Morphological Observation of LiCl Deliquescence in PDMS-Based Composite Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. LiCl-PDMS Foam Synthesis and Characterization
2.2. Hydration/Dehydration Cycle through Thermogravimetric Dynamic Vapor Sorption System
2.3. In-Situ Characterization of LiCl-PDMS Foam
2.4. Hydration Heat Capacity Measurement
3. Results
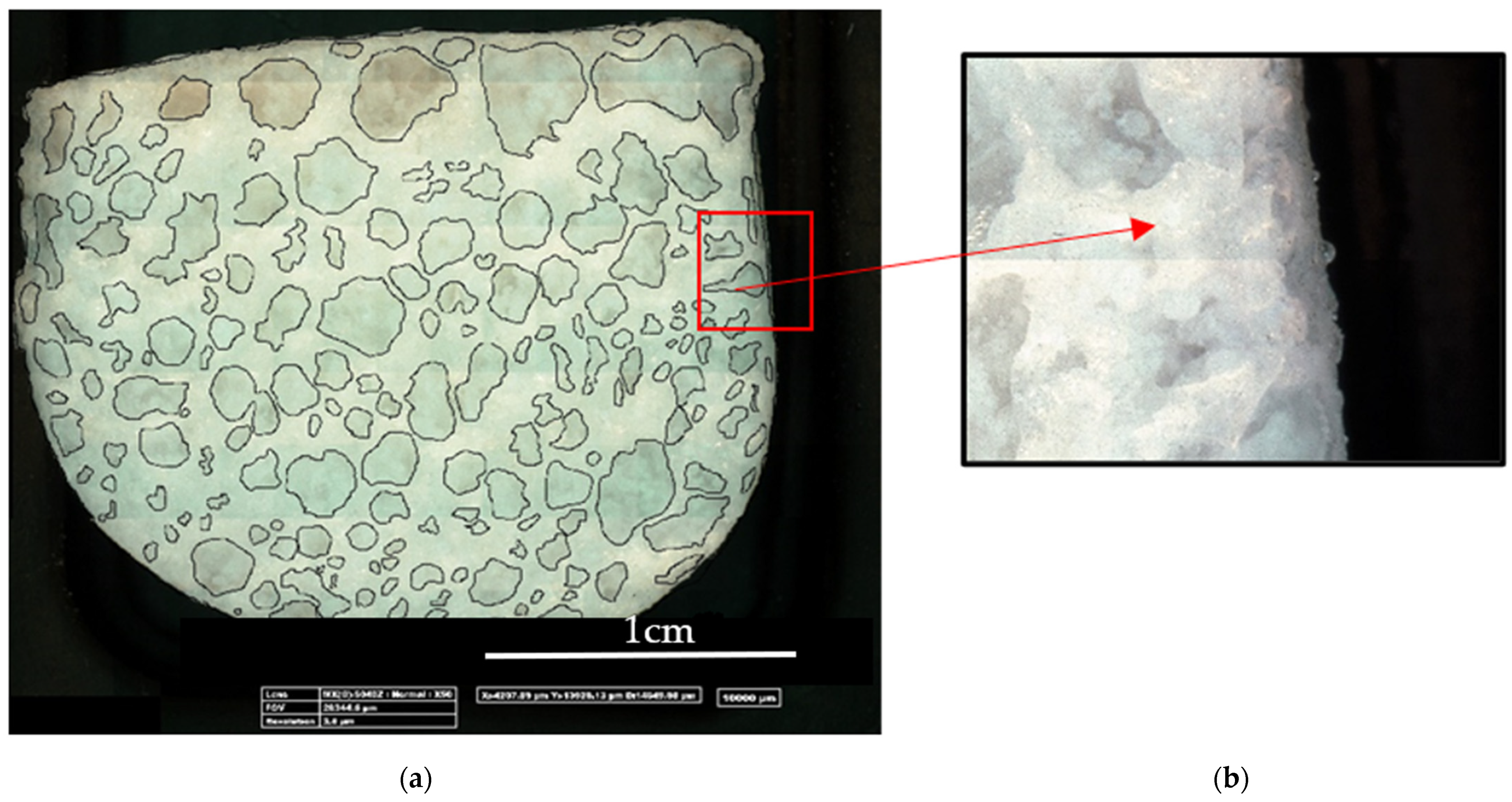
3.1. LiCl-PDMS Foam Optical Analysis
3.2. In-Situ X-ray Diffraction of LiCl-PDMS Foam during Dehydration Reaction
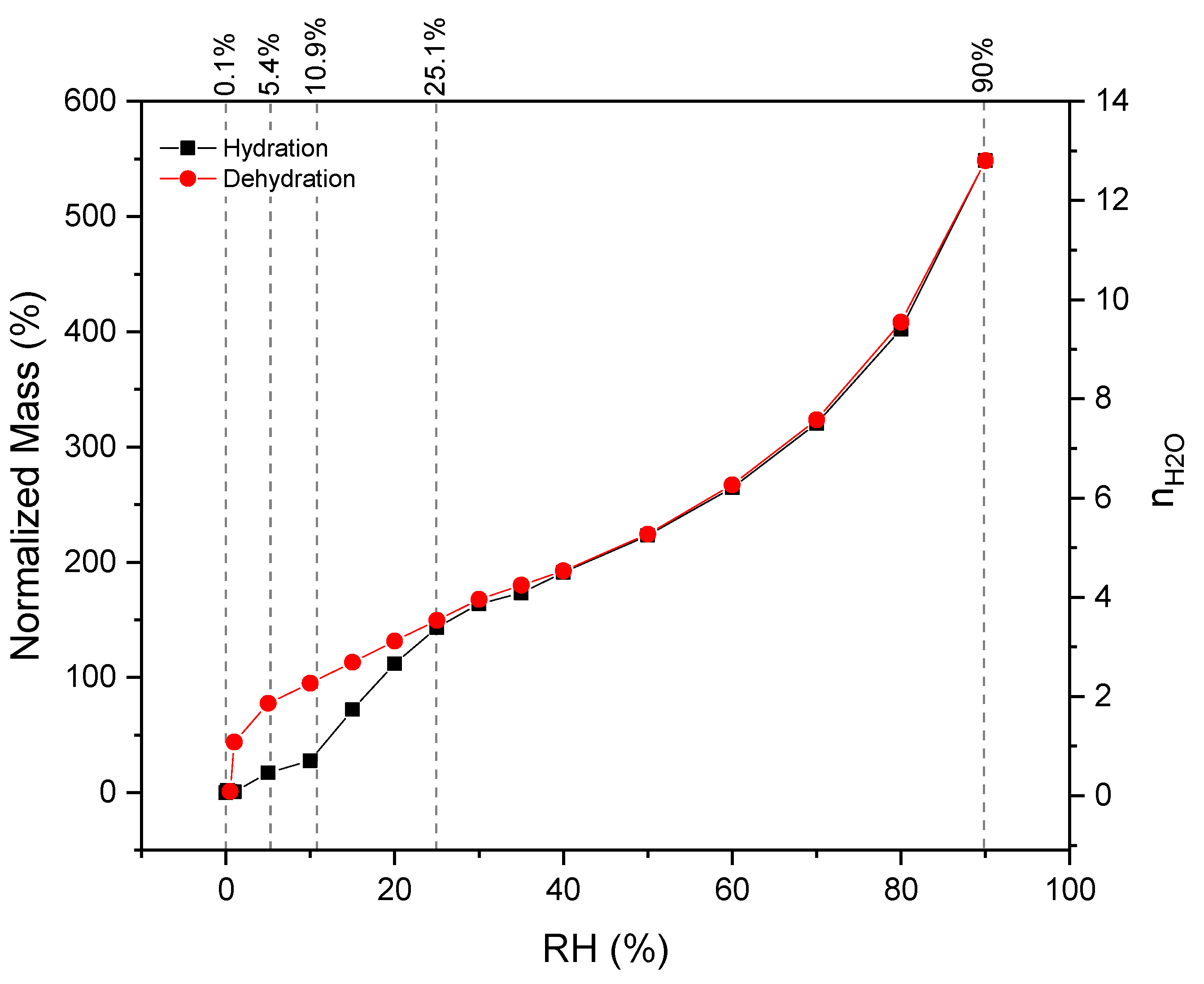
3.3. Hydration/Dehydration Thermochemical Behavior
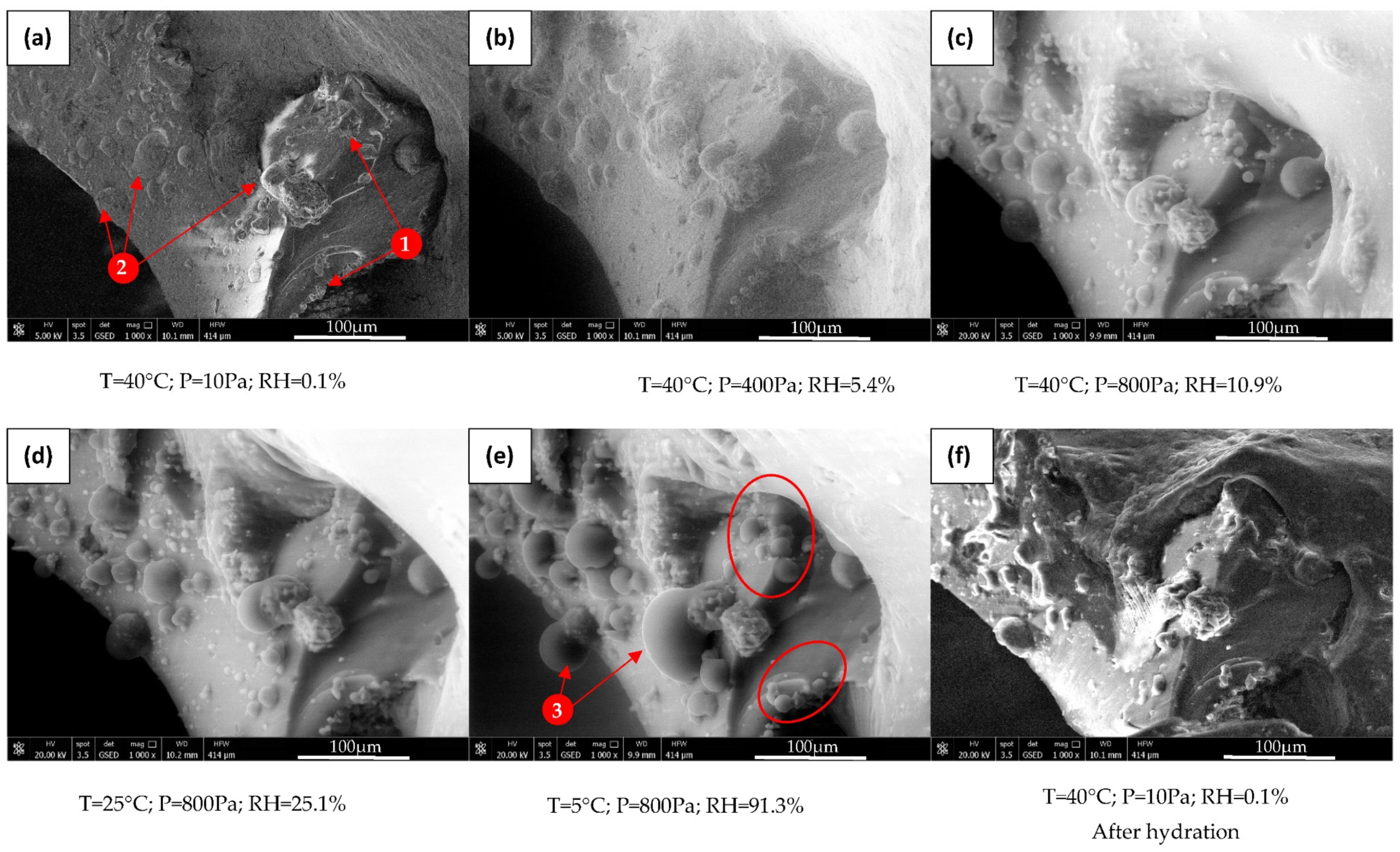
3.4. Morphological Characterization of LiCl-PDMS Foam during Hydration Reaction
3.5. Energy Storage/Release Density
4. Final Remarks
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- IRENA. Global Energy Transformation: A roadmap to 2050, International Renewable Energy Agency; IRENA: Abu Dhabi, United Arab Emirates, 2018; p. 12. [Google Scholar]
- Liu, H.; Wang, W.; Zhang, Y. Performance gap between thermochemical energy storage systems based on salt hydrates and materials. J. Clean. Prod. 2021, 313, 127908. [Google Scholar] [CrossRef]
- Donkers, P.; Sögütoglu, L.; Huinink, H.; Fischer, H.; Adan, O. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Trausel, F.; de Jong, A.