Austenitic Stainless Steel as a Catalyst Material for Photo-Fenton Degradation of Organic Dyes
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, J.; Kralj, M.B.; Dolenc, D.; Trebše, P. Removal of Flotation Collector O-Isopropyl-N-ethylthionocarbamate from Wastewater. Molecules 2021, 26, 6676. [Google Scholar] [CrossRef] [PubMed]
- Ahile, U.J.; Wuana, R.; Itodo, A.U.; Sha’Ato, R.; Dantas, R.F. A review on the use of chelating agents as an alternative to promote photo-Fenton at neutral pH: Current trends, knowledge gap and future studies. Sci. Total. Environ. 2020, 710, 134872. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, N.; An, J.; Ma, J.; Wu, Y.; Liu, H. Fenton-based technologies as efficient advanced oxidation processes for microcystin-LR degradation. Sci. Total Environ. 2021, 753, 141809. [Google Scholar] [CrossRef] [PubMed]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Oturan, N.; Oturan, M.A. Chapter 8—Electro-Fenton Process: Background, New Developments, and Applications. In Electrochemical Water and Wastewater Treatment; Matínez-Huitle, C.A., Rodrigo, M.A., Scialdone, O., Eds.; Butter-worth-Heinemann: Oxford, UK, 2018; pp. 193–221. ISBN 9780128131602. [Google Scholar] [CrossRef]
- O’Dowd, K.; Pillai, S.C. Photo-Fenton disinfection at near neutral pH: Process, parameter optimization and recent advances. J. Environ. Chem. Eng. 2020, 8, 104063. [Google Scholar] [CrossRef]
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.-R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochem. 2021, 71, 105367. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Naidu, R. Permeable reactive barrier for groundwater remediation. J. Ind. Eng. Chem. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- Rezaei, F.; Vione, D. Effect of pH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-Like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.; Mirbagheri, S.A.; Ye, F.; Dutta, J. Nano zero-valent iron on activated carbon cloth support as Fenton-like catalyst for efficient color and COD removal from melanoidin wastewater. Chemosphere 2021, 263, 127945. [Google Scholar] [CrossRef]
