Synthesis and Characterization of Anatase TiO2 Nanorods: Insights from Nanorods’ Formation and Self-Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Modified Synthesis of TiO2 NRs
2.3. Self-Assembly of TiO2 NRs
2.4. Characterization of TiO2 NCs
3. Results and Discussions
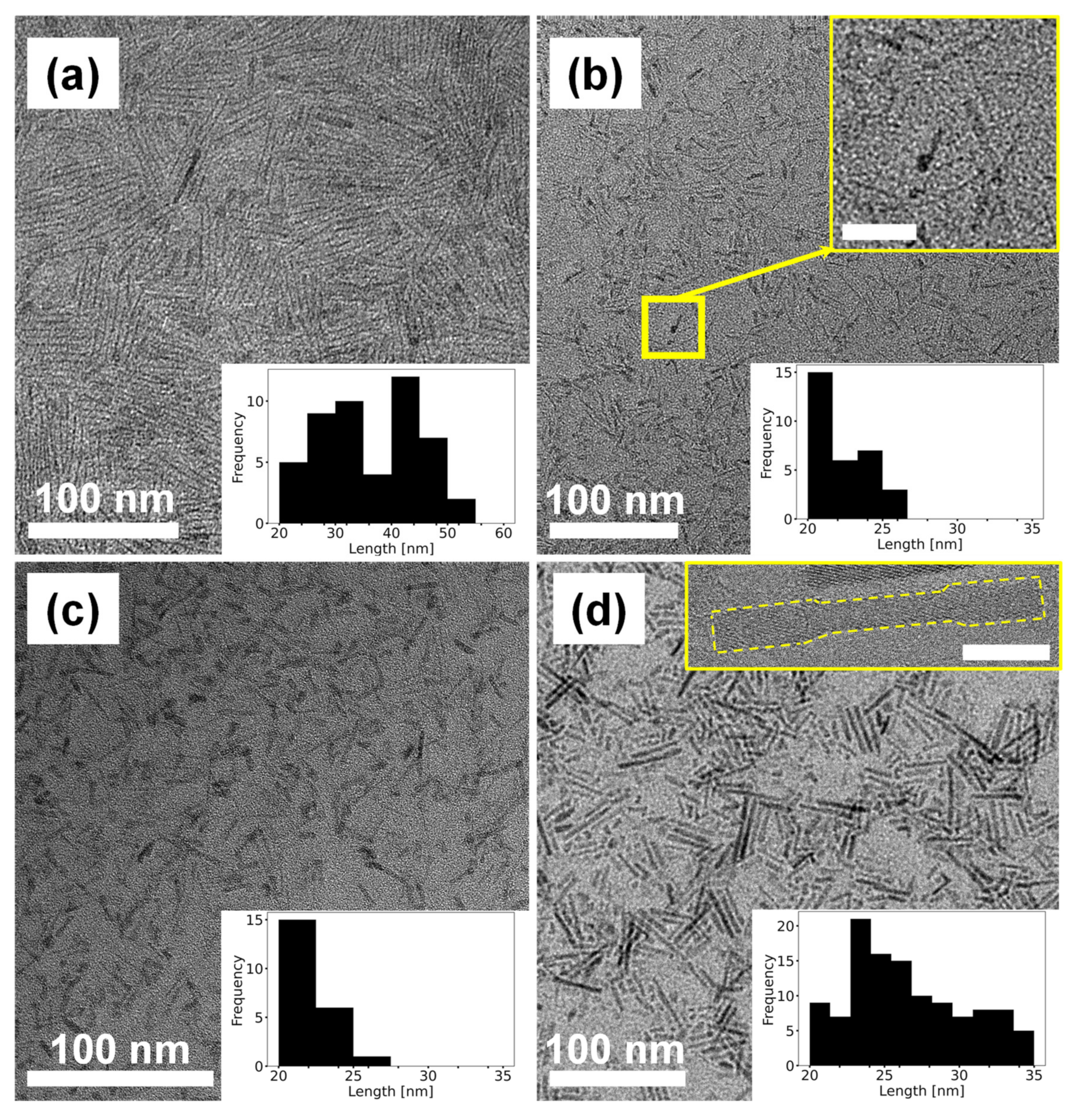
3.1. Growth Mechanism of TiO2 Nanocrystals
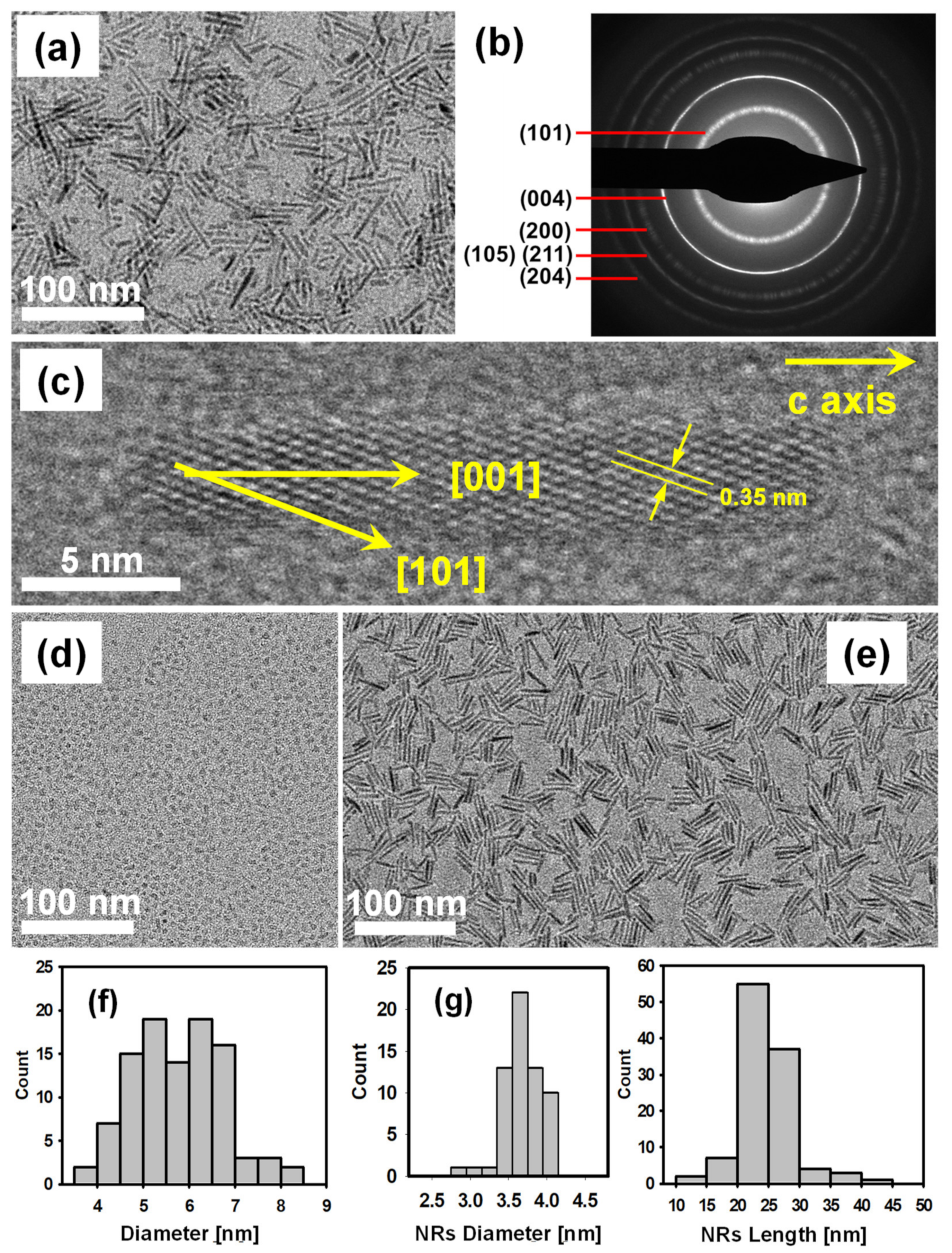
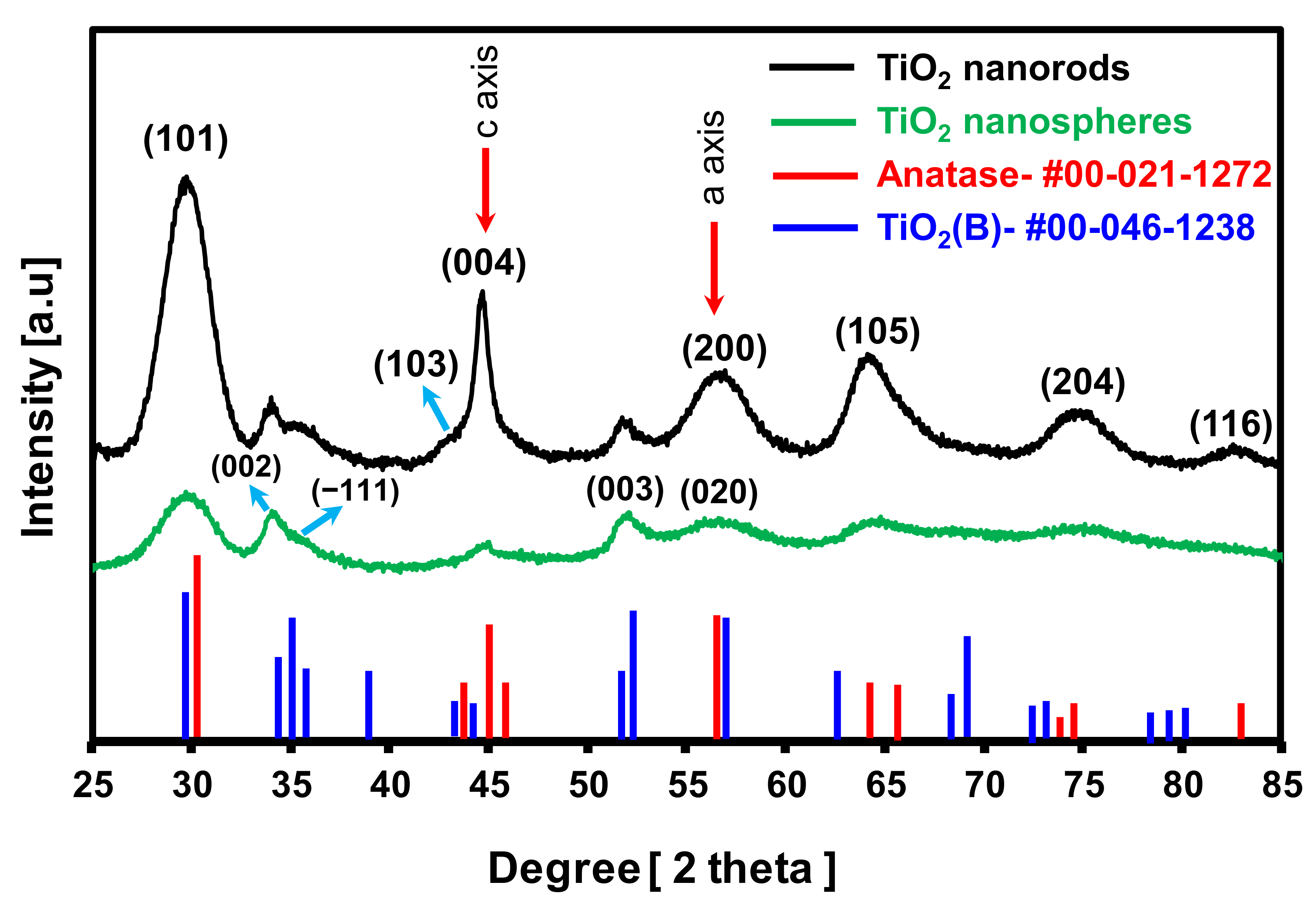
3.2. Characterization of Synthesized TiO2 Nanocrystals
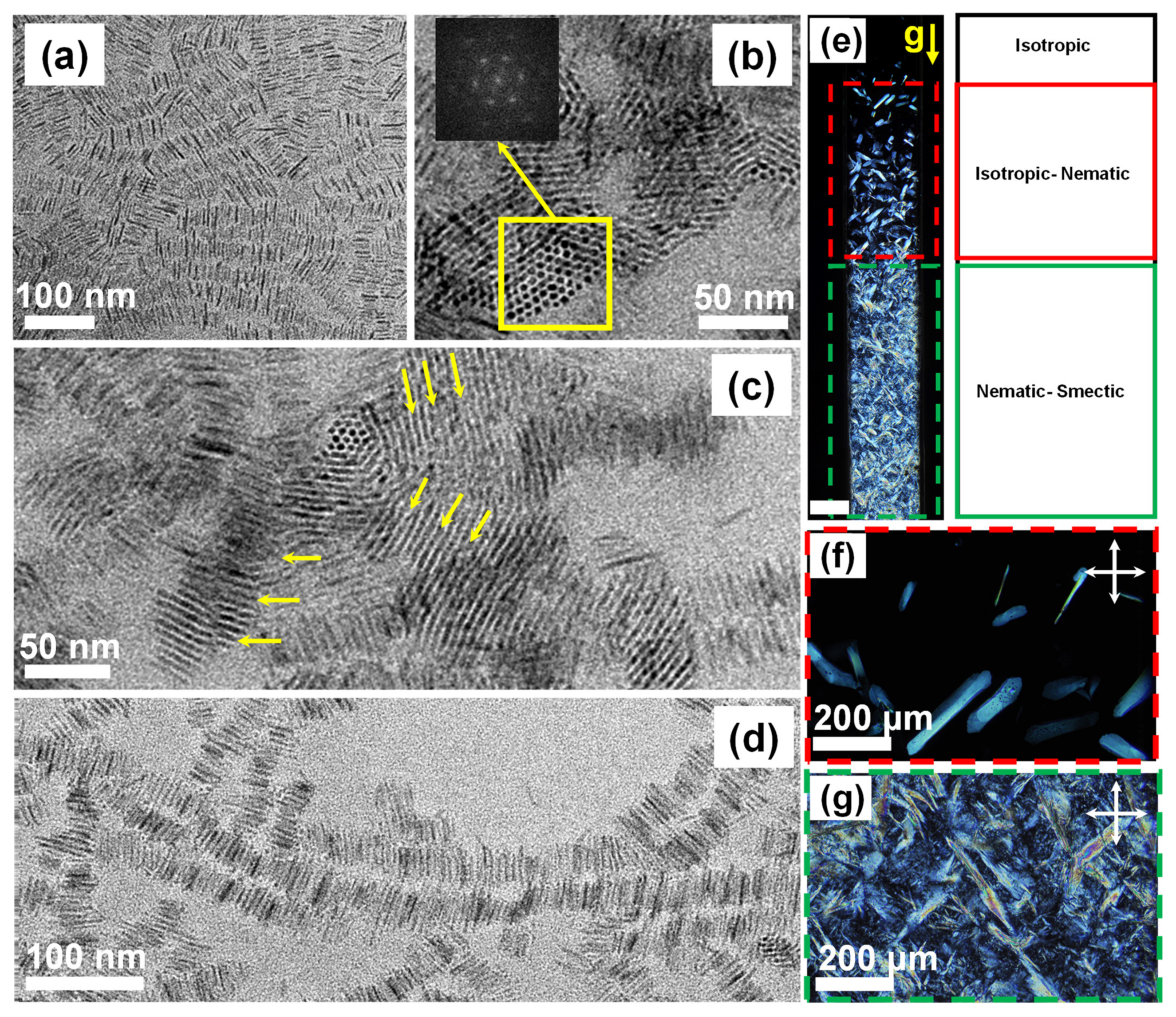
3.3. Liquid Crystalline Phases of TiO2 NRs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Y.; Mora-Sero, I.; De Angelis, F.; Bisquert, J.; Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef]
- Magalhães, P.; Andrade, L.; Nunes, O.C.; Mendes, A. Titanium dioxide photocatalysis: Fundamentals and application on photoinactivation. Rev. Adv. Mater. Sci. 2017, 51, 91–129. [Google Scholar]
- Cargnello, M.; Montini, T.; Smolin, S.Y.; Priebe, J.B.; Delgado Jaén, J.J.; Doan-Nguyen, V.V.T.; McKay, I.S.; Schwalbe, J.A.; Pohl, M.-M.; Gordon, T.R.; et al. Engineering titania nanostructure to tune and improve its photocatalytic activity. Proc. Natl. Acad. Sci. USA 2016, 113, 3966–3971. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium dioxide (Anatase and Rutile): Surface chemistry, liquid-solid interface chemistry, and scientific synthesis of supported catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Casavola, M.; Buonsanti, R.; Caputo, G.; Cozzoli, P.D. Colloidal strategies for preparing oxide-based hybrid nanocrystals. Eur. J. Inorg. Chem. 2008, 837–854. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Structural characteristics and mechanical and thermodynamic properties of nanocrystalline TiO2. Chem. Rev. 2014, 114, 9613–9644. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.L.; Jia, Y.S.; Chen, X.B.; Han, H.X.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Kern, P.; Schwaller, P.; Michler, J. Electrolytic deposition of titania films as interference coatings on biomedical implants: Microstructure, chemistry and nano-mechanical properties. Thin Solid Films 2006, 494, 279–286. [Google Scholar] [CrossRef]
