Carcass Lesion Severity and Pre-Slaughter Conditions in Heavy Pigs: A Prospective Study at a Commercial Abattoir in Northern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Pre-Slaughter and Slaughter Conditions
2.2. Data Management and Statistical Analyses
2.2.1. Data Management and Descriptive Statistics
2.2.2. Single- and Multivariable Regression Analysis
2.2.3. Regression Tree Analysis
3. Results
3.1. Description of Pre-Slaughter Conditions and Observations, and Prevalence of Carcass Skin Lesions
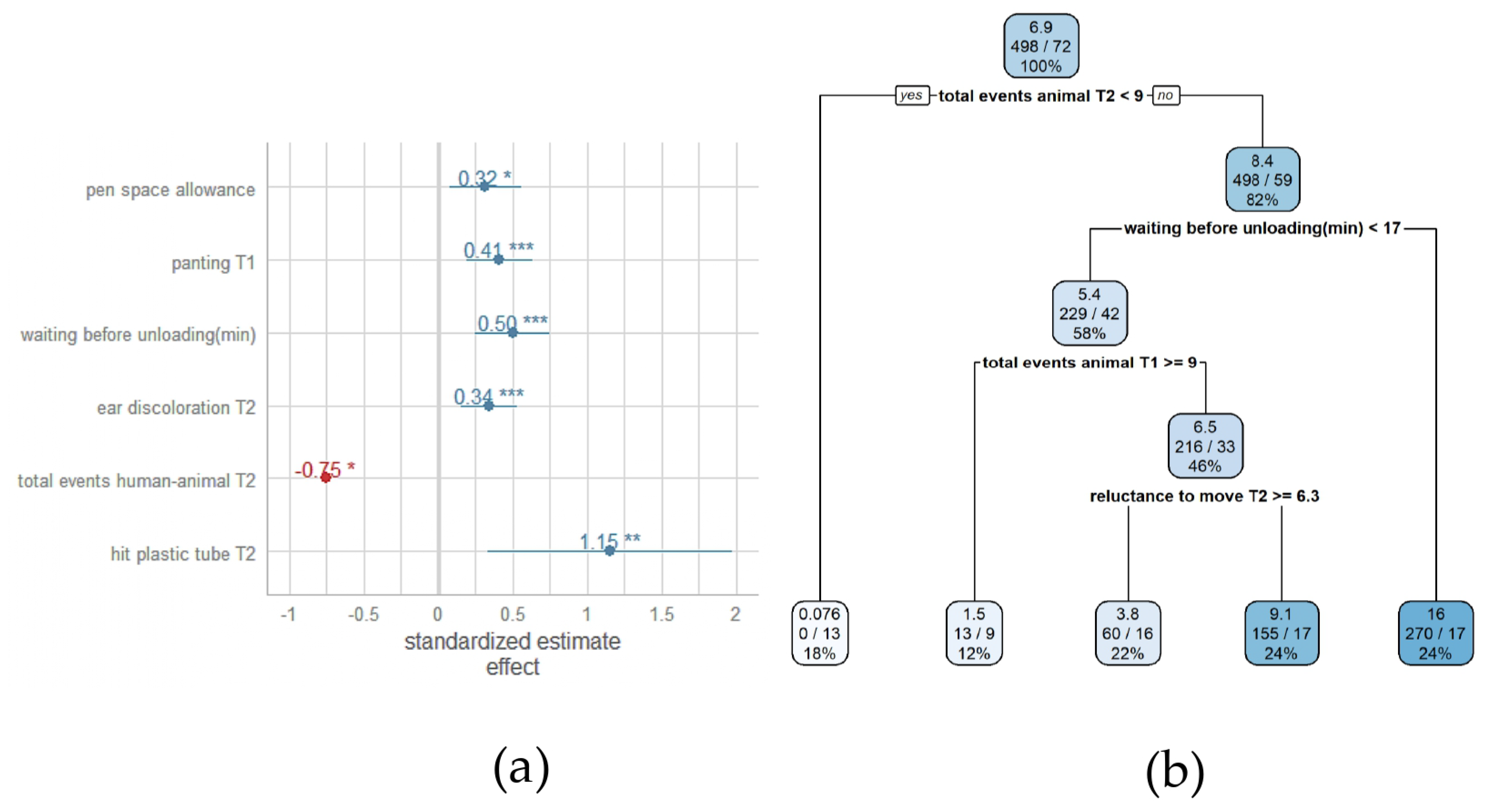
3.2. Association between Pre-Slaughter Conditions and Severe Lesions in the Rear Region of Heavy Pig Carcasses
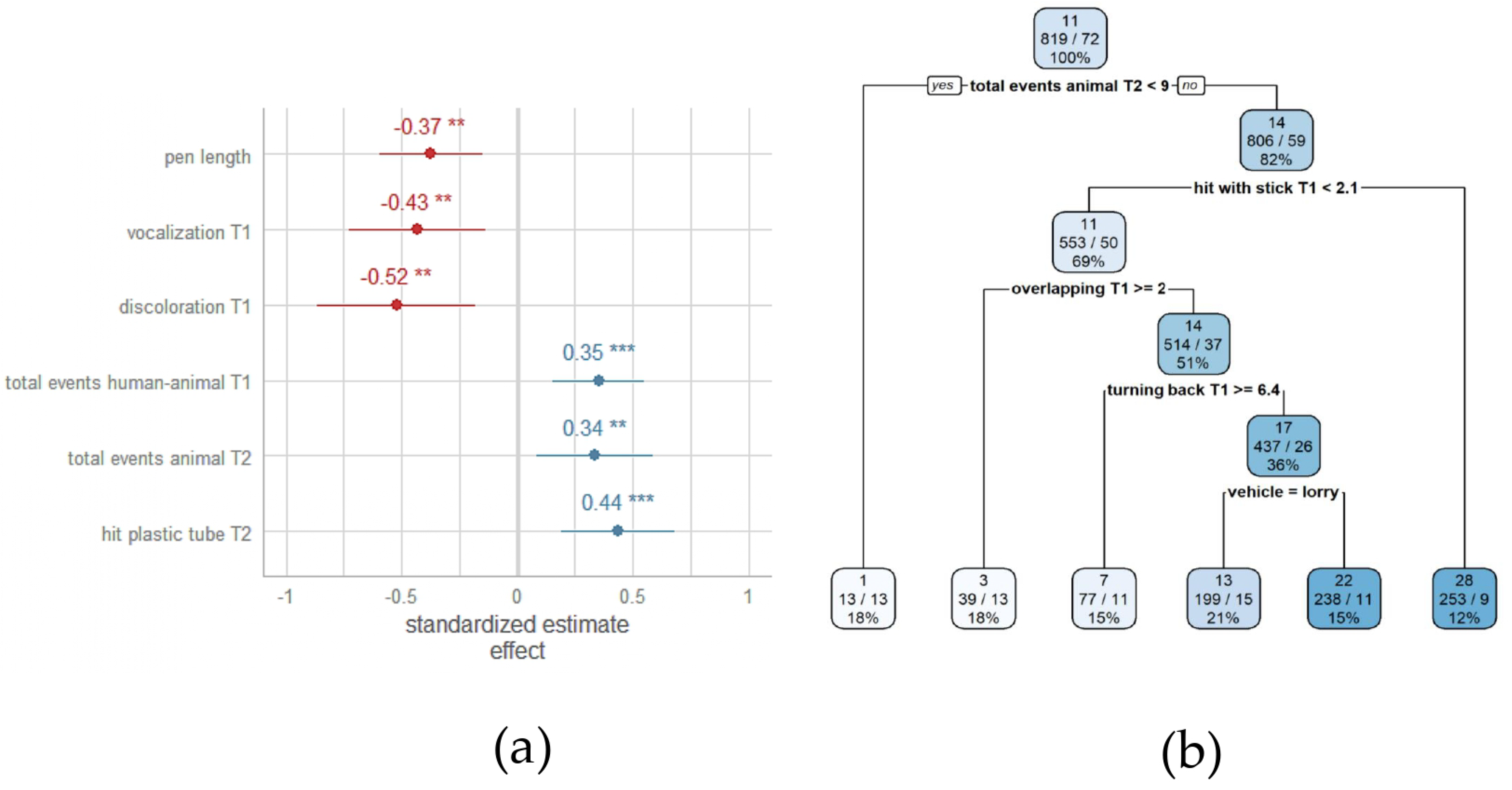
3.3. Association between Pre-Slaughter Conditions and Severe Lesions in the Middle Region of Heavy Pig Carcasses
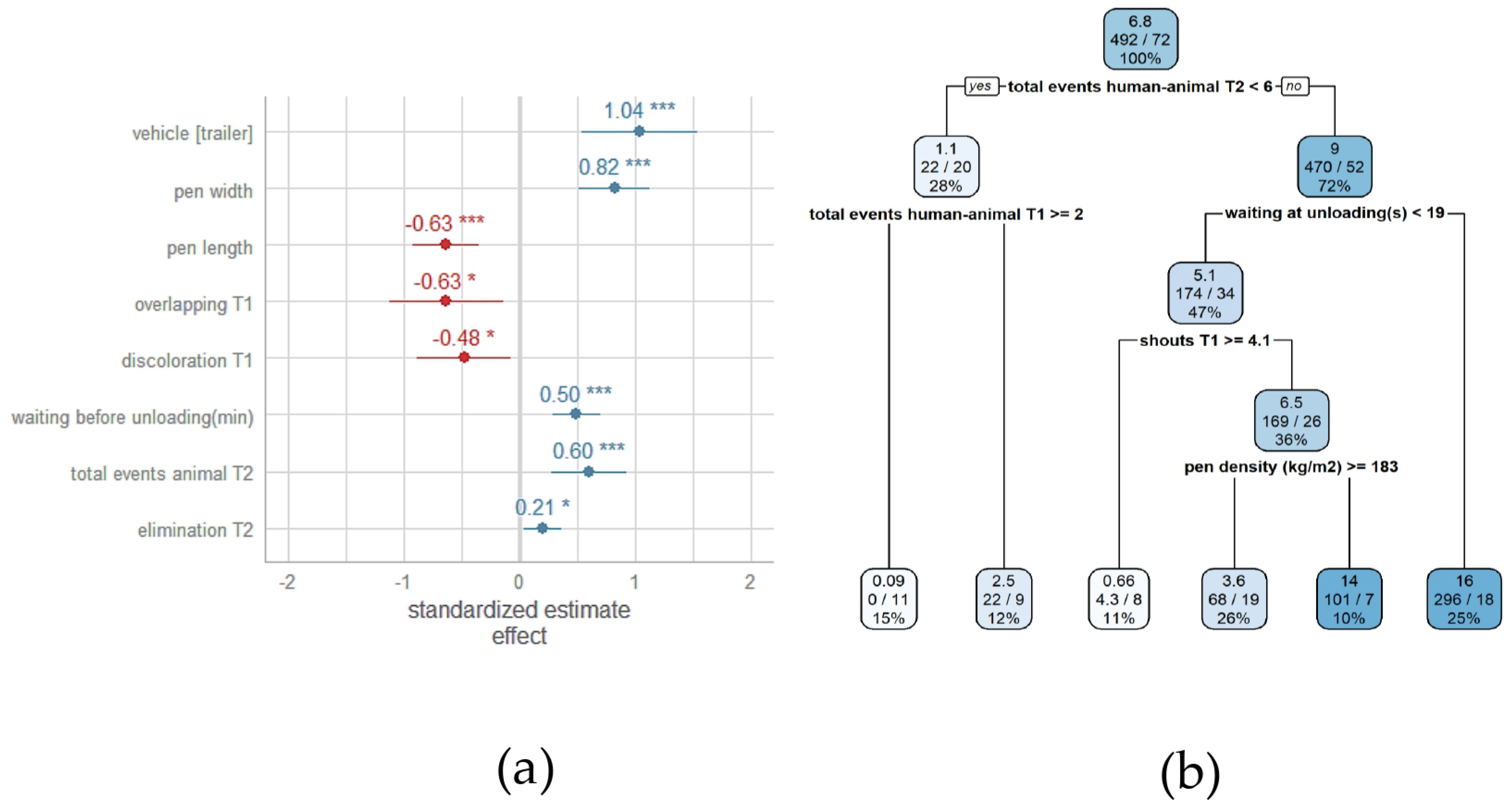
3.4. Association between Pre-Slaughter Conditions and Severe Lesions in the Front Region of Heavy Pig Carcasses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faucitano, L. Preslaughter Handling Practices and Their Effects on Animal Welfare and Pork Quality. J. Anim. Sci. 2018, 96, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Schwartzkopf-Genswein, K.S.; Faucitano, L.; Dadgar, S.; Shand, P.; González, L.A.; Crowe, T.G. Road Transport of Cattle, Swine and Poultry in North America and Its Impact on Animal Welfare, Carcass and Meat Quality: A Review. Meat Sci. 2012, 92, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Faucitano, L. Causes of Skin Damage to Pig Carcasses. Can. J. Anim. Sci. 2001, 81, 39–45. [Google Scholar] [CrossRef]
- Faucitano, L. Handling of Pigs Prior to Slaughter: Economical Impact of Good Practices. 2010. Available online: https://en.engormix.com/pig-industry/articles/handling-pigs-prior-slaughter-t34697.htm (accessed on 4 October 2021).
- Harley, S.; Boyle, L.; O’Connell, N.; More, S.; Teixeira, D.; Hanlon, A. Docking the Value of Pigmeat? Prevalence and Financial Implications of Welfare Lesions in Irish Slaughter Pigs. Anim. Welf. 2014, 23, 275–285. [Google Scholar] [CrossRef]