-J.; Cuypers, R. A Review on the Properties of Salt Hydrates for Thermochemical Storage. Energy Procedia 2014, 48, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Courbon, E.; D’Ans, P.; Skrylnyk, O.; Frère, M. New prominent lithium bromide-based composites for thermal energy storage. J. Energy Storage 2020, 32, 101699. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Y.; Ding, B.; Yu, G.; Ye, F.; Xu, C. Structure and hydration state characterizations of MgSO4-zeolite 13x composite materials for long-term thermochemical heat storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110047. [Google Scholar] [CrossRef]
- Casey, S.P.; Elvins, J.; Riffat, S.; Robinson, A. Salt impregnated desiccant matrices for ‘open’ thermochemical energy storage—Selection, synthesis and characterisation of candidate materials. Energy Build. 2014, 84, 412–425. [Google Scholar] [CrossRef]
- Farcot, L.; Le Pierrès, N.; Fourmigué, J.-F. Experimental investigation of a moving-bed heat storage thermochemical reactor with SrBr2/H2O couple. J. Energy Storage 2019, 26, 101009. [Google Scholar] [CrossRef]
- Liu, H.; Nagano, K.; Togawa, J. A composite material made of mesoporous siliceous shale impregnated with lithium chloride for an open sorption thermal energy storage system. Sol. Energy 2015, 111, 186–200. [Google Scholar] [CrossRef]