- Sungur, Ş. Titanium Dioxide Nanoparticles. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Cham, Switzerland, 2021; pp. 713–730. ISBN 978-3-030-36268-3. [Google Scholar]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Ranade, M.R.; Navrotsky, A.; Zhang, H.Z.; Banfield, J.F.; Elder, S.H.; Zaban, A.; Borse, P.H.; Kulkarni, S.K.; Doran, G.S.; Whitfield, H.J. Energetics of nanocrystalline TiO2. Proc. Natl. Acad. Sci. USA 2002, 99, 6476–6481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Mason, C.W.; Yeo, I.; Saravanan, K.; Balaya, P. Interconnected nanofibrous titanium dioxide bronze: An emerging lithium ion anode material for high rate performance. RSC Adv. 2013, 3, 2935–2941. [Google Scholar] [CrossRef]
- Voepel, P.; Seitz, C.; Waack, J.M.; Zahn, S.; Leichtweiß, T.; Zaichenko, A.; Mollenhauer, D.; Amenitsch, H.; Voggenreiter, M.; Polarz, S.; et al. Peering into the mechanism of low-temperature synthesis of bronze-type TiO2 in ionic liquids. Cryst. Growth Des. 2017, 17, 5586–5601. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Grau-Carbonell, A.; Nikolaenkova, A.G.; Xie, X.; Chen, X.; Imhof, A.; van Blaaderen, A.; Baesjou, P.J. Smectic Liquid Crystalline Titanium Dioxide Nanorods: Reducing Attractions by Optimizing Ligand Density. Adv. Funct. Mater. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Park, S.; Mundoor, H.; Fleury, B.; Davidson, P.; van de Lagemaat, J.; Smalyukh, I.I. Liquid Crystalline Order and Electric Switching of Upconversion Luminescence in Colloidal Nanorod Suspensions. Adv. Opt. Mater. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Kornowski, A.; Weller, H. Low-Temperature Synthesis of Soluble and Low-Temperature Synthesis of Soluble and Processable. J. Am. Chem. Soc. 2003, 125, 14539–14548. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-Phase Synthesis of Titanium Dioxide Nanoparticles and Nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, J.C.P.; Davidson, P. New trends in colloidal liquid crystals based on mineral moieties. Adv. Mater. 2000, 12, 9–20. [Google Scholar] [CrossRef]
- Sonin, A.S. Inorganic lyotropic liquid crystals. J. Mater. Chem. 1998, 8, 2557–2574. [Google Scholar] [CrossRef]
- Trentler, T.J.; Denler, T.E.; Bertone, J.F.; Agrawal, A.; Colvin, V.L. Synthesis of TiO2 nanocrystals by nonhydrolytic solution-based reactions. J. Am. Chem. Soc. 1999, 121, 1613–1614. [Google Scholar] [CrossRef]
- Chemseddine, A.; Moritz, T. Nanostructuring titania: Control over nanocrystal structure, size, shape, and organization. Eur. J. Inorg. Chem. 1999, 235–245. [Google Scholar] [CrossRef]