- Speer, N.C.; Slack, G.; Troyer, E. Economic Factors Associated with Livestock Transportation. J. Anim. Sci. 2001, 79, E166–E170. [Google Scholar] [CrossRef]
- Driessen, B.; Van Beirendonck, S.; Buyse, J. Effects of Transport and Lairage on the Skin Damage of Pig Carcasses. Animals 2020, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Barton-Gade, P.A.; Warriss, P.D.; Brown, S.N.; Lambooij, E. Methods of Improving Pig Welfare and Meat Quality by Reducing Stress and Discomfort before Slaughter—Methods of Measuring Meat Quality. In Proceedings of the Eu Seminar New Information on Welfare and Meat Quality of Pigs as Related to Handling Transport and Lairage Conditions; Schuette, A., Ed.; Braunschweig-Voelkenrode: Mariensee, Germany, 1996; pp. 23–34. [Google Scholar]
- Dalle Zotte, A.; Brugiapaglia, A.; Cullere, M. What Is Meat in Italy? Anim. Front. 2017, 7, 63–70. [Google Scholar] [CrossRef]
- Istituto di Servizi per il Mercato Agricolo Alimentare (ISMEA). Settore Suinicolo. Scheda di Settore. 2021. Available online: https://www.ismeamercati.it/analisi-e-studio-filiere-agroalimentari (accessed on 20 November 2021).
- Bosi, P.; Russo, V. The Production of the Heavy Pig for High Quality Processed Products. Ital. J. Anim. Sci. 2004, 3, 309–321. [Google Scholar] [CrossRef]
- Bottacini, M.; Scollo, A.; Edwards, S.A.; Contiero, B.; Veloci, M.; Pace, V.; Gottardo, F. Skin Lesion Monitoring at Slaughter on Heavy Pigs (170 Kg): Welfare Indicators and Ham Defects. PLoS ONE 2018, 13, e0207115. [Google Scholar] [CrossRef] [PubMed]
- Vitali, M.; Nannoni, E.; Sardi, L.; Martelli, G. Knowledge and Perspectives on the Welfare of Italian Heavy Pigs on Farms. Animals 2021, 11, 1690. [Google Scholar] [CrossRef]
- Grandin, T. Farm Animal Welfare during Handling, Transport, and Slaughter. J. Am. Vet. Med. Assoc. 1994, 204, 372–377. [Google Scholar]