- Minella, M.; Sappa, E.; Hanna, K.; Barsotti, F.; Maurino, V.; Minero, C.; Vione, D. Considerable Fenton and photo-Fenton reactivity of passivated zero-valent iron. RSC Adv. 2016, 6, 86752–86761. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hua, T.; Li, F.; Zhou, Q. Bio-electro-Fenton systems for sustainable wastewater treatment: Mechanisms, novel configurations, recent advances, LCA and challenges. An updated review. J. Chem. Technol. Biotechnol. 2020, 95, 2083–2097. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Minella, M.; Bertinetti, S.; Hanna, K.; Minero, C.; Vione, D. Degradation of ibuprofen and phenol with a Fenton-like process triggered by zero-valent iron (ZVI-Fenton). Environ. Res. 2019, 179, 108750. [Google Scholar] [CrossRef]
- Evgenidou, E.; Konstantinou, I.; Fytianos, K.; Poulios, I. Oxidation of two organophosphorous insecticides by the photo-assisted Fenton reaction. Water Res. 2007, 41, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.; Ghaly, M.Y.; Gad-Allah, T.A. Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. Desalination 2006, 194, 166–175. [Google Scholar] [CrossRef]
- Hamd, W.S.; Dutta, J. Heterogeneous photo-Fenton reaction and its enhancement upon addition of chelating agents. In Na-nomaterials for the Detection and Removal of Wastewater Pollutants; Bonelli, B., Freyria, F.S., Rossetti, I., Sethi, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–330. [Google Scholar]
- Xiong, X.; Sun, Y.; Sun, B.; Song, W.; Sun, J.; Gao, N.; Qiao, J.; Guan, X. Enhancement of the advanced Fenton process by weak magnetic field for the degradation of 4-nitrophenol. RSC Adv. 2015, 5, 13357–13365. [Google Scholar] [CrossRef]