- Marín, P.; Milian, Y.; Ushak, S.; Cabeza, L.; Grágeda, M.; Shire, G. Lithium compounds for thermochemical energy storage: A state-of-the-art review and future trends. Renew. Sustain. Energy Rev. 2021, 149, 111381. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C. Energy density and storage capacity cost comparison of conceptual solid and liquid sorption seasonal heat storage systems for low-temperature space heating. Renew. Sustain. Energy Rev. 2017, 76, 1314–1331. [Google Scholar] [CrossRef]
- Khvorostyanov, V.I.; Curry, J.A. Deliquescence and Efflorescence in Atmospheric Aerosols. In Thermodynamics, Kinetics, and Microphysics of Clouds; Cambridge University Press: Cambridge, UK, 2014; pp. 547–576. ISBN 9781139060004. [Google Scholar]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Kohler, T.; Biedermann, T.; Müller, K. Experimental Study of MgCl2 6 H2O as Thermochemical Energy Storage Material. Energy Technol. 2018, 6, 1935–1940. [Google Scholar] [CrossRef]
- Druske, M.-M.; Fopah-Lele, A.; Korhammer, K.; Rammelberg, H.U.; Wegscheider, N.; Ruck, W.; Schmidt, T. Developed Materials for Thermal Energy Storage: Synthesis and Characterization. Energy Procedia 2014, 61, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Zbair, M.; Bennici, S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies 2021, 14, 3105. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Li, T. Thermochemical characterizations of high-stable activated alumina/LiCl composites with multistage sorption process for thermal storage. Energy 2018, 156, 240–249. [Google Scholar] [CrossRef]