- Van Staaveren, N.; Doyle, B.; Manzanilla, E.G.; Calderón Díaz, J.A.; Hanlon, A.; Boyle, L.A. Validation of Carcass Lesions as Indicators for On-Farm Health and Welfare of Pigs. J. Anim. Sci. 2017, 95, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Hanlon, A.; O’Connell, N.; Boyle, L. Boar Carcass Skin Lesions Reflect Their Behaviour on Farm. In Proceedings of the 65th European Federation for Animal Science (EAAP) Annual meeting, Copenhagen, Denmark, 25–28 August 2014; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; p. 220. [Google Scholar]
- Camp Montoro, J.; Boyle, L.A.; Solà-Oriol, D.; Muns, R.; Gasa, J.; Garcia Manzanilla, E. Effect of Space Allowance and Mixing on Growth Performance and Body Lesions of Grower-Finisher Pigs in Pens with a Single Wet-Dry Feeder. Porc. Health Manag. 2021, 7, 7. [Google Scholar] [CrossRef]
- Godyń, D.; Nowicki, J.; Herbut, P. Effects of Environmental Enrichment on Pig Welfare—A Review. Animals 2019, 9, 383. [Google Scholar] [CrossRef]
- Terlouw, C.; Berne, A.; Astruc, T. Effect of Rearing and Slaughter Conditions on Behaviour, Physiology and Meat Quality of Large White and Duroc-Sired Pigs. Livest. Sci. 2009, 122, 199–213. [Google Scholar] [CrossRef]
- Tönepöhl, B.; Appel, A.K.; Welp, S.; Voß, B.; König von Borstel, U.; Gauly, M. Effect of Marginal Environmental and Social Enrichment during Rearing on Pigs’ Reactions to Novelty, Conspecifics and Handling. Appl. Anim. Behav. Sci. 2012, 140, 137–145. [Google Scholar] [CrossRef]
- Peden, R.S.E.; Turner, S.P.; Boyle, L.A.; Camerlink, I. The Translation of Animal Welfare Research into Practice: The Case of Mixing Aggression between Pigs. Appl. Anim. Behav. Sci. 2018, 204, 1–9. [Google Scholar] [CrossRef]
- Canario, L.; Bijma, P.; David, I.; Camerlink, I.; Martin, A.; Rauw, W.M.; Flatres-Grall, L.; van der Zande, L.; Turner, S.P.; Larzul, C.; et al. Prospects for the Analysis and Reduction of Damaging Behaviour in Group-Housed Livestock, with Application to Pig Breeding. Front. Genet. 2020, 11, 1560. [Google Scholar] [CrossRef]
- Grandin, T. Welfare of Pigs During Transport (Updated November 2014). Available online: http://www.grandin.com/welfare.pigs.during.transport.html (accessed on 10 November 2021).
