Competitive Detection of Volatile Compounds from Food Degradation by a Zinc Oxide Sensor
Abstract
:1. Introduction
2. Experimental
2.1. Sensor Preparation and Characterization
2.2. Sensing Experiments
3. Results
3.1. ZnO Characterization
3.2. Sensing Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gnisci, A.; Fotia, A.; Bonaccorsi, L.; Donato, A. Effect of Working Atmospheres on the Detection of Diacetyl by Resistive SnO2 Sensor. Appl. Sci. 2022, 12, 367. [Google Scholar] [CrossRef]
- Shahidi, F.; Pegg, R.B. Hexanal As an Indicator of Meat Flavor Deterioration. J. Food Lipids 1994, 1, 177–186. [Google Scholar] [CrossRef]
- Azarbad, M.H.; Jeleń, H. Determination of Hexanal—an Indicator of Lipid Oxidation by Static Headspace Gas Chromatography (SHS-GC) in Fat-Rich Food Matrices. Food Anal. Methods 2015, 8, 1727–1733. [Google Scholar] [CrossRef]
- Schindler, S.; Krings, U.; Berger, R.G.; Orlien, V. Aroma Development in High Pressure Treated Beef and Chicken Meat Compared to Raw and Heat Treated. Meat Sci. 2010, 86, 317–323. [Google Scholar] [CrossRef]
- Abd Wahab, N.Z.; Nainggolan, I.; Nasution, T.I.; Derman, M.N.; Shantini, D. Highly Response and Sensitivity Chitosan-Polyvinyl Alcohol Based Hexanal Sensors. MATEC Web. Conf. 2016, 78, 01072. [Google Scholar] [CrossRef] [Green Version]
- Jayasena, D.D.; Ahn, D.U.; Nam, K.C.; Jo, C. Flavour Chemistry of Chicken Meat: A Review. Asian-Australas. J. Anim. Sci. 2013, 26, 732–742. [Google Scholar] [CrossRef]
- Malara, A.; Bonaccorsi, L.; Donato, A.; Frontera, P.; Piscopo, A.; Poiana, M.; Leonardi, S.G.; Neri, G. Sensing Properties of Indium, Tin and Zinc Oxides for Hexanal Detection. In Sensors; Andò, B., Baldini, F., di Natale, C., Ferrari, V., Marletta, V., Marrazza, G., Militello, V., Miolo, G., Rossi, M., Scalise, L., et al., Eds.; Lecture Notes in Electrical Engineering; Springer International Publishing: Cham, Switzerland, 2019; Volume 539, pp. 39–44. ISBN 978-3-030-04323-0. [Google Scholar]
- Malara, A.; Bonaccorsi, L.; Donato, A.; Frontera, P.; Neri, G. Doped Zinc Oxide Sensors for Hexanal Detection; Springer International Publishing: Cham, Switzerland, 2020; Volume 629, ISBN 9783030375577. [Google Scholar]
- Ponzoni, A.; Comini, E.; Concina, I.; Ferroni, M.; Falasconi, M.; Gobbi, E.; Sberveglieri, V.; Sberveglieri, G. Nanostructured Metal Oxide Gas Sensors, a Survey of Applications Carried out at SENSOR Lab, Brescia (Italy) in the Security and Food Quality Fields. Sensors 2012, 12, 17023–17045. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.; Barsan, N. Design of Core–Shell Heterostructure Nanofibers with Different Work Function and Their Sensing Properties to Trimethylamine. ACS Appl. Mater. Interfaces 2016, 8, 19799–19806. [Google Scholar] [CrossRef]
- Faggio, G.; Gnisci, A.; Messina, G.D.S.; Lisi, N.; Capasso, A.; Lee, G.-H.; Armano, A.; Sciortino, A.; Messina, F.; Cannas, M.; et al. Carbon Dots Dispersed on Graphene/SiO2/Si: A Morphological Study. Phys. Status Solidi 2019, 216, 1800559. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Liu, X.; Zhang, J. Highly Sensitive and Selective Electronic Sensor Based on Co Catalyzed SnO2 Nanospheres for Acetone Detection. Sens. Actuators B Chem. 2020, 304, 127237. [Google Scholar] [CrossRef]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Bioelectronic Nose: Current Status and Perspectives. Biosens. Bioelectron. 2017, 87, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A Review of Gas Sensors Employed in Electronic Nose Applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Yang, L.; Wang, Z.; Liu, D.; Luo, L.; Zhu, X.; Xi, Y.; Yang, Z.; Han, N.; Wang, F.; et al. One-Step Electrospun SnO2/MOx Heterostructured Nanomaterials for Highly Selective Gas Sensor Array Integration. Sens. Actuators B Chem. 2019, 283, 793–801. [Google Scholar] [CrossRef]
- Soncin, S.; Chiesa, L.M.; Cantoni, C.; Biondi, P.A. Preliminary Study of the Volatile Fraction in the Raw Meat of Pork, Duck and Goose. J. Food Compos. Anal. 2007, 20, 436–439. [Google Scholar] [CrossRef]
- Covarrubias-Cervantes, M.; Mokbel, I.; Champion, D.; Jose, J.; Voilley, A. Saturated Vapour Pressure of Aroma Compounds at Various Temperatures. Food Chem. 2004, 85, 221–229. [Google Scholar] [CrossRef]
- Palczewska-Tulinska, M.; Oracz, P. Vapor Pressures of Hexanal, 2-Methylcyclohexanone, and 2-Cyclohexen-1-One. J. Chem. Eng. Data 2006, 51, 639–641. [Google Scholar] [CrossRef]
- Available online: Https://www.Cheric.Org/ (accessed on 18 November 2021).