- Jun, Y.W.; Casula, M.F.; Sim, J.H.; Kim, S.Y.; Cheon, J.; Alivisatos, A.P. Surfactant-Assisted Elimination of a High Energy Facet as a Means of Controlling the Shapes of TiO2 Nanocrystals. J. Am. Chem. Soc. 2003, 125, 15981–15985. [Google Scholar] [CrossRef]
- Niederberger, M.; Bartl, M.H.; Stucky, G.D. Benzyl alcohol and titanium tetrachloride - A versatile reaction system for the nonaqueous and low-temperature preparation of crystalline and luminescent titania nanoparticles. Chem. Mater. 2002, 14, 4364–4370. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. A Solution Chemistry Study of Nonhydrolytic Sol - Gel Routes to Titania. Chem. Mater. 1997, 9, 694–698. [Google Scholar] [CrossRef]
- Mutin, P.H.; Vioux, A. Nonhydrolytic processing of oxide-based materials: Simple routes to control homogeneity, morphology, and nanostructure. Chem. Mater. 2009, 21, 582–596. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, X.; Liu, S.; Li, D.; Han, M. Aminolysis route to monodisperse titania nanorods with tunable aspect ratio. Angew. Chem. Int. Ed. 2005, 44, 3466–3470. [Google Scholar] [CrossRef]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Kipp, T.; Cingolani, R.; Cozzoli, P.D. Nonhydrolytic synthesis of high-quality anisotropically shaped brookite TiO2 nanocrystals. J. Am. Chem. Soc. 2008, 130, 11223–11233. [Google Scholar] [CrossRef]
- Buonsanti, R.; Carlino, E.; Giannini, C.; Altamura, D.; De Marco, L.; Giannuzzi, R.; Manca, M.; Gigli, G.; Cozzoli, P.D. Hyperbranched Anatase TiO2 Nanocrystals: Nonaqueous Synthesis, Growth Mechanism, and Exploitation in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2011, 133, 19216–19239. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Kwon, S.G.; Yu, T.; Cho, M.; Lee, J.; Yoon, J. Large-Scale Synthesis of TiO2 Nanorods via Nonhydrolytic Sol - Gel Ester Elimination Reaction and Their Application to Photocatalytic Inactivation of E. coli. J. Phys. Chem. B 2005, 15297–15302. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Park, J.; Kim, Y.; Choi, S.H.; Sung, Y.E.; Hyeon, T. Simultaneous phase- and size-controlled synthesis of TiO2 nanorods via non-hydrolytic sol-gel reaction of syringe pump delivered precursors. J. Phys. Chem. B 2006, 110, 24318–24323. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Marcos, Z. The Sol-Gel Handbook; Levy, D., Marcos, Z., Eds.; Wiley-VCH: Weinheim, Germany, 2015; ISBN 9783527338443. [Google Scholar]