- Stewart, G.; Ritter, M.J.; Culbertson, M.; Mann, G.; Wofford, R. Effects of Previous Handling and Feed Withdrawal Prior to Loading on Transport Losses in Market Weight Pigs. In Proceedings of the 2008 American Association of Swine Veterinarians, San Diego, CA, USA, 8 March 2008; pp. 359–362. [Google Scholar]
- Dalla Costa, F.A.; Devillers, N.; Paranhos da Costa, M.J.R.; Faucitano, L. Effects of Applying Preslaughter Feed Withdrawal at the Abattoir on Behaviour, Blood Parameters and Meat Quality in Pigs. Meat Sci. 2016, 119, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Guàrdia, M.D.; Estany, J.; Balasch, S.; Oliver, M.; Gispert, M.; Diestre, A. Risk Assessment of Skin Damage Due to Pre-Slaughter Conditions and RYR1 Gene in Pigs. Meat Sci. 2009, 81, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Faucitano, L.; Goumon, S. Transport to Slaughter and Associated Handling. In Advances in Pig Welfare; Špinka, M., Ed.; Woodhead Publishing: London, UK, 2018; pp. 261–293. [Google Scholar]
- Edwards-Callaway, L.; Engle, T.; Grandin, T.; Ritter, M.; Sosnicki, A.A.; Carlson, B.A.; Anderson, D. The Effects of Distance Traveled during Loading, Lairage Time Prior to Slaughter, and Distance Traveled to the Stunning Area on Blood Lactate Concentration of Pigs in a Commercial Packing Plant. Prof. Anim. Sci. 2011, 27, 485–491. [Google Scholar] [CrossRef]
- Edwards, L.N.; Grandin, T.; Engle, T.E.; Porter, S.P.; Ritter, M.J.; Sosnicki, A.A.; Anderson, D.B. Use of Exsanguination Blood Lactate to Assess the Quality of Pre-Slaughter Pig Handling. Meat Sci. 2010, 86, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Guàrdia, M.D.; Estany, J.; Balasch, S.; Oliver, M.A.; Gispert, M.; Diestre, A. Risk Assessment of PSE Condition Due to Pre-Slaughter Conditions and RYR1 Gene in Pigs. Meat Sci. 2004, 67, 471–478. [Google Scholar] [CrossRef]
- Čobanović, N.; Bošković, M.; Vasilev, D.; Dimitrijević, M.; Parunović, N.; Djordjević, J.; Karabasil, N. Effects of Various Pre-Slaughter Conditions on Pig Carcasses and Meat Quality in a Low-Input Slaughter Facility. S. Afr. J. Anim. Sci. 2016, 46, 380–390. [Google Scholar] [CrossRef]
- Van Staaveren, N.; Teixeira, D.L.; Hanlon, A.; Boyle, L.A. The Effect of Mixing Entire Male Pigs Prior to Transport to Slaughter on Behaviour, Welfare and Carcass Lesions. PLoS ONE 2015, 10, e0122841. [Google Scholar] [CrossRef]
- Nanni Costa, L.; Lo Fiego, D.P.; Dall’olio, S.; Davoli, R.; Russo, V. Combined Effects of Pre-Slaughter Treatments and Lairage Time on Carcass and Meat Quality in Pigs of Different Halothane Genotype. Meat Sci. 2002, 61, 41–47. [Google Scholar] [CrossRef]
- De Luca, S.; Zanardi, E.; Alborali, G.L.; Ianieri, A.; Ghidini, S. Abattoir-Based Measures to Assess Swine Welfare: Analysis of the Methods Adopted in European Slaughterhouses. Animals 2021, 11, 226. [Google Scholar] [CrossRef]
- Gracey, J.F.; Collins, D.S. Meat Hygiene, 8th ed.; Ballière Tindall: London, UK, 1992. [Google Scholar]
- Dokmanović, M.; Velarde, A.; Tomović, V.; Glamočlija, N.; Marković, R.; Janjić, J.; Baltić, M.Ž. The Effects of Lairage Time and Handling Procedure Prior to Slaughter on Stress and Meat Quality Parameters in Pigs. Meat Sci. 2014, 98, 220–226. [Google Scholar] [CrossRef]
- Maisano, A.M.; Luini, M.; Vitale, N.; Rota Nodari, S.; Scali, F.; Alborali, G.L.; Vezzoli, F. Animal-Based Measures on Fattening Heavy Pigs at the Slaughterhouse and the Association with Animal Welfare at the Farm Level: A Preliminary Study. Animal 2020, 14, 108–118. [Google Scholar] [CrossRef]
- Arduini, A.; Redaelli, V.; Luzi, F.; Dall’Olio, S.; Pace, V.; Nanni Costa, L. Relationship between Deck Level, Body Surface Temperature and Carcass Damages in Italian Heavy Pigs after Short Journeys at Different Unloading Environmental Conditions. Animals 2017, 7, 10. [Google Scholar] [CrossRef]
- Vitali, M.; Bosi, P.; Santacroce, E.; Trevisi, P. The Multivariate Approach Identifies Relationships between Pre-Slaughter Factors, Body Lesions, Ham Defects and Carcass Traits in Pigs. PLoS ONE 2021, 16, e0251855. [Google Scholar] [CrossRef]