- Namkung, K.C.; Burgess, A.E.; Bremner, D.H. A Fenton-like Oxidation Process Using Corrosion of Iron Metal Sheet Surfaces in the Presence of Hydrogen Peroxide: A Batch Process Study Using Model Pollutants. Environ. Technol. 2005, 26, 341–352. [Google Scholar] [CrossRef]
- Loloei, M.; Rezaee, A. Decolorization of methylene blue by the electro-Fenton process using stainless steel mesh electrodes. Int. J. Environ. Health Eng. 2016, 5, 27. [Google Scholar] [CrossRef]
- Untea, I.; Orbeci, C.; Stanescu, R.; Segneanu, A.E.; Craciun, M.E. A new photo-fenton procedure applied in oxidative degradation of organic compounds from wastewater. Environ. Eng. Manag. J. 2012, 11, 141–146. [Google Scholar] [CrossRef]
- Di Cesare, A.; De Carluccio, M.; Eckert, E.M.; Fontaneto, D.; Fiorentino, A.; Corno, G.; Prete, P.; Cucciniello, R.; Proto, A.; Rizzo, L. Combination of flow cytometry and molecular analysis to monitor the effect of UVC/H2O2 vs UVC/H2O2/Cu-IDS processes on pathogens and antibiotic resistant genes in secondary wastewater effluents. Water Res. 2020, 184, 116194. [Google Scholar] [CrossRef]
- Silva, G.D.; Marson, E.O.; Batista, L.L.; Ueira-Vieira, C.; Starling, M.C.V.; Trovó, A.G. Contrasting the performance of photo-Fenton at neutral pH in the presence of different organic iron-complexes using hydrogen peroxide or persulfate as oxidants for naproxen degradation and removal of antimicrobial activity. Process. Saf. Environ. Prot. 2021, 147, 798–807. [Google Scholar] [CrossRef]
- O’Dell, J.W. The Determination of Chemical Oxygen Demand by Semi-Automated Colorimetry; Elsevier: Amsterdam, The Netherlands, 1996; pp. 509–521. [Google Scholar]