- Grekova, A.; Gordeeva, L.; Aristov, Y. Composite sorbents “Li/Ca halogenides inside Multi-wall Carbon Nano-tubes” for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2016, 155, 176–183. [Google Scholar] [CrossRef]
- Grekova, A.D.; Gordeeva, L.G.; Lu, Z.; Wang, R.; Aristov, Y.I. Composite “LiCl/MWCNT” as advanced water sorbent for thermal energy storage: Sorption dynamics. Sol. Energy Mater. Sol. Cells 2018, 176, 273–279. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.; Lu, Z.; Wang, L. Development and characterization of silica gel–LiCl composite sorbents for thermal energy storage. Chem. Eng. Sci. 2014, 111, 73–84. [Google Scholar] [CrossRef]
- Frazzica, A.; Brancato, V.; Capri’, A.; Cannilla, C.; Gordeeva, L.; Aristov, Y. Development of “salt in porous matrix” composites based on LiCl for sorption thermal energy storage. Energy 2020, 208, 118338. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Bruzzaniti, P.; Brancato, V.; Palomba, V.; Caprì, A.; Frazzica, A.; Cabeza, L.F.; Proverbio, E.; Milone, C. Morphological and Structural Evaluation of Hydration/Dehydration Stages of MgSO4 Filled Composite Silicone Foam for Thermal Energy Storage Applications. Appl. Sci. 2020, 10, 453. [Google Scholar] [CrossRef] [Green Version]