- Strappini, A.C.; Metz, J.H.M.; Gallo, C.; Frankena, K.; Vargas, R.; de Freslon, I.; Kemp, B. Bruises in Culled Cows: When, Where and How Are They Inflicted? Animal 2013, 7, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Welfare Quality Consortium. Welfare Quality Assessment Protocol for Pigs (Sows and Piglets, Growing and Finishing Pigs). 2009. Available online: http://www.welfarequalitynetwork.net/media/1018/pig_protocol.pdf (accessed on 20 November 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Grümpel, A.; Krieter, J.; Veit, C.; Dippel, S. Factors Influencing the Risk for Tail Lesions in Weaner Pigs (Sus Scrofa). Livest. Sci. 2018, 216, 219–226. [Google Scholar] [CrossRef]
- Prosciutto Di Parma (Parma Ham) Protected Designation of Origin. (Specifications and Dossier Pursuant to Article 4 of Council Regulation EEC No. 2081/92 Dated 14 July 1992). 1992, p. 38. Available online: https://politico.eu/wp-content/uploads/2017/06/parma.pdf (accessed on 20 November 2021).
- Gispert, M.; Faucitano, L.; Oliver, M.A.; Guàrdia, M.D.; Coll, C.; Siggens, K.; Harvey, K.; Diestre, A. A Survey of Pre-Slaughter Conditions, Halothane Gene Frequency, and Carcass and Meat Quality in Five Spanish Pig Commercial Abattoirs. Meat Sci. 2000, 55, 97–106. [Google Scholar] [CrossRef]
- Di Pasquale, J.; Nannoni, E.; Del Duca, I.; Adinolfi, F.; Capitanio, F.; Sardi, L.; Vitali, M.; Martelli, G. What Foods Are Identified as Animal Friendly by Italian Consumers? Ital. J. Anim. Sci. 2014, 13, 3582. [Google Scholar] [CrossRef]
- Fitzgerald, R.F.; Stalder, K.J.; Matthews, J.O.; Schultz Kaster, C.M.; Johnson, A.K. Factors Associated with Fatigued, Injured, and Dead Pig Frequency during Transport and Lairage at a Commercial Abattoir. J. Anim. Sci. 2009, 87, 1156–1166. [Google Scholar] [CrossRef]
- Urrea, V.M.; Bridi, A.M.; Ceballos, M.C.; Paranhos da Costa, M.J.R.; Faucitano, L. Behavior, Blood Stress Indicators, Skin Lesions, and Meat Quality in Pigs Transported to Slaughter at Different Loading Densities. J. Anim. Sci. 2021, 99, skab119. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Consortium of the Animal Transport Guides Project. Guide to Good Practices for the Transport of Pigs. 2017. Available online: http://animaltransportguides.eu/wp-content/uploads/2016/05/D3-Pigs-Revised-Final.pdf (accessed on 20 November 2021).
- Robertshaw, D. Mechanisms for the Control of Respiratory Evaporative Heat Loss in Panting Animals. J. Appl. Physiol. 2006, 101, 664–668. [Google Scholar] [CrossRef]
- Entin, P.L.; Robertshaw, D.; Rawson, R.E. Thermal Drive Contributes to Hyperventilation during Exercise in Sheep. J. Appl. Physiol. 1998, 85, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Fortin, A. The Effect of Transport Time from the Assembly Yard to the Abattoir and Resting Time at the Abattoir on Pork Quality. Can. J. Anim. Sci. 2002, 82, 141–150. [Google Scholar] [CrossRef]
- Pérez, M.P.; Palacio, J.; Santolaria, M.P.; Aceña, M.C.; Chacón, G.; Gascón, M.; Calvo, J.H.; Zaragoza, P.; Beltran, J.A.; García-Belenguer, S. Effect of Transport Time on Welfare and Meat Quality in Pigs. Meat Sci. 2002, 61, 425–433. [Google Scholar] [CrossRef]
- Petrolli, T.G.; Junqueira, O.M.; Pereira, A.S.; Domingues, C.H.; Artoni, S.M.; Santos, E.T. Lesión En La Carne y Adicción de Nutrientes En El Ayuno Antes Del Sacrificio de Cerdos. Rev. MVZ Córdoba 2017, 5619–5630. [Google Scholar] [CrossRef][Green Version]
- Aaslyng, M.D.; Brandt, P.; Blaabjerg, L.; Støier, S. Assessment and Incidence of Skin Damage in Slaughter Pigs. In International Congress of Meat Science and Technology (ICoMST) Proceedings; Izmir, Turkey, 2013; Available online: http://icomst-proceedings.helsinki.fi/papers/2013_04_13.pdf (accessed on 20 November 2021).
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; et al. Welfare of Pigs at Slaughter. EFSA J. 2020, 18, e06148. [Google Scholar] [CrossRef]
- Tarrant, P.V. The Effects of Handling, Transport, Slaughter and Chilling on Meat Quality and Yield in Pigs: A Review. Ir. J. Food Sci. Technol. 1989, 13, 79–107. [Google Scholar]
- Gerritzen, M.A.; Marahrens, M.; Kongsted, H.; Bracke, M.B.M. Review of Pig Welfare in Slaughterhouses at Stunning and Bleeding. EURCAW Pigs 2021. Available online: https://edepot.wur.nl/546026 (accessed on 20 November 2021).