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Oprea, A.; Degler, D.; Barsan, N.; Hemeryck, A.; Rebholz, J. Basics of Semiconducting Metal Oxide–Based Gas Sensors; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128112243. [Google Scholar]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Brewer, M.S.; Vega, J.D. Detectable Odor Thresholds of Selected Lipid Oxidation Compounds in a Meat Model System. J. Food Sci. 1995, 60, 592–595. [Google Scholar] [CrossRef]
- Ajuyah, A.O.; Fenton, T.W.; Hardin, R.T.; Sim, J.S. Measuring Lipid Oxidation Volatiles in Meats. J. Food Sci. 1993, 58, 270–273. [Google Scholar] [CrossRef]
- Schmidt, O.; Kiesel, P.; van de Walle, C.G.; Johnson, N.M.; Nause, J.; Dohler, G.H. Effects of an Electrically Conducting Layer at the Zinc Oxide Surface. Jpn. J. Appl. Phys. 2005, 44, 7271–7274. [Google Scholar] [CrossRef]
- Schmidt, O.; Geis, A.; Kiesel, P.; van de Walle, C.G.; Johnson, N.M.; Bakin, A.; Waag, A.; Döhler, G.H. Analysis of a Conducting Channel at the Native Zinc Oxide Surface. Superlattices Microstruct. 2006, 39, 8–16. [Google Scholar] [CrossRef]
- Janotti, A.; van de Walle, C.G. Fundamentals of Zinc Oxide as a Semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines Our Choice? Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Understanding the Fundamental Principles of Metal Oxide Based Gas Sensors; the Example of CO Sensing with SnO2 Sensors in the Presence of Humidity. J. Phys. Condens. Matter 2003, 15, R813–R839. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Hjiri, M.; el Mir, L.; Leonardi, S.G.; Pistone, A.; Mavilia, L.; Neri, G. Al-Doped ZnO for Highly Sensitive CO Gas Sensors. Sens. Actuators B Chem. 2014, 196, 413–420. [Google Scholar] [CrossRef]