- Gernjak, W.; Krutzler, T.; Glaser, A.; Malato, S.; Caceres, J.; Bauer, R.; Fernández-Alba, A. Photo-Fenton treatment of water containing natural phenolic pollutants. Chemosphere 2003, 50, 71–78. [Google Scholar] [CrossRef]
- Gibbs, M.M. A simple method for the rapid determination of iron in natural waters. Water Res. 1979, 13, 295–297. [Google Scholar] [CrossRef]
- Ahmed, N.; Vione, D.; Rivoira, L.; Carena, L.; Castiglioni, M.; Bruzzoniti, M. A Review on the Degradation of Pollutants by Fenton-Like Systems Based on Zero-Valent Iron and Persulfate: Effects of Reduction Potentials, pH, and Anions Occurring in Waste Waters. Molecules 2021, 26, 4584. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Shin, D.-S. Treatment of Diesel-Contaminated Soil by Fenton and Persulfate Oxidation with Zero-Valent Iron. Soil Sediment Contam. Int. J. 2013, 23, 180–193. [Google Scholar] [CrossRef]
- De la Cruz, N.; Gimenez, J.; Esplugas, S.; Grandjean, D.; de Alencastro, L.; Pulgarín, C. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Res. 2012, 46, 1947–1957. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; Sdiri, A.; Ltaief, W.; Da Costa, P.; Gálvez, M.E.; Ben Zina, M. Efficient removal of cadmium and 2-chlorophenol in aqueous systems by natural clay: Adsorption and photo-Fenton degradation processes. Comptes Rendus Chim. 2018, 21, 253–262. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P. Degradation and mineralization of methylene blue using a heterogeneous photo-Fenton catalyst under visible and solar light irradiation. Catal. Sci. Technol. 2016, 6, 1222–1232. [Google Scholar] [CrossRef]
- Gao, Y.; Champagne, P.; Blair, D.; He, O.; Song, T. Activated persulfate by iron-based materials used for refractory organics degradation: A review. Water Sci. Technol. 2020, 81, 853–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbari, F.; Moradi, M.; Manshouri, M. Textile wastewater decolorization by zero valent iron activated peroxymonosulfate: Compared with zero valent copper. J. Environ. Chem. Eng. 2014, 2, 1846–1851. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef]