- Kobayashi, M.; Saito, H.; Boury, B.; Matsukawa, K.; Sugahara, Y. Epoxy-based hybrids using TiO2 nanoparticles prepared via a non-hydrolytic sol-gel route. Appl. Organomet. Chem. 2013, 27, 673–677. [Google Scholar] [CrossRef]
- Lee, B.; Littrell, K.; Sha, Y.; Shevchenko, E.V. Revealing the Effects of the Non-solvent on the Ligand Shell of Nanoparticles and Their Crystallization. J. Am. Chem. Soc. 2019, 141, 16651–16662. [Google Scholar] [CrossRef]
- Kuijk, A.; Byelov, D.V.; Petukhov, A.V.; van Blaaderen, A.; Imhof, A. Phase behavior of colloidal silica rods. Faraday Discuss. 2012, 159, 181–199. [Google Scholar] [CrossRef]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application; Springer: London, UK, 2009; ISBN 9781848826700. [Google Scholar]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis And Characterization Of Monodisperse Nanocrystals And Close-Packed Nanocrystal Assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123919274. [Google Scholar]
- Penn, R.L.; Banfield, J.F. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from titania. Geochim. Cosmochim. Acta 1999, 63, 1549–1557. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G.; Pinna, N.; Neri, G. Non-aqueous routes to crystalline metal oxide nanoparticles: Formation mechanisms and applications. Prog. Solid State Chem. 2005, 33, 59–70. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous Sol – Gel Routes to Metal Oxide Nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Niederberger, M. Nonhydrolytic Sol – Gel Methods. In The Sol–Gel Handbook: Synthesis, Characterization, and Applications; Wiley-VCH: Weinheim, Germany, 2015; pp. 29–69. [Google Scholar]
- Dalmaschio, C.J.; Leite, E.R. Detachment induced by rayleigh-instability in metal oxide nanorods: Insights from TiO2. Cryst. Growth Des. 2012, 12, 3668–3674. [Google Scholar] [CrossRef]
- Niederberger, M.; Bartl, M.H.; Stucky, G.D. Benzyl alcohol and transition metal chlorides as a versatile reaction system for the nonaqueous and low-temperature synthesis of crystalline nano-objects with controlled dimensionality. J. Am. Chem. Soc. 2002, 124, 13642–13643. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Peng, Q.; Yi, J.X.; Wang, X.; Li, Y. Near monodisperse TiO2 nanoparticles and nanorods. Chem. A Eur. J. 2006, 12, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xu, Y.; Hou, B.; Wu, D.; Sun, Y. A simple non-aqueous route to anatase TiO2. Eur. J. Inorg. Chem. 2008, 1236–1240. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Chen, F.; Anpo, M. Preparation of high photocatalytic activity TiO2 with a bicrystalline phase containing anatase and TiO2 (B). Mater. Lett. 2005, 59, 3378–3381. [Google Scholar] [CrossRef]