- Galstyan, V.; Bhandari, M.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Agirre, I.; Barrio, V.L.; Güemez, M.B.; Cambra, J.F.; Arias, P.L. Acetals as possible diesel additives. Econ. Eff. Biofuel Prod. 2011, 15, 299–316. [Google Scholar]

| Mixtures | Composition (w%) | |||
|---|---|---|---|---|
| Liquid | Vapor | Liquid | Vapor | |
| Hexanal/1-pentanol | 70/30 | 75/25 | 95/5 | 98/2 |
| Hexanal/1-octen-3-ol | 70/30 | 75/25 | 95/5 | 98/2 |
| Hexanal /1-pentanol/1-octen-3-ol | - | - | 90/5/5 | 96/2/2 |
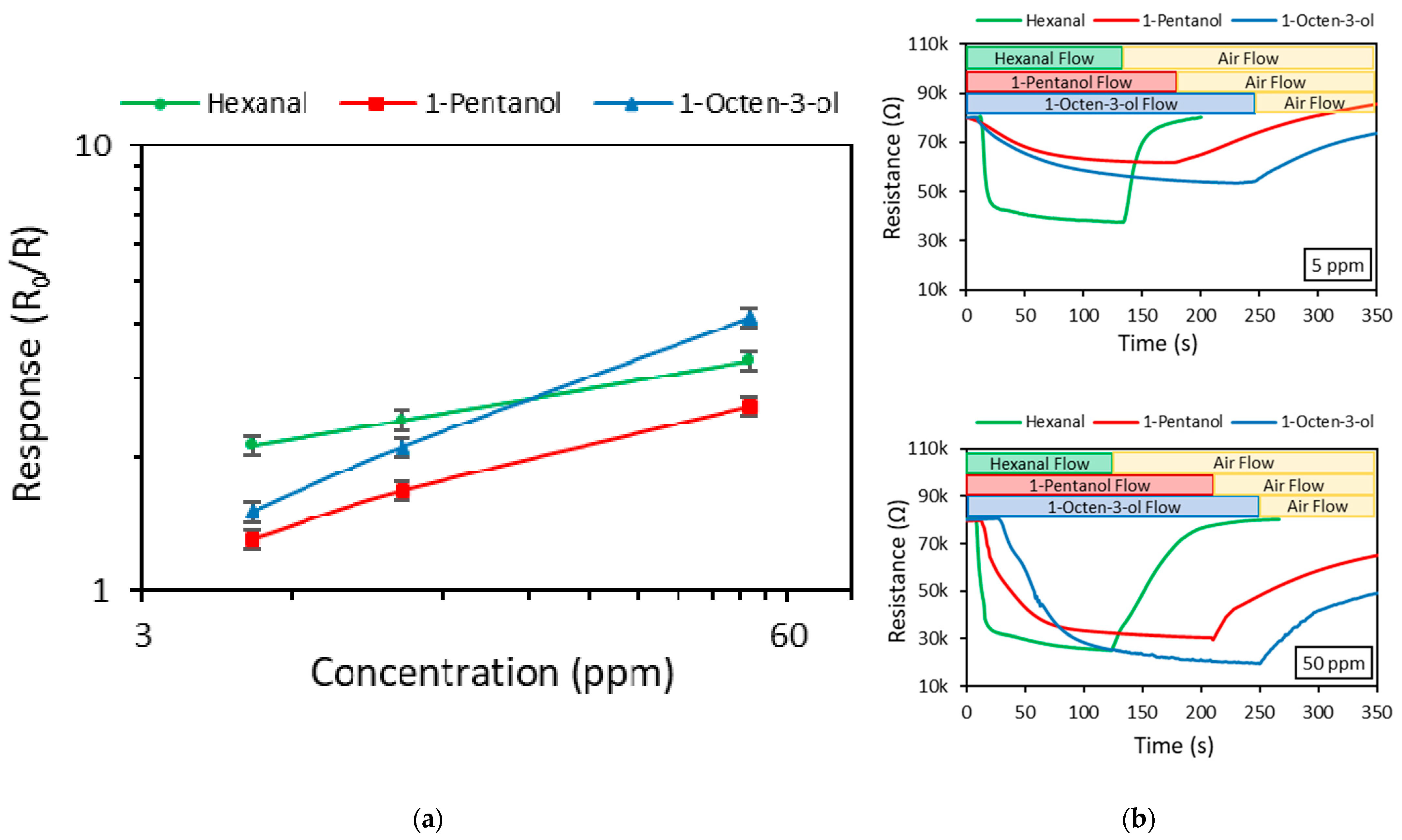
| Chemical Compound | Response (R0/R) | ||
|---|---|---|---|
| C = 5 ppm | C = 10 ppm | C = 50 ppm | |
| Hexanal | 2.12 | 2.42 | 3.28 |
| 1-pentanol | 1.30 | 1.68 | 2.60 |
| 1-octen-3-ol | 1.50 | 2.10 | 4.10 |