- Su, S.; Liu, Y.; Liu, X.; Jin, W.; Zhao, Y. Transformation pathway and degradation mechanism of methylene blue through β-FeOOH@GO catalyzed photo-Fenton-like system. Chemosphere 2019, 218, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Effect of Photo-Fenton Operating Conditions on the Performance of Photo-Fenton-SBR Process for Recalcitrant Wastewater Treatment. J. Appl. Sci. 2010, 10, 3236–3242. [Google Scholar] [CrossRef] [Green Version]
- Vermilyea, A.W.; Voelker, B.M. Photo-Fenton Reaction at Near Neutral pH. Environ. Sci. Technol. 2009, 43, 6927–6933. [Google Scholar] [CrossRef]
- Singh, J.; Chang, Y.-Y.; Koduru, J.R.; Yang, J.-K. Potential degradation of methylene blue (MB) by nano-metallic particles: A kinetic study and possible mechanism of MB degradation. Environ. Eng. Res. 2018, 23, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Taufik, A. Photo-Fenton degradation of methylene blue in the presence of Au-Fe3O4/graphene composites under UV and visible light at near neutral pH: Effect of coexisting inorganic anion. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100221. [Google Scholar] [CrossRef]
- Choquehuanca, A.; Ruiz-Montoya, J.G.; Gómez, A.L.R.-T. Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides. Open Chem. 2021, 19, 1009–1020. [Google Scholar] [CrossRef]
- Gil Cortes, J.; Dantas, R.F. Optimization of photo-Fenton to work at neutral pH using NTA-Fe2+. Desalin. Water Treat. 2019, 169, 287–293. [Google Scholar] [CrossRef]
- Kirchon, A.; Zhang, P.; Li, J.; Joseph, E.A.; Chen, W.; Zhou, H.-C. Effect of Isomorphic Metal Substitution on the Fenton and Photo-Fenton Degradation of Methylene Blue Using Fe-Based Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 9292–9299. [Google Scholar] [CrossRef]