- Brancato, V.; Calabrese, L.; Palomba, V.; Frazzica, A.; Fullana-Puig, M.; Solé, A.; Cabeza, L.F. MgSO4·7H2O filled macro cellular foams: An innovative composite sorbent for thermo-chemical energy storage applications for solar buildings. Sol. Energy 2018, 173, 1278–1286. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Magnesium sulphate-silicone foam composites for thermochemical energy storage: Assessment of dehydration behaviour and mechanical stability. Sol. Energy Mater. Sol. Cells 2019, 200, 109992. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Frazzica, A.; Sapienza, A.; Freni, A. Novel experimental methodology for the characterization of thermodynamic performance of advanced working pairs for adsorptive heat transformers. Appl. Therm. Eng. 2014, 72, 229–236. [Google Scholar] [CrossRef]
- Brancato, V.; Gordeeva, L.G.; Sapienza, A.; Palomba, V.; Vasta, S.; Grekova, A.D.; Frazzica, A.; Aristov, Y.I. Experimental characterization of the LiCl/vermiculite composite for sorption heat storage applications. Int. J. Refrig. 2019, 105, 92–100. [Google Scholar] [CrossRef]
- Tieger, E.; Kiss, V.; Pokol, G.; Finta, Z.; Dušek, M.; Rohlíček, J.; Skořepová, E.; Brázda, P. Studies on the crystal structure and arrangement of water in sitagliptinl-tartrate hydrates. CrystEngComm 2016, 18, 3819–3831. [Google Scholar] [CrossRef] [Green Version]
- Brancato, V.; Gordeeva, L.; Caprì, A.; Grekova, A.; Frazzica, A. Experimental Comparison of Innovative Composite Sorbents for Space Heating and Domestic Hot Water Storage. Crystals 2021, 11, 476. [Google Scholar] [CrossRef]