- Cargnello, M.; Cargnello, M.; Doan-nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of Metal Nanocrystal Size Role for Ceria Catalysts. Science 2014, 771, 771–774. [Google Scholar] [CrossRef]
- Casavola, M.; Grillo, V.; Carlino, E.; Giannini, C.; Gozzo, F.; Pinel, E.F.; Garcia, M.A.; Manna, L.; Cingolani, R.; Cozzoli, P.D. Topologically controlled growth of magnetic-metal-functionalized semiconductor oxide nanorods. Nano Lett. 2007, 7, 1386–1395. [Google Scholar] [CrossRef]
- Barnard, A.S.; Curtiss, L.A. Prediction of TiO2 nanoparticle phase and shape transitions controlled by surface chemistry. Nano Lett. 2005, 5, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.O.; Gonalves, R.H.; Stroppa, D.G.; Ramirez, A.J.; Leite, E.R. Synthesis of recrystallized anatase TiO2 mesocrystals with Wulff shape assisted by oriented attachment. Nanoscale 2011, 3, 1910–1916. [Google Scholar] [CrossRef]
- Dalmaschio, C.J.; Ribeiro, C.; Leite, E.R. Impact of the colloidal state on the oriented attachment growth mechanism. Nanoscale 2010, 2, 2336–2345. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, F.; Lin, Z. Progress of nanocrystalline growth kinetics based on oriented attachment. Nanoscale 2010, 2, 18–34. [Google Scholar] [CrossRef]
- Nichols, F.A.; Mullins, W.W. Contributions to Morphological Changes Driven by Capillarity. Trans. Met. Soc. AIME 1965, 233. [Google Scholar]
- Karim, S.; Toimil-Molares, M.E.; Ensinger, W.; Balogh, A.G.; Cornelius, T.W.; Khan, E.U.; Neumann, R. Influence of crystallinity on the Rayleigh instability of gold nanowires. J. Phys. D. Appl. Phys. 2007, 40, 3767–3770. [Google Scholar] [CrossRef]
- Qin, Y.; Lee, S.M.; Pan, A.; Gösele, U.; Knez, M. Rayleigh-instability-induced metal nanoparticle chains encapsulated in nanotubes produced by atomic layer deposition. Nano Lett. 2008, 8, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, L.; Yang, R.; Gösele, U.; Knez, M. General assembly method for linear metal nanoparticle chains embedded in nanotubes. Nano Lett. 2008, 8, 3221–3225. [Google Scholar] [CrossRef]