- Holmes, R.; Gerritzen, M.A.; Herskin, M.S.; Schwarzlose, I.; Ruis, M.A.W. Review on Arrival and Lairage Management at Pig Slaughterhouses. EURCAW Pigs 2020. Available online: https://edepot.wur.nl/526511 (accessed on 20 November 2021).
- Brandt, P.; Rousing, T.; Herskin, M.S.; Aaslyng, M.D. Identification of Post-Mortem Indicators of Welfare of Finishing Pigs on the Day of Slaughter. Livest. Sci. 2013, 157, 535–544. [Google Scholar] [CrossRef]
- Hambrecht, E.; Eissen, J.J.; Nooijen, R.I.J.; Ducro, B.J.; Smits, C.H.M.; den Hartog, L.A.; Verstegen, M.W.A. Preslaughter Stress and Muscle Energy Largely Determine Pork Quality at Two Commercial Processing Plants. J. Anim. Sci. 2004, 82, 1401–1409. [Google Scholar] [CrossRef]
- Warriss, P.D.; Brown, S.N.; Adams, S.J.M.; Corlett, I.K. Relationships between Subjective and Objective Assessments of Stress at Slaughter and Meat Quality in Pigs. Meat Sci. 1994, 38, 329–340. [Google Scholar] [CrossRef]
- Correa, J.A.; Torrey, S.; Devillers, N.; Laforest, J.P.; Gonyou, H.W.; Faucitano, L. Effects of Different Moving Devices at Loading on Stress Response and Meat Quality in Pigs1. J. Anim. Sci. 2010, 88, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, O.A.; Faucitano, L.; Coldebella, A.; Ludke, J.V.; Peloso, J.V.; dalla Roza, D.; Paranhos da Costa, M.J.R. Effects of the Season of the Year, Truck Type and Location on Truck on Skin Bruises and Meat Quality in Pigs. Livest. Sci. 2007, 107, 29–36. [Google Scholar] [CrossRef]
- Guise, H.J.; Penny, R.H.C. Factors Influencing the Welfare and Carcass and Meat Quality of Pigs 1. The Effects of Stocking Density in Transport and the Use of Electric Goads. Anim. Sci. 1989, 49, 511–515. [Google Scholar] [CrossRef]
- Barton Gade, P.; Christensen, L.; Brown, S.N.; Warris, P.D. Effect of Tier and Ventilation during Transport on Blood Parameters and Meat Quality in Slaughter Pigs. In Proceedings of the Eu Seminar New Information on Welfare and Meat Quality of Pigs as Related to Handling Transport and Lairage Conditions; Schuette, A., Ed.; Braunschweig-Voelkenrode: Mariensee, Germany, 1996; pp. 101–116. [Google Scholar]
- Randall, J.M.; Stiles, M.A.; Geers, A.; Schutte, A.; Christensen, L.; Bradshaw, R.H. Vibration on Pig Transporters: Implications for Reducing Stress. In Proceedings of the Eu Seminar New Information on Welfare and Meat Quality of Pigs as Related to Handling Transport and Lairage Conditions; Schuette, A., Ed.; Braunschweig-Voelkenrode: Mariensee, Germany, 1996; pp. 143–159. [Google Scholar]
- Dalla Costa, F.A.; Lopes, L.S.; Dalla Costa, O.A. Effects of the Truck Suspension System on Animal Welfare, Carcass and Meat Quality Traits in Pigs. Animals 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
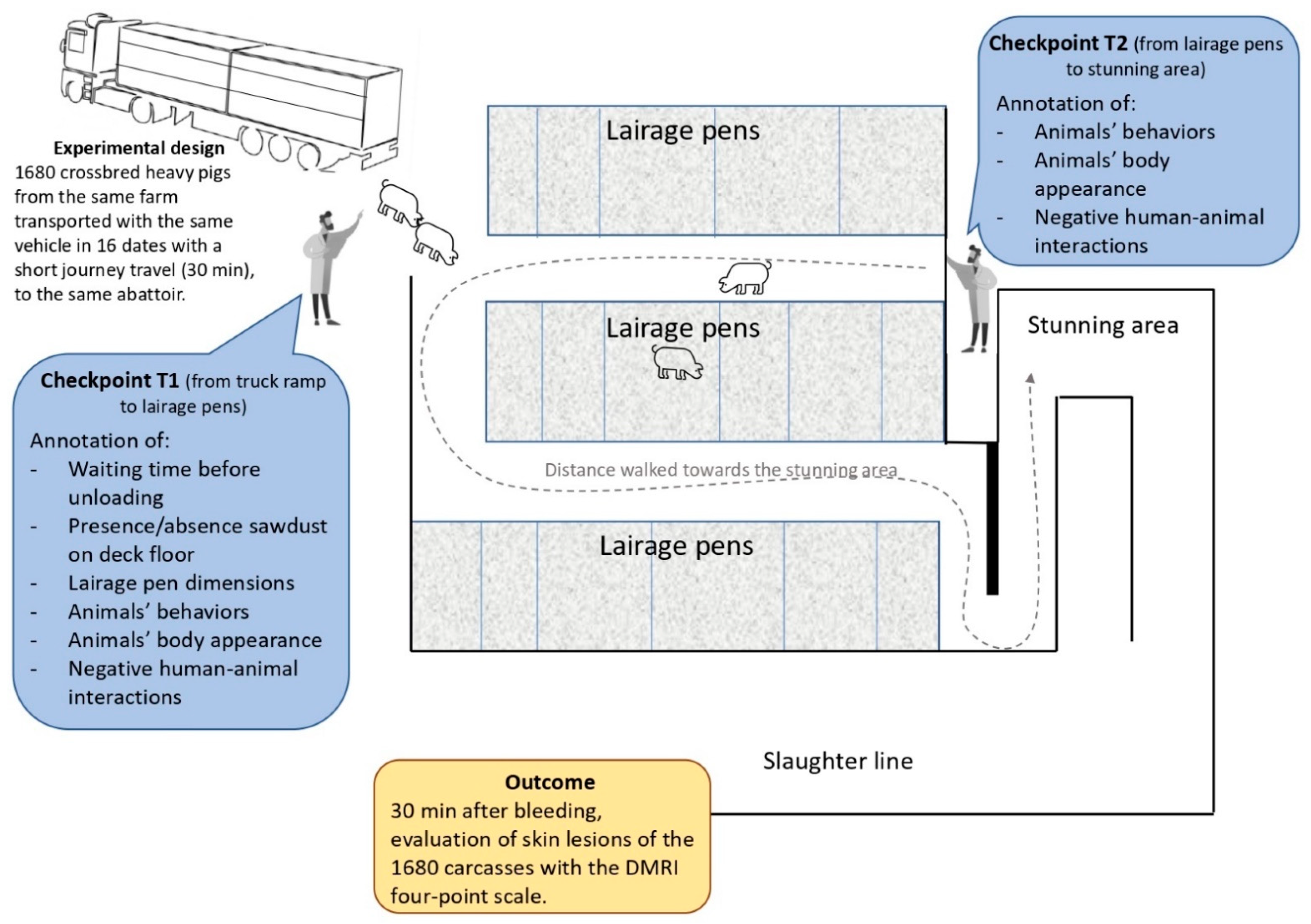
| Type of Event | Event | Description | Checkpoint |
|---|---|---|---|
| Animal | Falling | The pig loses balance and touch the ground with a shoulder, thigh, trunk or another part of the body that is not one leg [41]. | T1, T2 |
| Animal | Slipping | The pig loses balance without a part of the body touching the floor [41]. | T1, T2 |
| Animal | Reluctance to move | The pig refuses to walk and stops without moving the head or the body for more than 2 s [41]. | T1, T2 |
| Animal | Turning back | The pig turns away and moves in the direction opposite of the desired [41]. | T1, T2 |