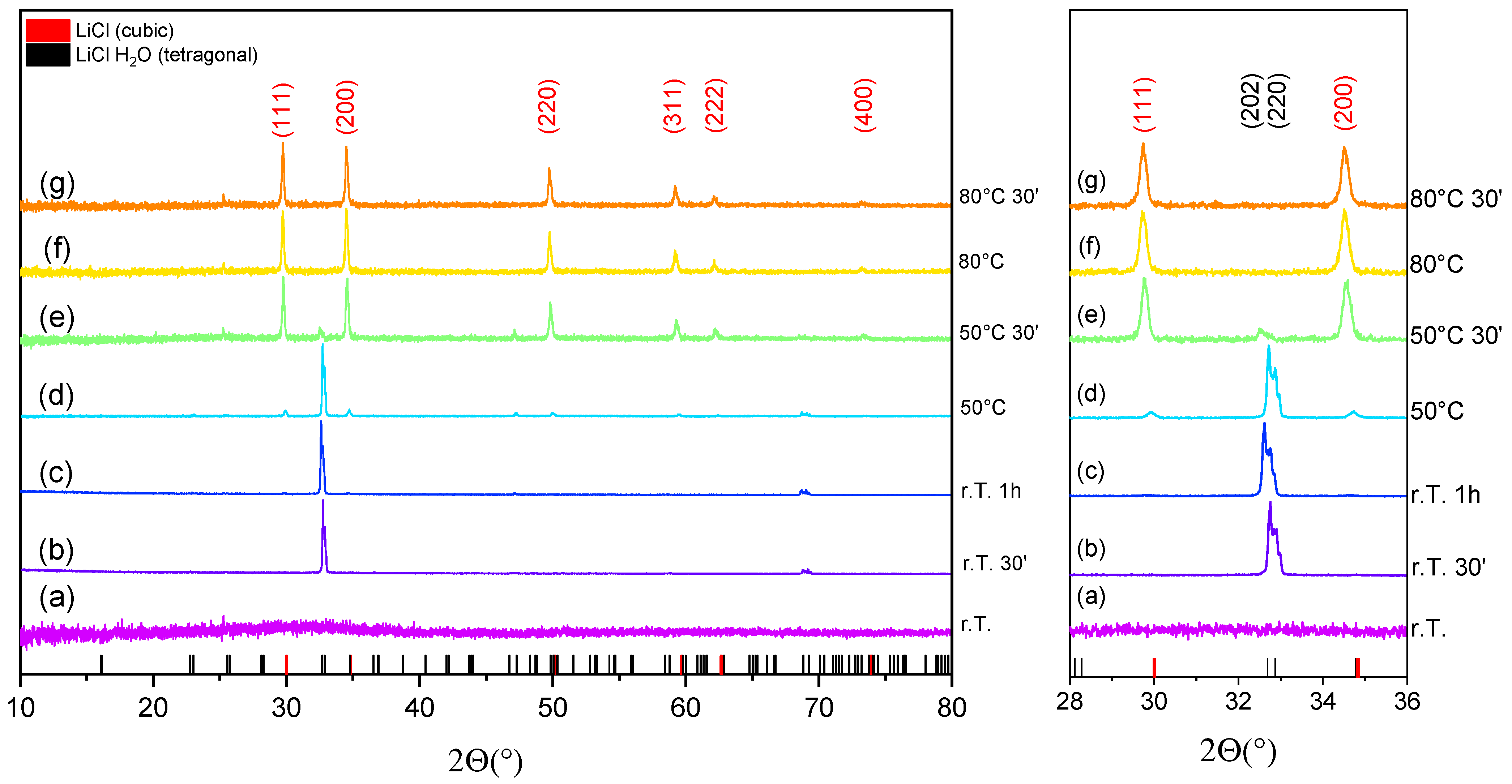


| Pattern Code | Experimental Conditions | LiCl·H2O Phase (Tetragonal P42/nmc) | LiCl Phase (Cubic Fm-3m) | |||||
|---|---|---|---|---|---|---|---|---|
| Lattice Parameters | Volume (Å3) | Fraction (%) | Lattice Parameters | Volume (Å3) | Fraction (%) | |||
| a/√2 (Å) | c/√2 (Å) | a (=b = c) (Å) | ||||||
| a | r.T., exposed air | - | - | - | - | - | - | - |
| b | r.T., N2 flow | 5.422 (3) | 5.474 (4) | 455.33 (6) | 100 | - | - | 0 |
| c | r.T. 30′, N2 flow | 5.417 (5) | 5.465 (9) | 453.68 (8) | 96.3 | 5.106 (3) | 133.15 (1) | 3.7 |
| d | 50 °C, N2 flow | 5.401 (6) | 5.458 (2) | 450.44 (8) | 80.8 | 5.136 (5) | 135.48 (4) | 19.2 |
| e | 50 °C 30′, N2 flow | 5.374 (1) | 5.442 (6) | 444.57 (7) | 21.7 | 5.140 (6) | 135.79 (7) | 78.3 |
| f | 80 °C, N2 flow | - | - | - | - | 5.144 (8) | 136.11 (4) | 100 |
| g | 80 °C 30′, N2 flow | - | - | - | - | 5.161 (1) | 137.46 (8) | 100 |
| h | r.T., N2 flow, after cooling | - | - | - | - | 5.157 (3) | 137.14 (8) | 100 |
| i | r.T. 30′, N2 flow, after cooling | - | - | - | - | 5.155 (2) | 136.99 (9) | 100 |
| j | r.T. 1 h exposed air, after cooling | - | - | - | - | - | - | - |
| Li Composite | LiCl Content (wt.%) | Stored/Released Heat (kJ/kgcomposite) | Energy Storage Density (kWh/m3) | Charging/Discharging Conditions | Ref. | ||