- Dinh, C.; Nguyen, T.; Kleitz, F.; Do, T. Shape-Controlled Synthesis of Highly Crystalline Titania Nanocrystals. ACS Nano 2009, 3, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Penn Lee, R.; Banfield, F.J. Formation of rutile nuclei at anatase (112) twin interfaces and the phase transformation mechanism in nanocrystalline titania. Am. Mineral. 1999, 84, 871–876. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the “Debye-Scherrer equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Gonzalo-Juan, I.; McBride, J.R.; Dickerson, J.H. Ligand-mediated shape control in the solvothermal synthesis of titanium dioxide nanospheres, nanorods and nanowires. Nanoscale 2011, 3, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Kandiel, T.A.; Feldhoff, A.; Robben, L.; Dillert, R.; Bahnemann, D.W. Tailored titanium dioxide nanomaterials: Anatase nanoparticles and brookite nanorods as highly active photocatalysts. Chem. Mater. 2010, 22, 2050–2060. [Google Scholar] [CrossRef]
- Li, J.G.; Ishigaki, T.; Sun, X. Anatase, brookite, and rutile nanocrystals via redox reactions under mild hydrothermal conditions: Phase-selective synthesis and physicochemical properties. J. Phys. Chem. C 2007, 111, 4969–4976. [Google Scholar] [CrossRef]
- Andreev, Y.G.; Panchmatia, P.M.; Liu, Z.; Parker, S.C.; Islam, M.S.; Bruce, P.G. The shape of TiO2-B nanoparticles. J. Am. Chem. Soc. 2014, 136, 6306–6312. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, P.; Frenkel, D. Tracing the phase boundaries of hard spherocylinders. J. Chem. Phys. 1997, 106, 666–687. [Google Scholar] [CrossRef]
- Onsager, L. The Effects of Shape on the Interaction of Colloidal Particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Buining, P.A.; Lekkerkerker, H.N.W. Isotropic-Nematic Phase Separation of Dispersion of Organophilic Boehmite Rods D/L. J. Phys. Chem. 1993, 97, 11510–11516. [Google Scholar] [CrossRef]
- Bohle, A.M.; Hołyst, R.; Vilgis, T. Polydispersity and Ordered Phases in Solutions of Rodlike Macromolecules. Phys. Rev. Lett. 1996, 76, 1396–1399. [Google Scholar] [CrossRef]
- Van Bruggen, M.P.B.; Dhont, J.K.G.; Lekkerkerker, H.N.W. Morphology and kinetics of the isotropic-nematic phase transition in dispersions of hard rods. Macromolecules 1999, 32, 2256–2264. [Google Scholar] [CrossRef]
- Vroege, G.J.; Petukhov, A.V.; Lemaire, B.J.; Davidson, P. Smectic liquid-crystalline order in suspensions of highly polydisperse goethite nanorods. Adv. Mater. 2006, 18, 2565–2568. [Google Scholar] [CrossRef]
- Wensink, H.H.; Vroege, G.J. Isotropic – nematic phase behavior of length- polydisperse hard rods. J. Chem. Phys. 2003, 119, 6868–6882. [Google Scholar] [CrossRef]