- Lam, F.; Hu, X. A high performance bimetallic catalyst for photo-Fenton oxidation of Orange II over a wide pH range. Catal. Commun. 2007, 8, 2125–2129. [Google Scholar] [CrossRef]
- Devi, L.G.; Kumar, S.G.; Raju, K.S.A.; Rajashekhar, K.E. Photo-Fenton and photo-Fenton-like processes for the degradation of methyl orange in aqueous medium: Influence of oxidation states of iron. Chem. Pap. 2010, 64, 378–385. [Google Scholar] [CrossRef]
- Devi, L.G.; Srinivas, M.; ArunaKumari, M. Heterogeneous advanced photo- Fenton process using peroxymonosulfate and peroxydisulfate in presence of zero valent metallic iron: A comparative study with hydrogen peroxide photo-Fenton process. J. Water Process Eng. 2016, 13, 117–126. [Google Scholar] [CrossRef]
- Kočanová, V.; Dušek, L. Electrochemical dissolution of steel as a typical catalyst for electro-Fenton oxidation. Monatshefte Chem Chem. Mon. 2016, 147, 935–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.-F.; Hutchison, M.J.; Santucci, J.R.J.; Scully, J.R.; Rondinelli, J.M. Improved Electrochemical Phase Diagrams from Theory and Experiment: The Ni–Water System and Its Complex Compounds. J. Phys. Chem. C 2017, 121, 9782–9789. [Google Scholar] [CrossRef]
- Buerge, I.J.; Hug, S.J. Kinetics and pH Dependence of Chromium(VI) Reduction by Iron(II). Environ. Sci. Technol. 1997, 31, 1426–1432. [Google Scholar] [CrossRef]
- Mahringer, D.; Polenz, C.; El-Athman, F. Stabilization of Chromium (VI) in the Presence of Iron (II): Method Development and Validation. Water 2020, 12, 924. [Google Scholar] [CrossRef] [Green Version]
- Ohmi, T. Formation of chromium oxide on 316L austenitic stainless steel. J. Vac. Sci. Technol. A 1996, 14, 2505–2510. [Google Scholar] [CrossRef]