|---|---|---|---|---|---|---|---|
| Tchar (°C) | Tdis (°C) | RHdis (%) | |||||
| Li-PDMS foam | 40 | 1854 | 323 | 80 | 35 | 22 | this work |
| LiCl/activated alumina | 14.68 | 874 | 318 | 120 | 20 | 80 | [18] |
| LiCl/Siogel | 31.10 | 1000 | 166 | 66 | 35 | 15 | [30] |
| LiCl/Vermiculite | 45.20 | 1250 | 111 | 66 | 35 | 15 | [30] |
| LiCl/MWCNTs | 41–42 | 1700 | n.a. | 75–46 | 65–35 | 15 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastronardo, E.; Piperopoulos, E.; Palamara, D.; Frazzica, A.; Calabrese, L. Morphological Observation of LiCl Deliquescence in PDMS-Based Composite Foams. Appl. Sci. 2022, 12, 1510. https://doi.org/10.3390/app12031510
Mastronardo E, Piperopoulos E, Palamara D, Frazzica A, Calabrese L. Morphological Observation of LiCl Deliquescence in PDMS-Based Composite Foams. Applied Sciences. 2022; 12(3):1510. https://doi.org/10.3390/app12031510
Chicago/Turabian StyleMastronardo, Emanuela, Elpida Piperopoulos, Davide Palamara, Andrea Frazzica, and Luigi Calabrese. 2022. "Morphological Observation of LiCl Deliquescence in PDMS-Based Composite Foams" Applied Sciences 12, no. 3: 1510. https://doi.org/10.3390/app12031510
APA StyleMastronardo, E., Piperopoulos, E., Palamara, D., Frazzica, A., & Calabrese, L. (2022). Morphological Observation of LiCl Deliquescence in PDMS-Based Composite Foams. Applied Sciences, 12(3), 1510. https://doi.org/10.3390/app12031510