- Savenko, S.V.; Dijkstra, M. Sedimentation and multiphase equilibria in suspensions of colloidal hard rods. Phys. Rev. E 2004, 70, 51401. [Google Scholar] [CrossRef] [PubMed]
- Savenko, S.V.; Dijkstra, M. Phase behavior of a suspension of colloidal hard rods and nonadsorbing polymer. J. Chem. Phys. 2006, 124. [Google Scholar] [CrossRef] [PubMed]
- Pietra, F.; Rabouw, F.T.; Evers, W.H.; Byelov, D.V.; Petukhov, A.V.; De Mello Donegá, C.; Vanmaekelbergh, D. Semiconductor nanorod self-assembly at the liquid/air interface studied by in situ GISAXS and ex situ TEM. Nano Lett. 2012, 12, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Qian, G.; Fan, X.; Wang, Z. Self-Assembled Superlattices from Colloidal TiO2 Nanorods. Curr. Nanosci. 2010, 6, 262–268. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, C.; Hu, R.; Mai, K.; Qian, G.; Wang, Z. Two-step self-assembly and lyotropic liquid crystal behavior of TiO2 nanorods. J. Nanomater. 2012, 2012, 180989. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.M. Large-scale superlattices from colloidal TiO2nanorods: A facile self-assembly approach. Appl. Surf. Sci. 2016, 367, 559–562. [Google Scholar] [CrossRef]
- Ryan, K.M.; Mastroianni, A.; Stancil, K.A.; Liu, H.; Alivisatos, A.P. Electric-field-assisted assembly of perpendicularly oriented nanorod superlattices. Nano Lett. 2006, 6, 1479–1482. [Google Scholar] [CrossRef]
- Li, L.S.; Alivisatos, A.P. Semiconductor nanorod liquid crystals and their assembly on a substrate. Adv. Mater. 2003, 15, 408–411. [Google Scholar] [CrossRef]
- An, K.; Lee, N.; Park, J.; Kim, S.C.; Hwang, Y.; Park, J.G.; Kim, J.Y.; Park, J.H.; Han, M.J.; Yu, J.; et al. Synthesis, characterization, and self-assembly of pencil-shaped CoO nanorods. J. Am. Chem. Soc. 2006, 128, 9753–9760. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Kwan, S.; Akana, J.; Yang, P. Langmuir-Blodgett nanorod assembly. J. Am. Chem. Soc. 2001, 123, 4360–4361. [Google Scholar] [CrossRef] [PubMed]
- Diroll, B.T.; Greybush, N.J.; Kagan, C.R.; Murray, C.B. Smectic nanorod superlattices assembled on liquid subphases: Structure, orientation, defects, and optical polarization. Chem. Mater. 2015, 27, 2998–3008. [Google Scholar] [CrossRef]
