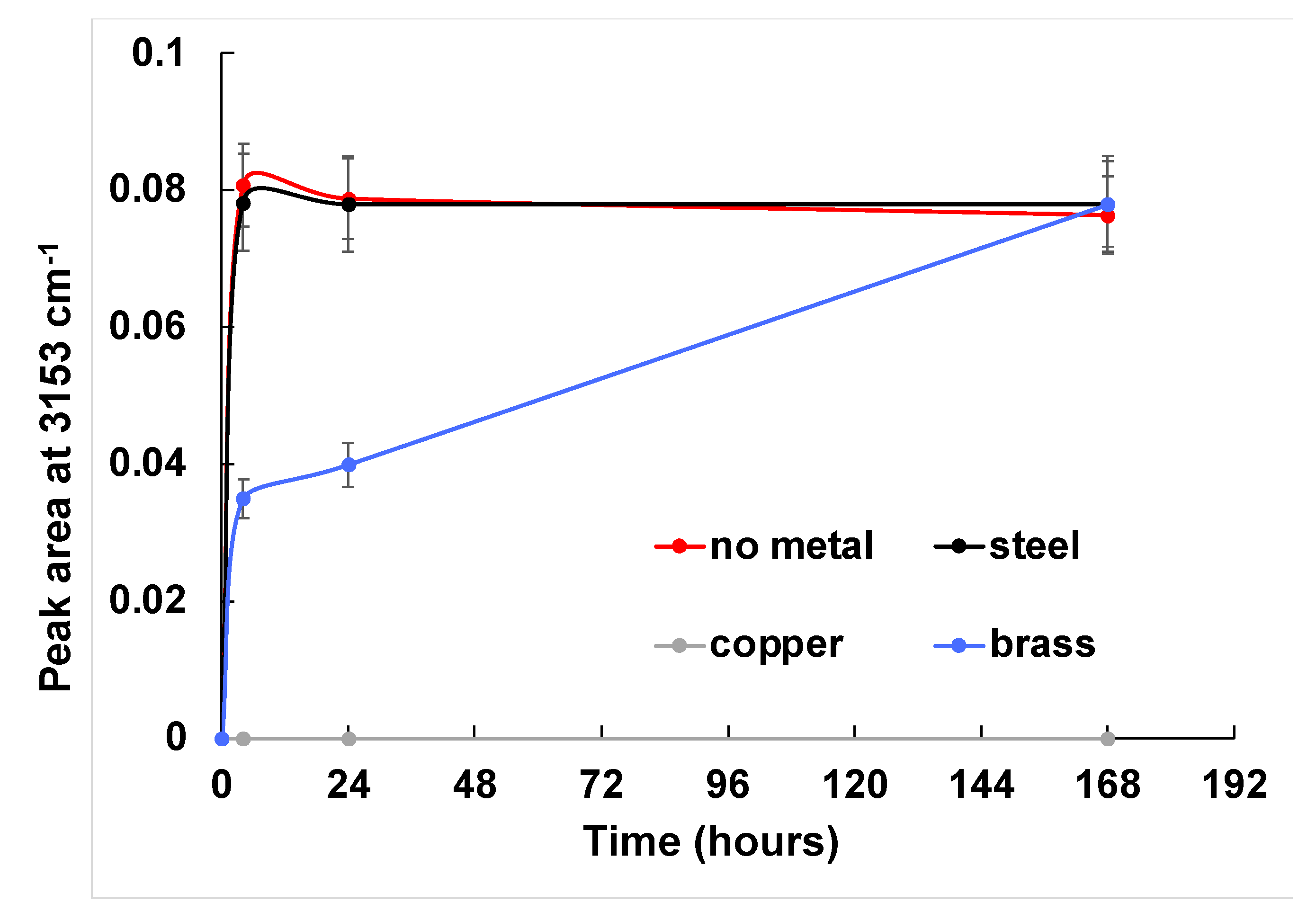
2.2. FTIR Spectroscopy
2.3. HS-GC-MS Analysis
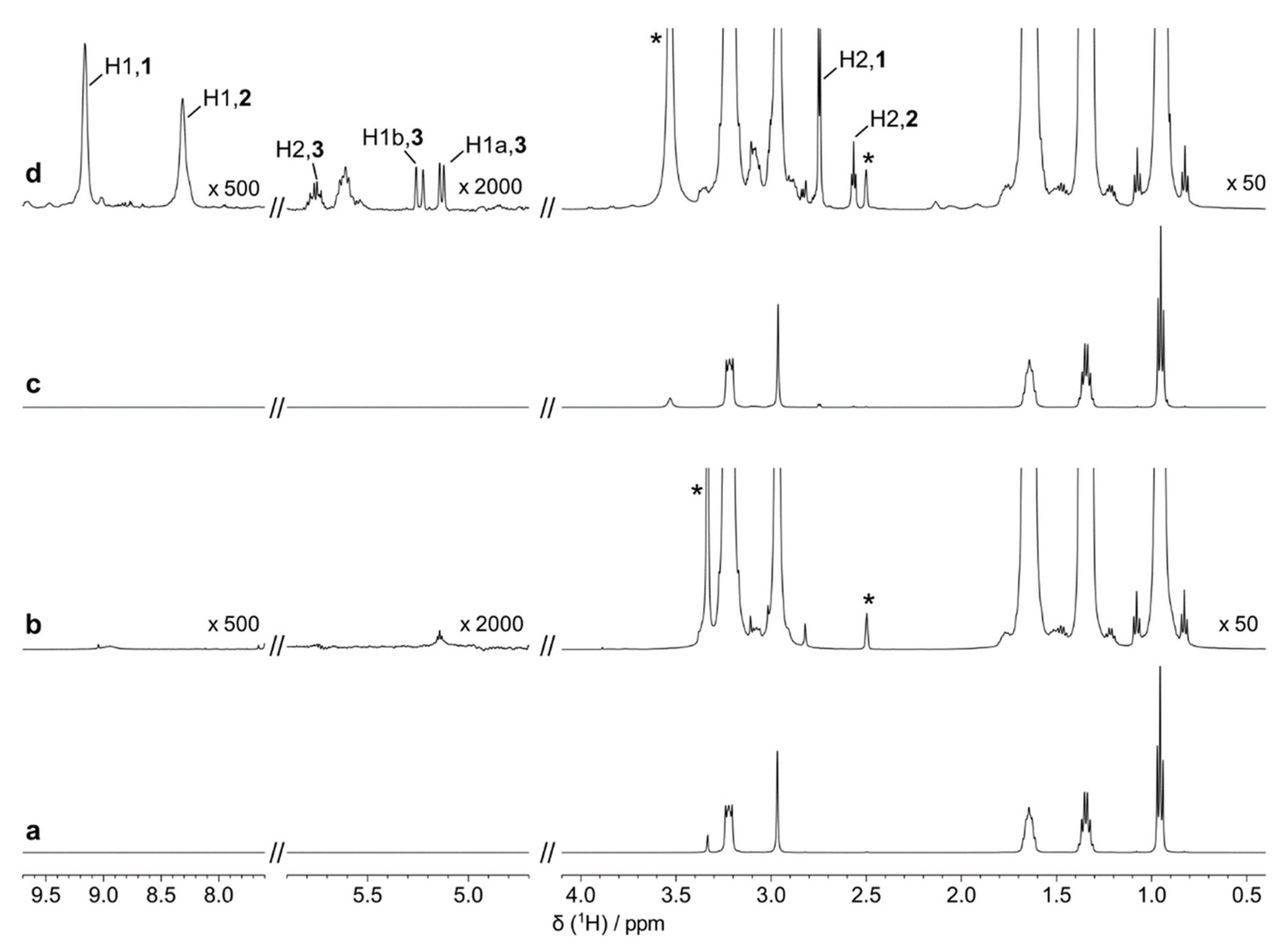
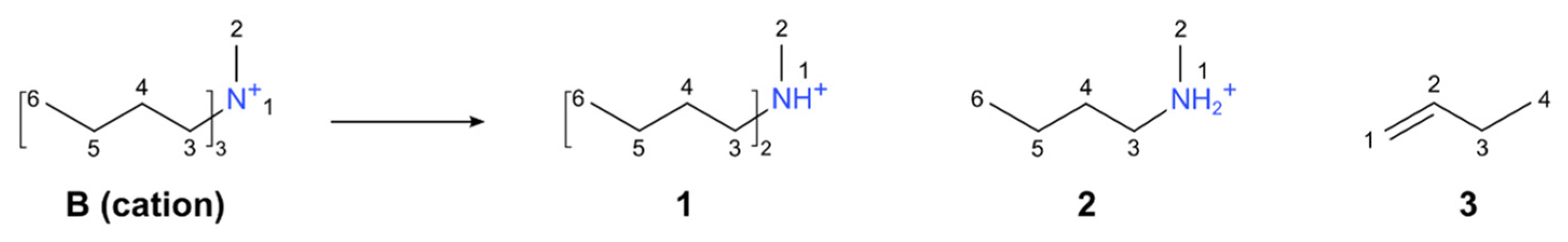
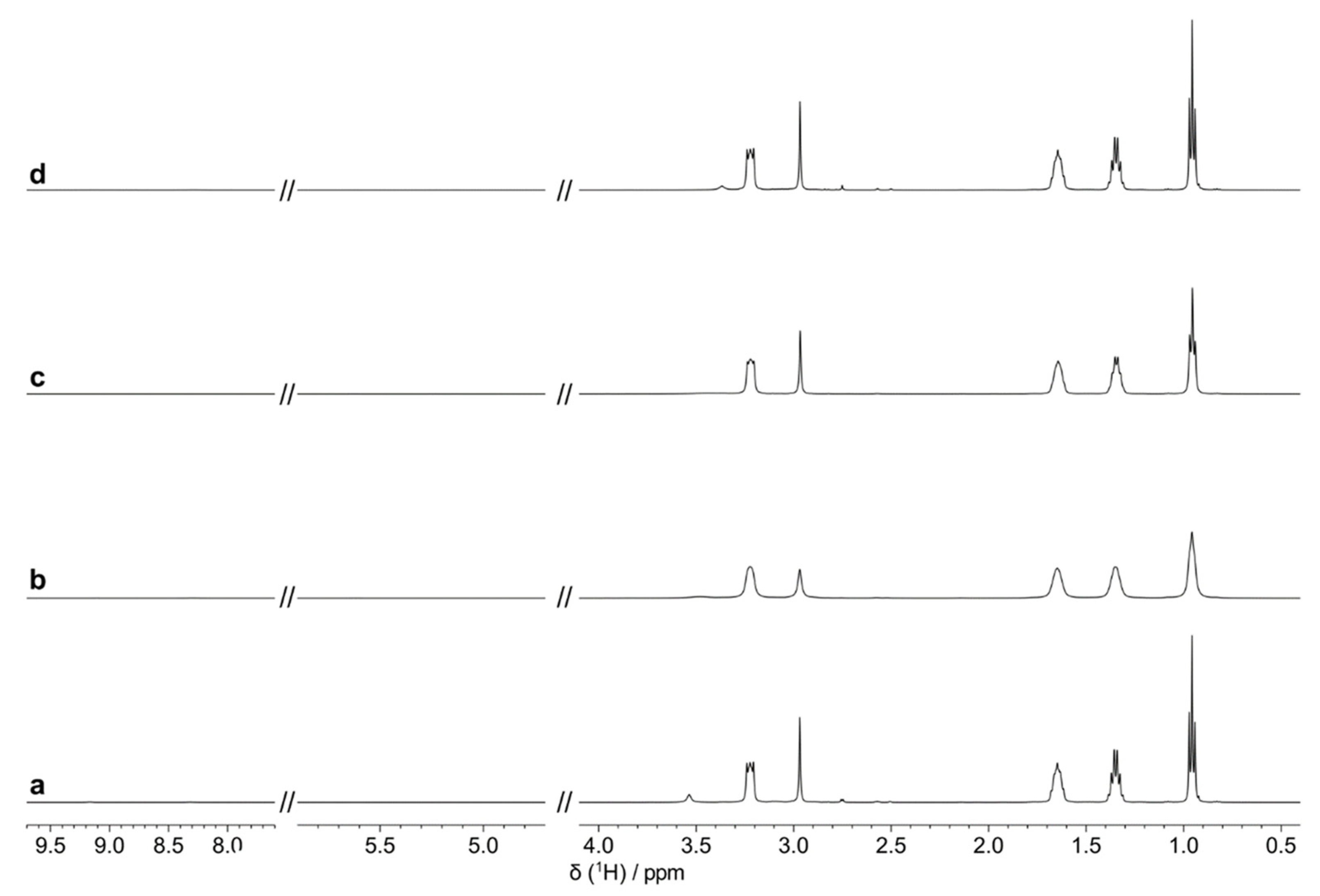
2.4. HRMAS NMR Spectroscopy
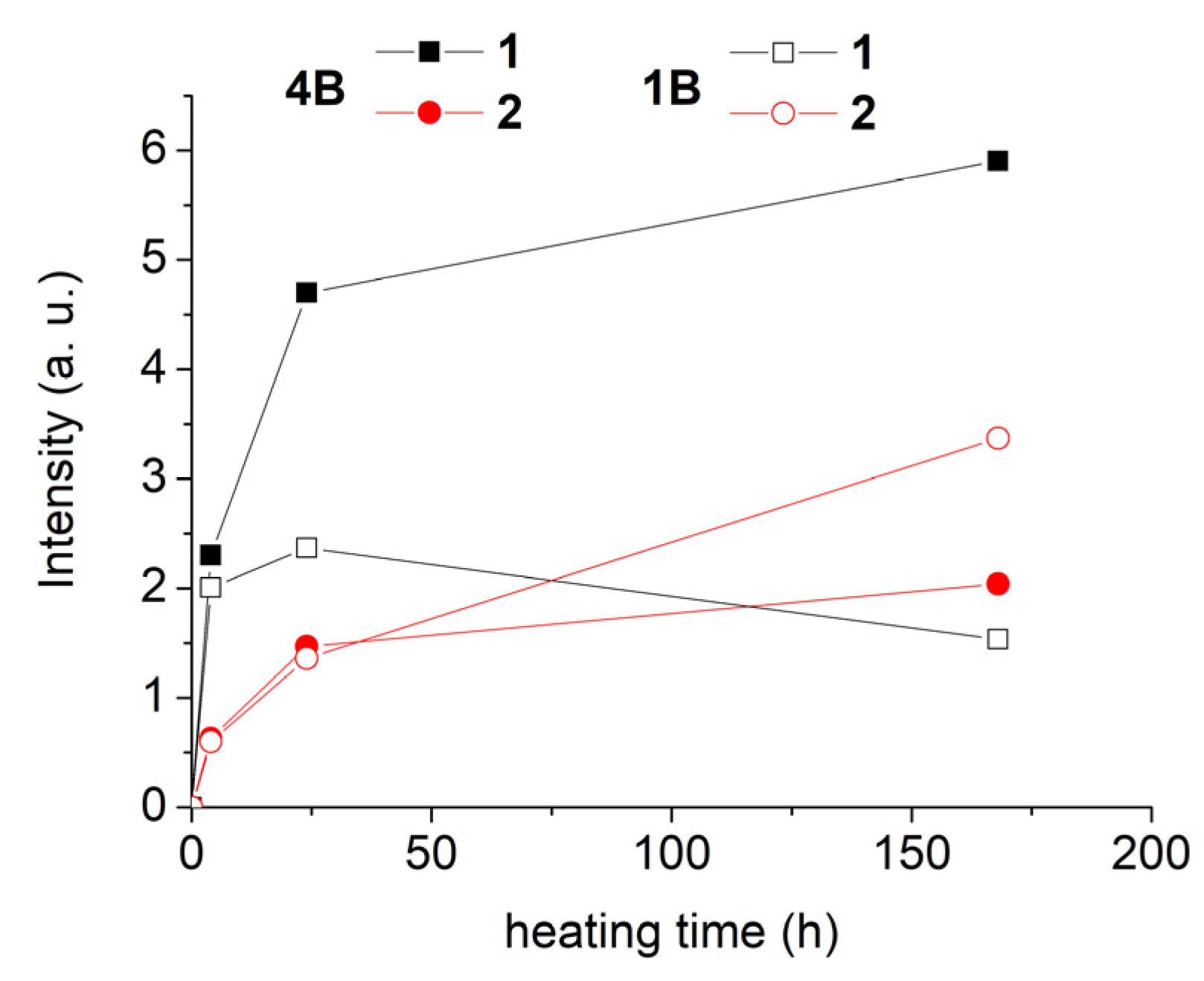
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Visser, A.E.; Bridges, N.J.; Khan, J.A. Thermal performance of ionic liquids for solar thermal applications. Exp. Therm. Fluid Sci. 2014, 59, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Salih, A.A.M.; Li, M.; Yang, B. Synthesis and characterization of functionalized ionic liquids for thermal storage. Energy Fuels 2014, 28, 2802–2810. [Google Scholar] [CrossRef]