| Animal | Jumping | The pig does a sudden movement and jumps. | T1, T2 |
| Animal | Elimination | The pig defecates and/or urinates. | T1, T2 |
| Animal | Vocalization | The pig vocalizes. | T1, T2 |
| Animal | Panting | The pig breaths loudly and fast with mouth open. | T1, T2 |
| Animal | Wounds on body | The pig has wounds on the body [41]. | T1 |
| Animal | Hematoma | The pig has one or more hematomas. | T1 |
| Animal | Discoloration | The pig shows skin discoloration. | T1 |
| Animal | Ear discoloration | The pig shows skin discoloration on ears. | T2 |
| Animal | Thigh discoloration | The pig shows skin discoloration on thighs. | T2 |
| Animal | Back discoloration | The pig shows skin discoloration on the back part of the trunk. | T2 |
| Animal | Side discoloration | The pig shows skin discoloration on the side part of the trunk. | T2 |
| Animal | Lameness | The pig fails to walk normally. It can vary in severity, from reduced ability to inability to bear weight [41]. | T1 |
| Animal | Shivering | The pig trembles. Slow or irregular vibration of a body part or the whole body [41]. | T1 |
| Animal | Overlapping | The pig mounts another one with its front legs on the back of the other. | T1 |
| Animal | Pushes | The pig presses the head on the body of another pig lying or walking ahead. | T2 |
| Animal | Huddling | More pigs huddle and create a group. | T1, T2 |
| Human–animal | Hit with stick | An operator beats the pig with a stick [40]. | T1 |
| Human–animal | Hit with plastic tube | An operator beats the pig with a plastic tube [40]. | T1, T2 |
| Human–animal | Hit with rubber stick | An operator beats the pig with a rubber stick [40]. | T1 |
| Human–animal | Poke with electric goad | An operator touches the pig with the electric goad [40]. | T1 |
| Human–animal | Shouts | An operator shouts at animals to encourage them proceeding. | T1, T2 |
| Variables | Mean | Median | S.D. | Min | Max |
|---|---|---|---|---|---|
| Frequency of events annotated at T1 | |||||
| Animal events: | |||||
| Falling (%) | 1.73 | 0.00 | 2.76 | 0.00 | 8.70 |
| Slipping (%) | 3.59 | 4.17 | 3.72 | 0.00 | 17.39 |
| Turning back (%) | 6.46 | 4.35 | 7.75 | 0.00 | 34.78 |
| Reluctance to move (%) | 2.79 | 0.00 | 3.45 | 0.00 | 13.04 |
| Overlapping (%) | 1.97 | 0.00 | 4.09 | 0.00 | 21.74 |
| Jumping (%) | 0.12 | 0.00 | 0.69 | 0.00 | 4.17 |
| Huddling (%) | 2.21 | 0.00 | 3.91 | 0.00 | 21.74 |
| Elimination (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vocalization (%) | 9.04 | 4.35 | 12.53 | 0.00 | 60.87 |
| Panting (%) | 1.72 | 0.00 | 4.53 | 0.00 | 21.74 |
| Wounds on body (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hematoma (%) | 0.06 | 0.00 | 0.51 | 0.00 | 4.35 |
| Discoloration (%) | 1.96 | 0.00 | 3.93 | 0.00 | 17.39 |
| Shivering (%) | 0.18 | 0.00 | 0.87 | 0.00 | 4.35 |
| Lameness (%) | 0.06 | 0.00 | 0.51 | 0.00 | 4.35 |
| Total number of animal events at T1 (N) | 7.43 | 7.00 | 4.54 | 2.00 | 32.00 |
| Human–animal events: | |||||
| Hit with stick (%) | 0.71 | 0.00 | 2.04 | 0.00 | 8.70 |
| Hit with plastic tube (%) | 1.91 | 0.00 | 2.87 | 0.00 | 8.70 |
| Poke with electric goad (%) | 0.36 | 0.00 | 1.19 | 0.00 | 4.35 |
| Shouts (%) | 2.38 | 2.00 | 2.58 | 0.00 | 8.70 |
| Hit with rubber stick (%) | 1.61 | 0.00 | 2.35 | 0.00 | 9.09 |
| Total number of human–animal events at T1 (N) | 1.62 | 1.00 | 1.66 | 0.00 | 6.00 |
| Frequency of events annotated at T2 | |||||