| Chemical Compound | Tb [K] | P° (293 K) [kPa] | P° (258 K) [kPa] |
|---|---|---|---|
| Hexanal | 402 | 2 | 1.1 × 10−1 |
| 1-pentanol | 411 | 5 × 10−1 | 3.7 × 10−2 |
| 1-octen-3-ol | 447 | 3 × 10−2 | 3.1 × 10−2 |
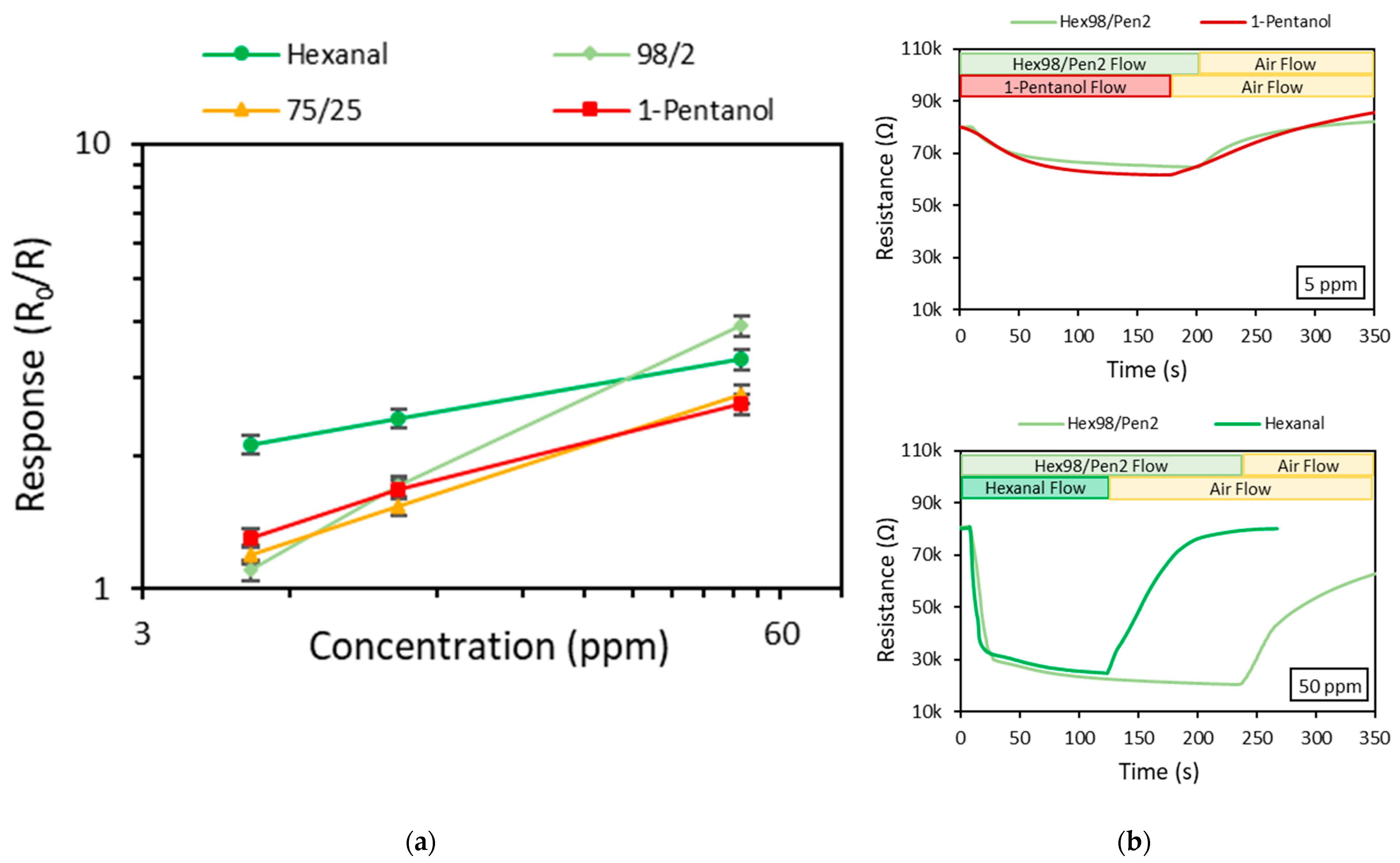
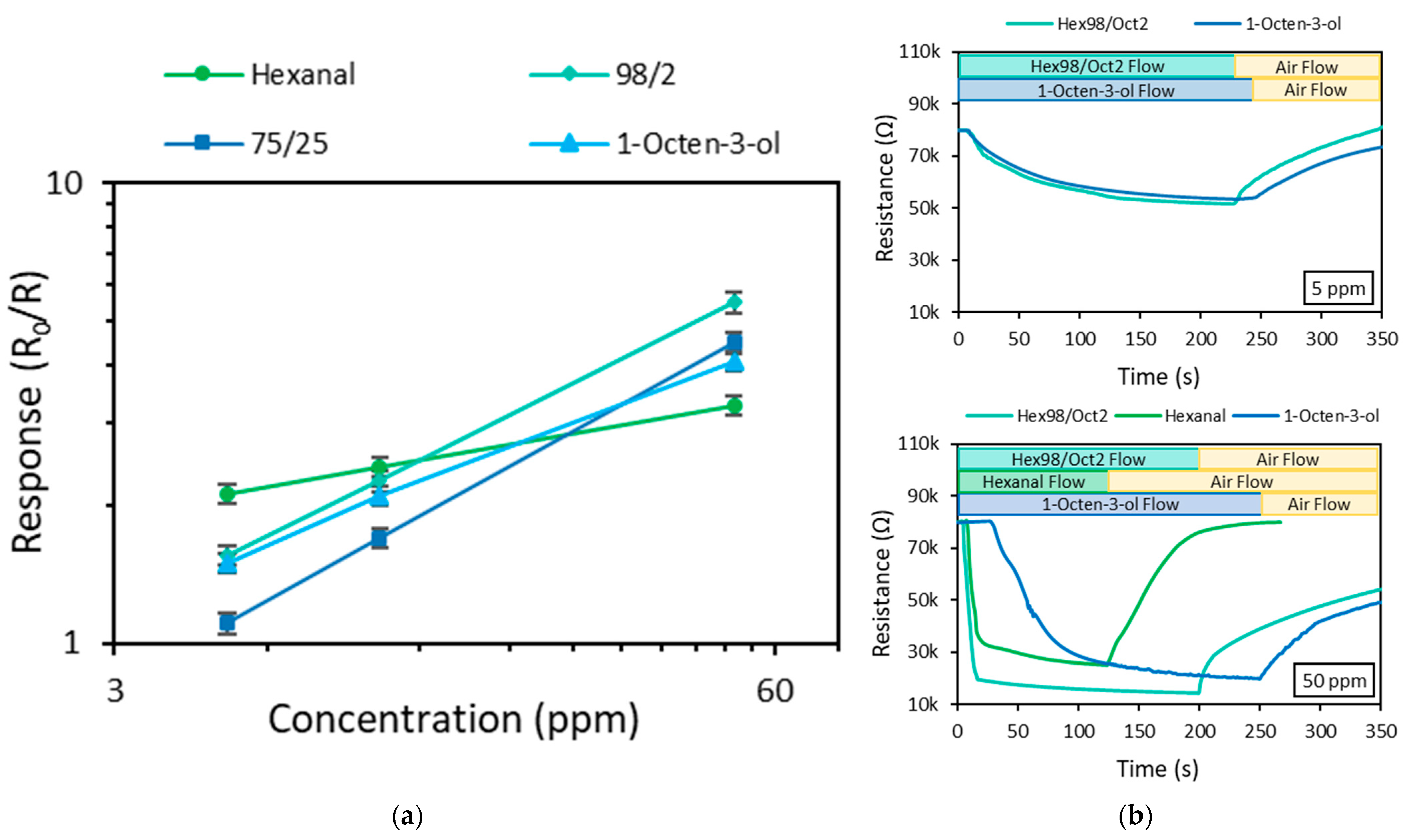
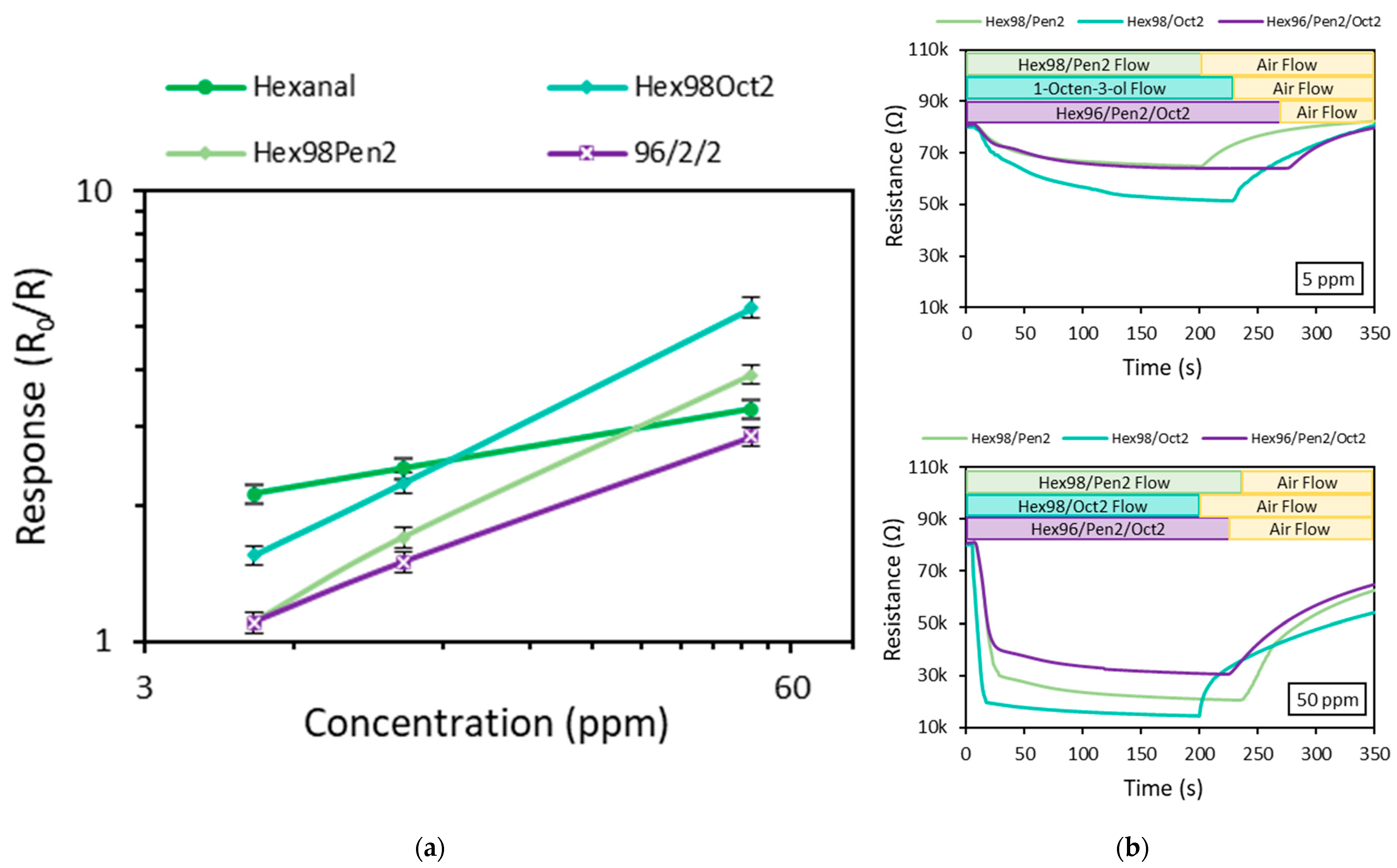
| Vapor Mixture | Response (R0/R) | |||
|---|---|---|---|---|
| Composition (w%) | C = 5 ppm | C = 10 ppm | C = 50 ppm | |
| Hexanal/1-pentanol | 98/2 | 1.10 | 1.69 | 3.90 |
| 75/25 | 1.20 | 1.53 | 2.74 | |
| Hexanal/1-octen-3-ol | 98/2 | 1.55 | 2.25 | 5.50 |
| 75/25 | 1.11 | 1.71 | 4.50 | |
| Hexanal /1-pentanol/1-octen-3-ol | 96/2/2 | 1.10 | 1.50 | 2.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaccorsi, L.; Donato, A.; Fotia, A.; Frontera, P.; Gnisci, A. Competitive Detection of Volatile Compounds from Food Degradation by a Zinc Oxide Sensor. Appl. Sci. 2022, 12, 2261. https://doi.org/10.3390/app12042261
Bonaccorsi L, Donato A, Fotia A, Frontera P, Gnisci A. Competitive Detection of Volatile Compounds from Food Degradation by a Zinc Oxide Sensor. Applied Sciences. 2022; 12(4):2261. https://doi.org/10.3390/app12042261
Chicago/Turabian StyleBonaccorsi, Lucio, Andrea Donato, Antonio Fotia, Patrizia Frontera, and Andrea Gnisci. 2022. "Competitive Detection of Volatile Compounds from Food Degradation by a Zinc Oxide Sensor" Applied Sciences 12, no. 4: 2261. https://doi.org/10.3390/app12042261
APA StyleBonaccorsi, L., Donato, A., Fotia, A., Frontera, P., & Gnisci, A. (2022). Competitive Detection of Volatile Compounds from Food Degradation by a Zinc Oxide Sensor. Applied Sciences, 12(4), 2261. https://doi.org/10.3390/app12042261