- Wadekar, V. V Ionic liquids as heat transfer fluids—An assessment using industrial exchanger geometries. Appl. Therm. Eng. 2017, 111, 1581–1587. [Google Scholar] [CrossRef]
- Das, L.; Rubbi, F.; Habib, K.; Aslfattahi, N.; Saidur, R.; Baran Saha, B.; Algarni, S.; Irshad, K.; Alqahtani, T. State-of-the-art ionic liquid & ionanofluids incorporated with advanced nanomaterials for solar energy applications. J. Mol. Liq. 2021, 336, 116563. [Google Scholar]
- Macfarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Valkenburg, M.E.V.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Salgado, J.; Parajó, J.J.; Fernández, J.; Villanueva, M. Long-term thermal stability of some 1-butyl-1-methylpyrrolidinium ionic liquids. J. Chem. Thermodyn. 2014, 74, 51–57. [Google Scholar] [CrossRef]
- Monteiro, B.; Maria, L.; Cruz, A.; Carretas, J.M.; Marçalo, J.; Leal, J.P. Thermal stability and specific heats of coordinating ionic liquids. Thermochim. Acta 2020, 684, 178482. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew. Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Crosthwaite, J.M.; Aki, S.N.V.K.; Brennecke, J.F. Thermal stability of ionic liquids in nitrogen and air environments. J. Chem. Thermodyn. 2021, 161, 106560. [Google Scholar] [CrossRef]
- Kosmulski, M.; Gustafsson, J.; Rosenholm, J.B. Thermal stability of low temperature ionic liquids revisited. Thermochim. Acta 2004, 412, 47–53. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Perissi, I.; Bardi, U.; Caporali, S.; Fossati, A.; Lavacchi, A.; Vizza, F. Ionic liquids: Electrochemical investigation on corrosion activity of ethyl-dimethyl-propylammonium bis(trifluoromethylsulfonyl)imide at high temperature. Russ. J. Electrochem. 2012, 48, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Perissi, I.; Bardi, U.; Caporali, S.; Fossati, A.; Lavacchi, A. Ionic liquids as diathermic fluids for solar trough collectors’ technology: A corrosion study. Sol. Energy Mater. Sol. Cells 2008, 92, 510–517. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors: Design, performance and industrial scale applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Perissi, I.; Bardi, U.; Caporali, S.; Lavacchi, A. High temperature corrosion properties of ionic liquids. Corros. Sci. 2006, 48, 2349–2362. [Google Scholar] [CrossRef]
- Pisarova, L.; Gabler, C.; Dörr, N.; Pittenauer, E.; Allmaier, G. Thermo-oxidative stability and corrosion properties of ammonium based ionic liquids. Tribol. Int. 2012, 46, 73–83. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Alam, M.T.; Jenkins, E.J. HR-MAS NMR Spectroscopy in Material Science. In Advanced Aspects of Spectroscopy; Farrukh, M.A., Ed.; IntechOpen: London, UK, 2012; pp. 279–306. [Google Scholar]
- Ilharco, L.M.; Garcia, A.R.; Hargreaves, E.C.; Chesters, M.A. Comparative reflection-absorption infrared spectroscopy study of the thermal decomposition of 1-hexene