| Animal events: | |||||
| Falling (%) | 0.06 | 0.00 | 0.49 | 0.00 | 4.17 |
| Slipping (%) | 1.01 | 0.00 | 3.45 | 0.00 | 26.09 |
| Turning back (%) | 12.02 | 8.70 | 10.67 | 0.00 | 40.91 |
| Reluctance to move (%) | 6.87 | 4.55 | 7.77 | 0.00 | 44.00 |
| Pushes (%) | 1.94 | 0.00 | 4.03 | 0.00 | 20.00 |
| Jumping (%) | 2.03 | 0.00 | 3.47 | 0.00 | 13.64 |
| Huddling (%) | 1.71 | 0.00 | 3.79 | 0.00 | 17.39 |
| Elimination (%) | 0.36 | 0.00 | 1.41 | 0.00 | 8.70 |
| Vocalization (%) | 7.31 | 8.33 | 4.90 | 0.00 | 13.04 |
| Panting (%) | 0.66 | 0.00 | 2.48 | 0.00 | 17.39 |
| Side discoloration (%) | 9.54 | 8.51 | 8.87 | 0.00 | 30.43 |
| Ear discoloration (%) | 3.94 | 2.00 | 5.21 | 0.00 | 25.00 |
| Thigh discoloration (%) | 11.15 | 8.89 | 10.65 | 0.00 | 43.48 |
| Back discoloration (%) | 11.32 | 8.70 | 12.22 | 0.00 | 47.83 |
| Human–animal events: | |||||
| Hit with plastic tube (%) | 7.61 | 8.70 | 3.67 | 0.00 | 13.04 |
| Shouts (%) | 7.50 | 8.70 | 3.71 | 0.00 | 13.04 |
| Total number of human–animal events at T2 (N) | 7.03 | 7.00 | 3.22 | 0.00 | 12.00 |
| Skin lesion score | |||||
| Rear | |||||
| 1 (%) | 66.86 | 65.22 | 24.19 | 13.04 | 100.00 |
| 2 (%) | 26.22 | 25.00 | 18.93 | 0.00 | 70.83 |
| 3 (%) | 6.03 | 4.26 | 7.65 | 0.00 | 30.43 |
| 4 (%) | 0.89 | 0.00 | 2.48 | 0.00 | 13.04 |
| Moderate lesions (1 + 2) | 93.08 | 95.65 | 8.79 | 65.22 | 100.00 |
| Severe lesions (3 + 4) | 6.92 | 4.35 | 8.79 | 0.00 | 34.78 |
| Middle | |||||
| 1 (%) | 52.05 | 47.92 | 27.77 | 8.70 | 100.00 |
| 2 (%) | 36.58 | 39.13 | 19.71 | 0.00 | 73.91 |
| 3 (%) | 10.06 | 6.44 | 10.46 | 0.00 | 39.13 |
| 4 (%) | 1.31 | 0.00 | 3.41 | 0.00 | 16.67 |
| Moderate lesions (1 + 2) | 88.63 | 91.49 | 12.07 | 52.17 | 100.00 |
| Severe lesions (3 + 4) | 11.37 | 8.51 | 12.07 | 0.00 | 47.83 |
| Shoulder | |||||
| 1 (%) | 72.94 | 78.26 | 21.52 | 21.74 | 100.00 |
| 2 (%) | 20.23 | 17.39 | 14.31 | 0.00 | 56.52 |
| 3 (%) | 4.94 | 0.00 | 7.63 | 0.00 | 30.43 |
| 4 (%) | 1.90 | 0.00 | 3.97 | 0.00 | 16.67 |
| Moderate lesions (1 + 2) | 93.17 | 98.00 | 10.38 | 60.87 | 100.00 |
| Severe lesions (3 + 4) | 6.83 | 2.00 | 10.38 | 0.00 | 39.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappaterra, M.; Padalino, B.; Menchetti, L.; Arduini, A.; Pace, V.; Nanni Costa, L. Carcass Lesion Severity and Pre-Slaughter Conditions in Heavy Pigs: A Prospective Study at a Commercial Abattoir in Northern Italy. Appl. Sci. 2022, 12, 1078. https://doi.org/10.3390/app12031078
Zappaterra M, Padalino B, Menchetti L, Arduini A, Pace V, Nanni Costa L. Carcass Lesion Severity and Pre-Slaughter Conditions in Heavy Pigs: A Prospective Study at a Commercial Abattoir in Northern Italy. Applied Sciences. 2022; 12(3):1078. https://doi.org/10.3390/app12031078
Chicago/Turabian StyleZappaterra, Martina, Barbara Padalino, Laura Menchetti, Agnese Arduini, Vincenzo Pace, and Leonardo Nanni Costa. 2022. "Carcass Lesion Severity and Pre-Slaughter Conditions in Heavy Pigs: A Prospective Study at a Commercial Abattoir in Northern Italy" Applied Sciences 12, no. 3: 1078. https://doi.org/10.3390/app12031078
APA StyleZappaterra, M., Padalino, B., Menchetti, L., Arduini, A., Pace, V., & Nanni Costa, L. (2022). Carcass Lesion Severity and Pre-Slaughter Conditions in Heavy Pigs: A Prospective Study at a Commercial Abattoir in Northern Italy. Applied Sciences, 12(3), 1078. https://doi.org/10.3390/app12031078