- Xie, Y.; Liang, Y.; Chen, D.; Wu, X.; Dai, L.; Liu, Q. Vortical superlattices in a gold nanorods’ self-assembled monolayer. Nanoscale 2014, 6, 3064–3068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Querner, C.; Fischbein, M.D.; Heiney, P.A.; Drndić, M. Millimeter-scale assembly of CdSe nanorods into smectic superstructures by solvent drying kinetics. Adv. Mater. 2008, 20, 2308–2314. [Google Scholar] [CrossRef]
- Modlinska, A.; Alsayed, A.M.; Gibaud, T. Condensation and dissolution of nematic droplets in dispersions of colloidal rods with thermo-sensitive depletants. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Prinsen, P.; van der Schoot, P. Shape and director-field transformation of tactoids. Phys. Rev. E 2003, 68, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; MacLachlan, M.J. Liquid crystalline tactoids: Ordered structure, defective coalescence and evolution in confined geometries. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170042. [Google Scholar] [CrossRef] [PubMed]
- Jamali, V.; Behabtu, N.; Senyuk, B.; Lee, J.A.; Smalyukh, I.I.; van Der Schoot, P.; Pasquali, M. Experimental realization of crossover in shape and director field of nematic tactoids. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2015, 91, 1–7. [Google Scholar] [CrossRef]
- Green, M.J.; Parra-Vasquez, A.N.G.; Behabtu, N.; Pasquali, M. Modeling the phase behavior of polydisperse rigid rods with attractive interactions with applications to single-walled carbon nanotubes in superacids. J. Chem. Phys. 2009, 131. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, S.N.; Chen, X.; Baesjou, P.J.; Imhof, A.; van Blaaderen, A. Synthesis and Characterization of Anatase TiO2 Nanorods: Insights from Nanorods’ Formation and Self-Assembly. Appl. Sci. 2022, 12, 1614. https://doi.org/10.3390/app12031614
Hosseini SN, Chen X, Baesjou PJ, Imhof A, van Blaaderen A. Synthesis and Characterization of Anatase TiO2 Nanorods: Insights from Nanorods’ Formation and Self-Assembly. Applied Sciences. 2022; 12(3):1614. https://doi.org/10.3390/app12031614
Chicago/Turabian StyleHosseini, Seyed Naveed, Xiaodan Chen, Patrick J. Baesjou, Arnout Imhof, and Alfons van Blaaderen. 2022. "Synthesis and Characterization of Anatase TiO2 Nanorods: Insights from Nanorods’ Formation and Self-Assembly" Applied Sciences 12, no. 3: 1614. https://doi.org/10.3390/app12031614
APA StyleHosseini, S. N., Chen, X., Baesjou, P. J., Imhof, A., & van Blaaderen, A. (2022). Synthesis and Characterization of Anatase TiO2 Nanorods: Insights from Nanorods’ Formation and Self-Assembly. Applied Sciences, 12(3), 1614. https://doi.org/10.3390/app12031614