on Ru(0001) and on Pt(111). Surf. Sci. 2000, 459, 115–123. [Google Scholar] [CrossRef]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.C.; Chu, Y.H. On the chemical stabilities of ionic liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, C.H.C.; Macías-Ruvalcaba, N.A.; Aguilar-Martínez, M.; Kobrak, M.N. Copper extraction using protic ionic liquids: Evidence of the Hofmeister effect. Sep. Purif. Technol. 2016, 168, 275–283. [Google Scholar] [CrossRef]
- Totarella, G.; Beerthuis, R.; Masoud, N.; Louis, C.; Delannoy, L.; De Jongh, P.E. Supported Cu Nanoparticles as Selective and Stable Catalysts for the Gas Phase Hydrogenation of 1,3-Butadiene in Alkene-Rich Feeds. J. Phys. Chem. C 2021, 125, 366–375. [Google Scholar] [CrossRef] [PubMed]
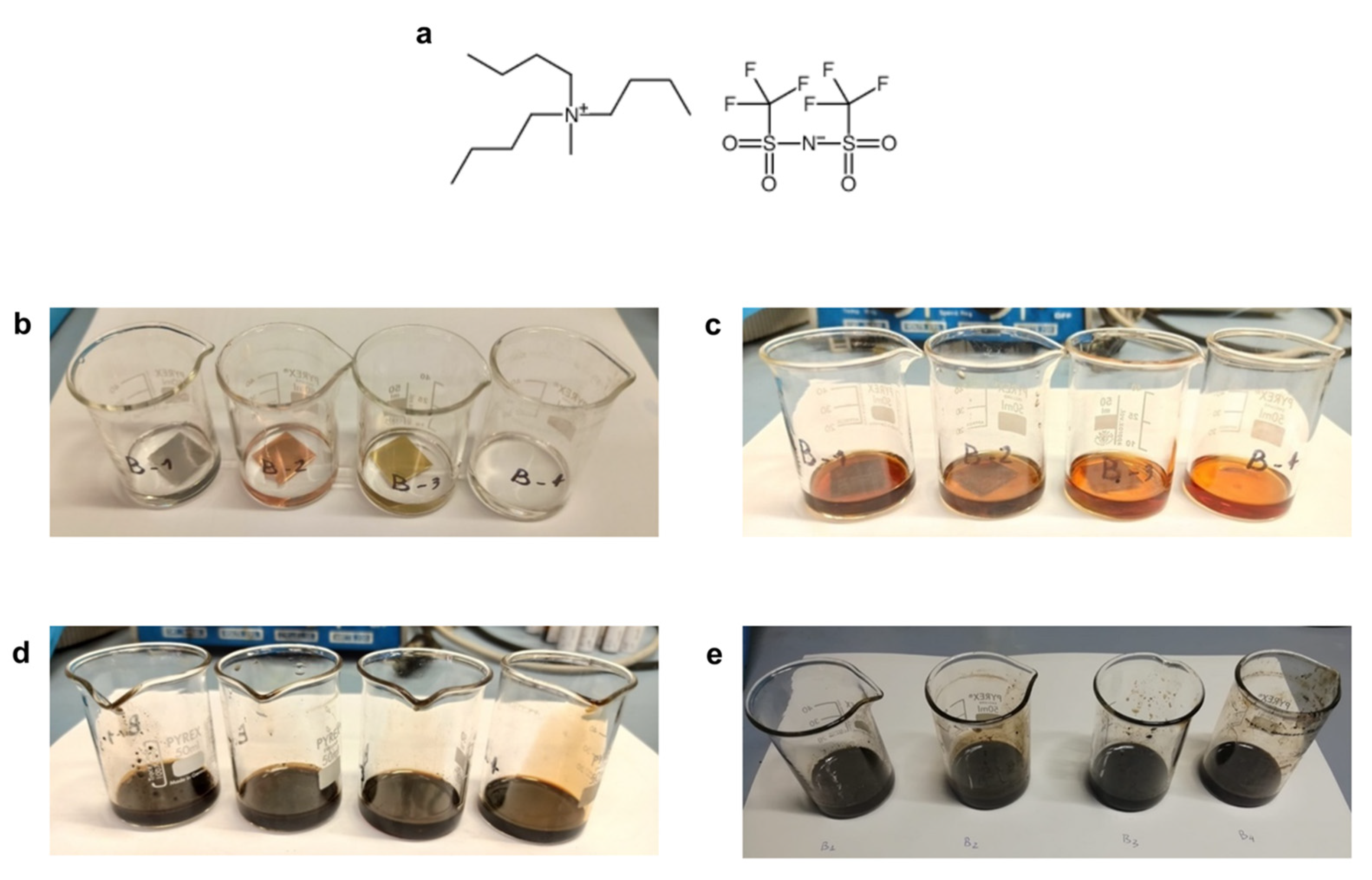
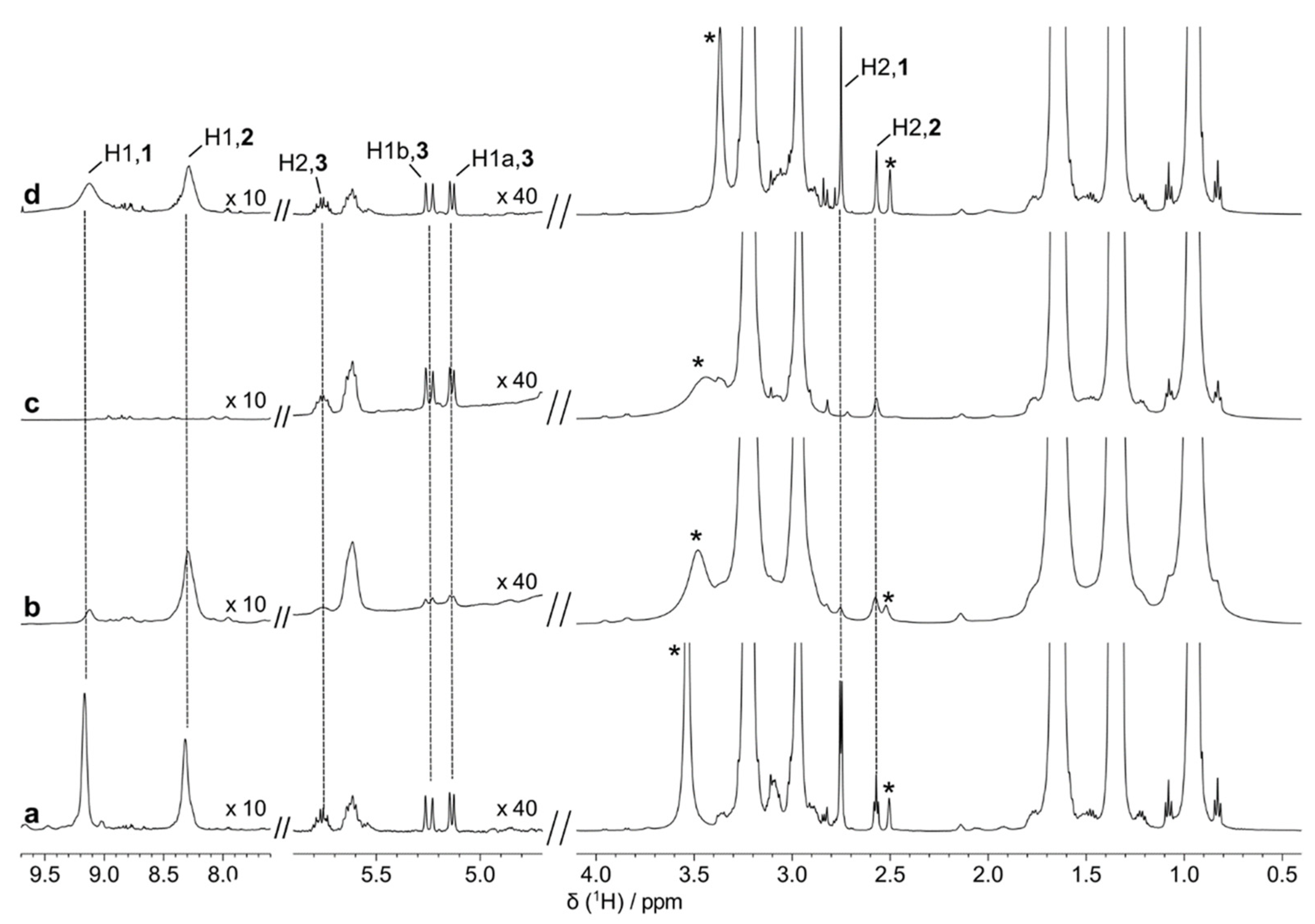
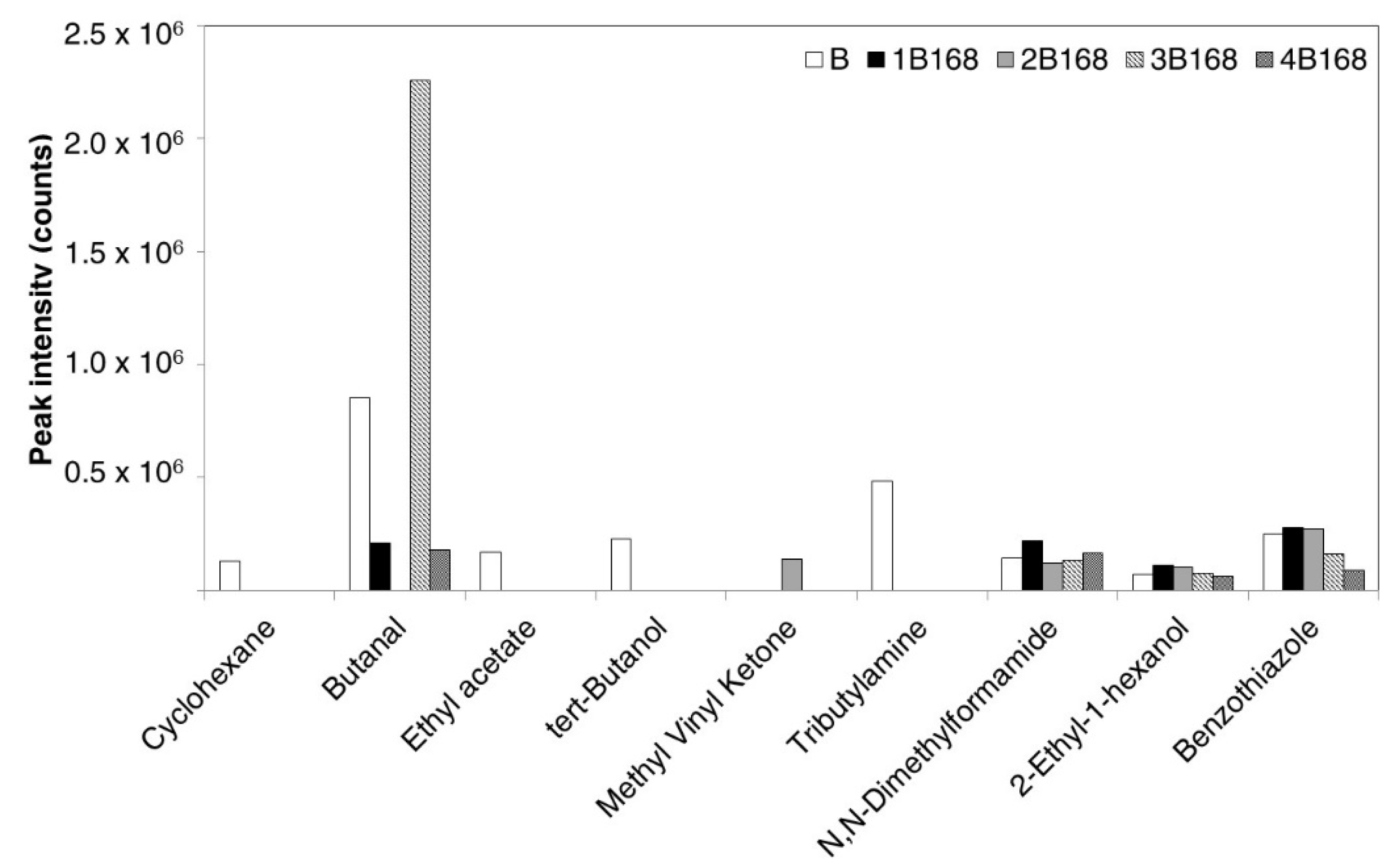
| Metal | 4 h at 200 °C | 24 h at 200 °C | 168 h at 200 °C |
|---|---|---|---|
| 1 (steel) | 1B4 | 1B24 | 1B168 |
| 2 (copper) | 2B4 | 2B24 | 2B168 |
| 3 (brass) | 3B4 | 3B24 | 3B168 |
| 4 (no metal) | 4B4 | 4B24 | 4B168 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardelli, F.; Bramanti, E.; Lavacchi, A.; Pizzanelli, S.; Campanella, B.; Forte, C.; Berretti, E.; Freni, A. Thermal Stability of Ionic Liquids: Effect of Metals. Appl. Sci. 2022, 12, 1652. https://doi.org/10.3390/app12031652
Nardelli F, Bramanti E, Lavacchi A, Pizzanelli S, Campanella B, Forte C, Berretti E, Freni A. Thermal Stability of Ionic Liquids: Effect of Metals. Applied Sciences. 2022; 12(3):1652. https://doi.org/10.3390/app12031652
Chicago/Turabian StyleNardelli, Francesca, Emilia Bramanti, Alessandro Lavacchi, Silvia Pizzanelli, Beatrice Campanella, Claudia Forte, Enrico Berretti, and Angelo Freni. 2022. "Thermal Stability of Ionic Liquids: Effect of Metals" Applied Sciences 12, no. 3: 1652. https://doi.org/10.3390/app12031652
APA StyleNardelli, F., Bramanti, E., Lavacchi, A., Pizzanelli, S., Campanella, B., Forte, C., Berretti, E., & Freni, A. (2022). Thermal Stability of Ionic Liquids: Effect of Metals. Applied Sciences, 12(3), 1652. https://doi.org/10.3390/app12031652