- Huttenlochner, K.; Müller-Renno, C.; Ziegler, C.; Merz, R.; Merz, B.; Kopnarski, M.; Chodorski, J.; Schlegel, C.; Ulber, R. Removing biofilms from stainless steel without changing surface properties relevant for bacterial attachment. Biointerphases 2017, 12, 02C404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Jiang, E.Y.; Bai, H.L. Fabrication of ultrathin epitaxial γ-Fe2O3films by reactive sputtering. J. Phys. D Appl. Phys. 2011, 44, 075003. [Google Scholar] [CrossRef]
- Wu, H.; Gao, G.; Zhou, X.; Zhang, Y.; Guo, S. Control on the formation of Fe3O4nanoparticles on chemically reduced graphene oxide surfaces. CrystEngComm 2012, 14, 499–504. [Google Scholar] [CrossRef]
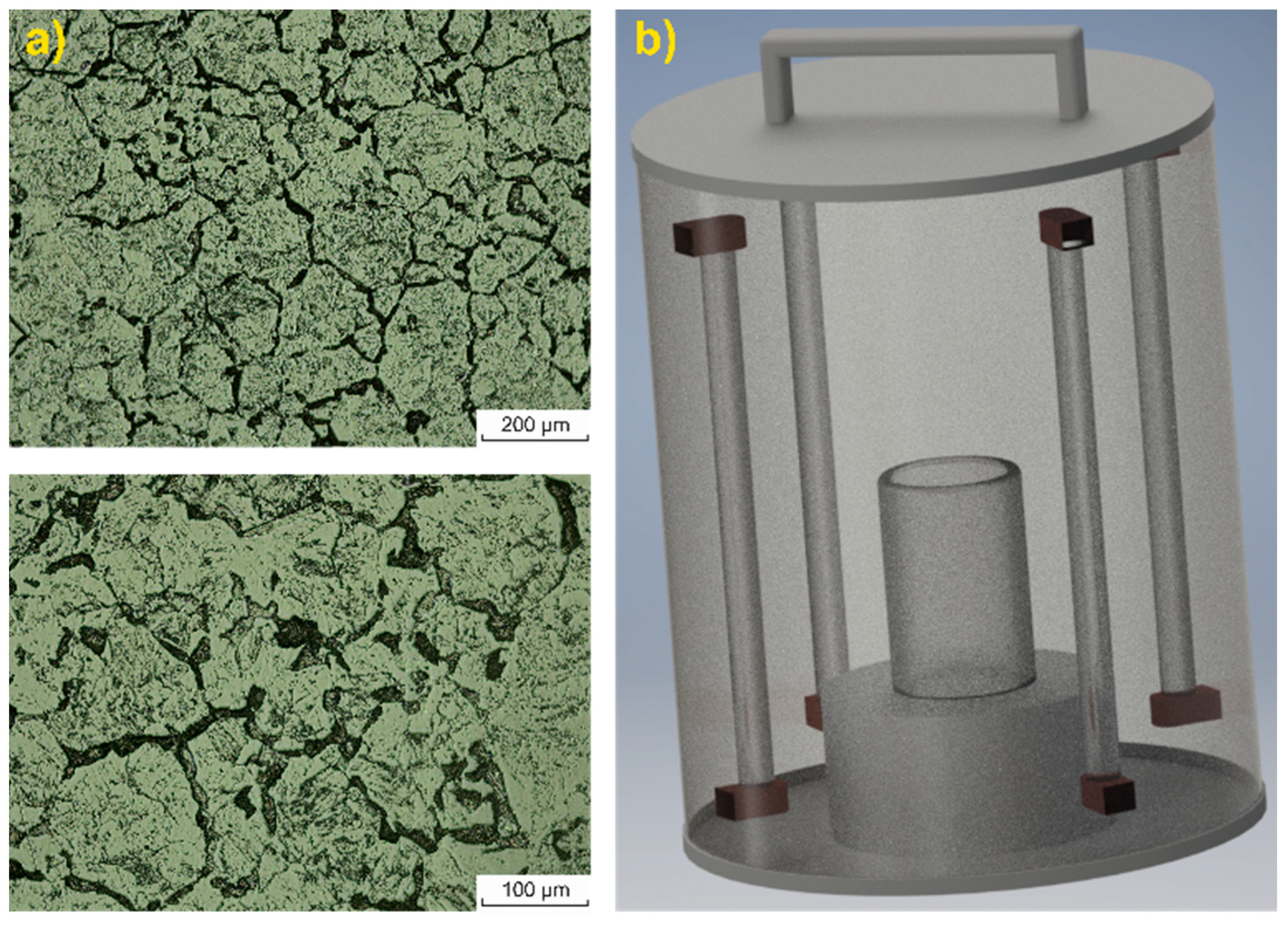
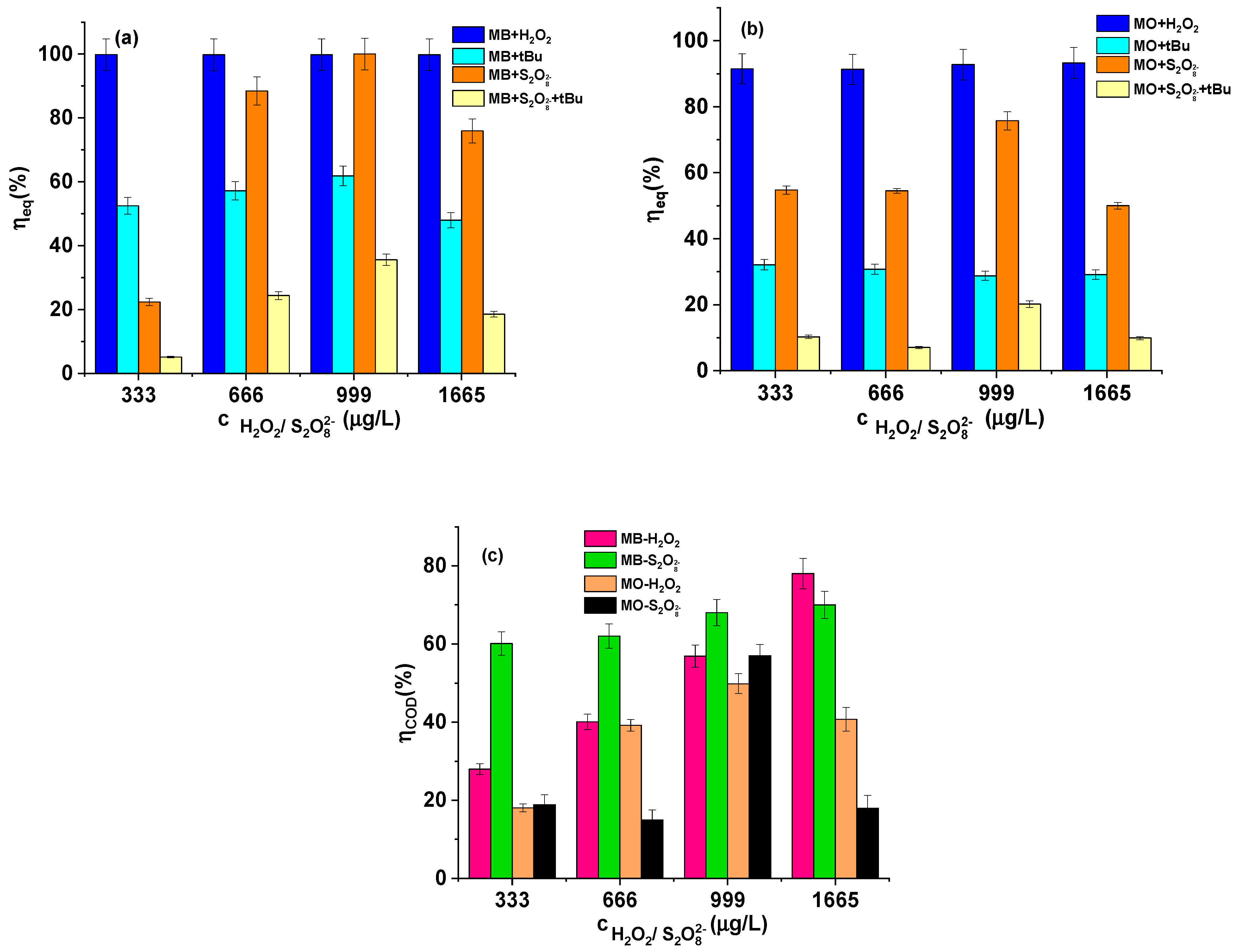
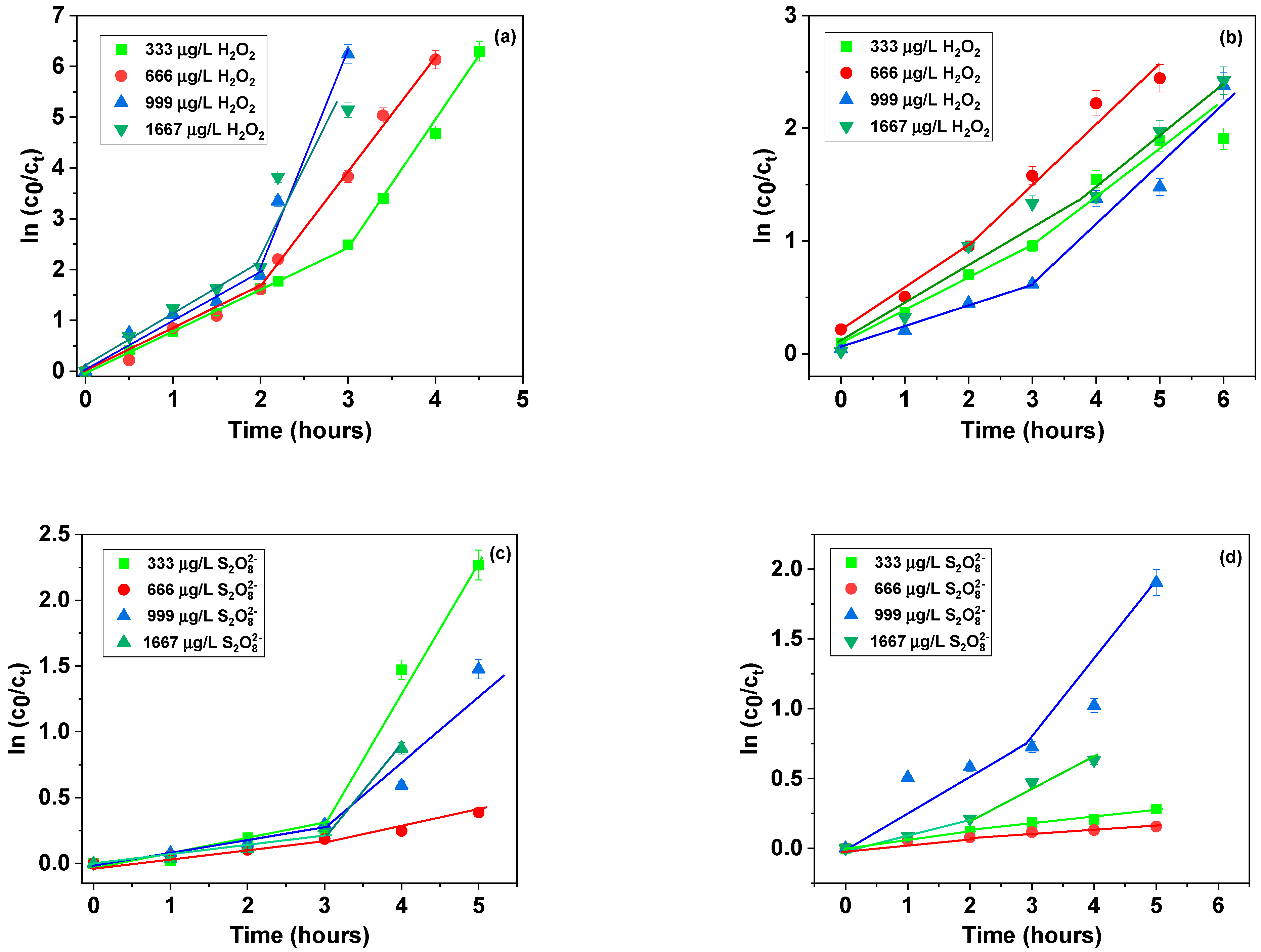
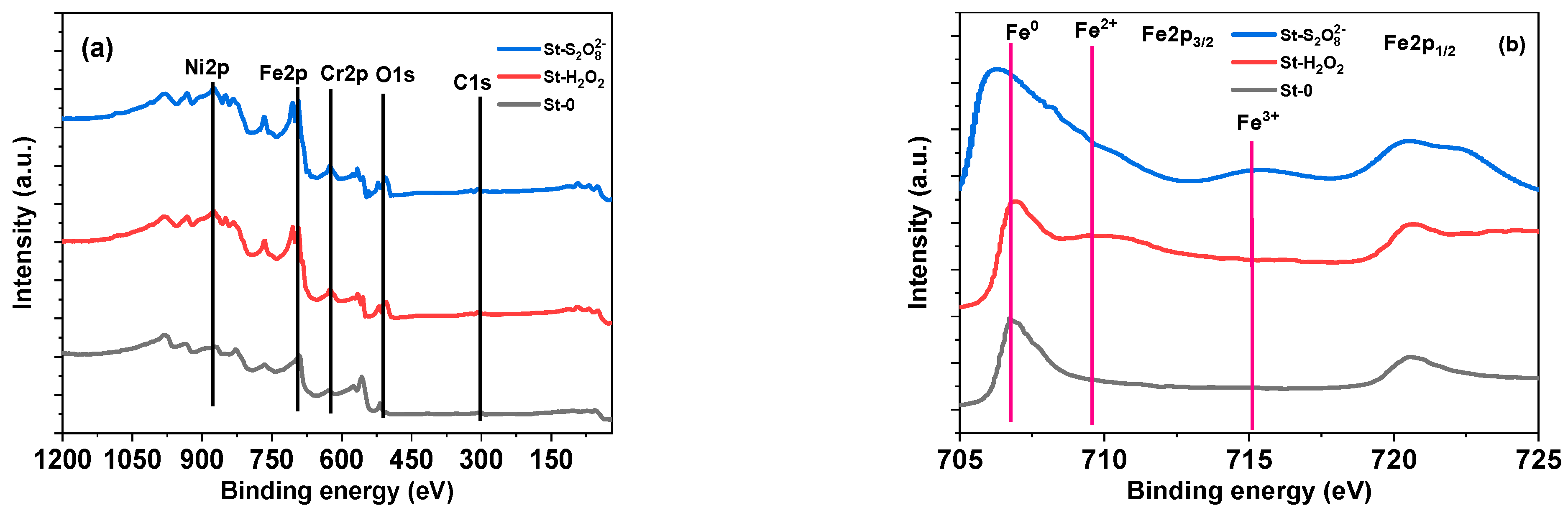
| cH2O2 (μg/L) | Kinetic Data for MB | Kinetic Data for MO | ||||
|---|---|---|---|---|---|---|
| Total Fe (mg/L) | Rate Constants | Total Fe (mg/L) | Rate Constants | |||
| k1 (h−1) | k2 (h−1) | k1 (h−1) | k2 (h−1) | |||
| 333 | 0.13 | 0.77 (0.992) | 2.35 (0.995) | 0.15 | 0.33 (0.962) | 0.35 (0.983) |
| 666 | 0.16 | 1.00 (0.987) | 2.39 (0.998) | 0.18 | 0.34 (0.996) | 0.87 (0.980) |
| 999 | 0.17 | 1.23 (0.985) | 3.21 (0.996) | 0.21 | 0.39 (0.993) | 1.03 (0.984) |
| 1665 | 0.28 | 1.26 (0.992) | 4.05 (0.978) | 0.32 | 0.47 (0.983) | 1.33 (0.989) |
| cS2O82 (μg/L) | Kinetic Data for MB | Kinetic Data for MO | ||||
|---|---|---|---|---|---|---|
| Total Fe (mg/L) | Rate Constants | Total Fe (mg/L) | Rate Constants | |||
| k1 (h−1) | k2 (h−1) | k1 (h−1) | k2 (h−1) | |||
| 333 | 0.94 | 0.35 (0.892) | 1.36 (0.996) | 0.98 | 0.20 (0.936) | 0.35 (0.983) |
| 666 | 1.36 | 0.10 (0.951) | 0.38 (0.995) | 1.32 | 0.23 (0.984) | 0.36 (0.990) |
| 999 | 2.96 | 0.15 (0.980) | 0.24 (0.992) | 3.04 | 0.10 (0.989) | 0.33 (0.999) |
| 1665 | 3.68 | 0.12 (0.996) | 0.30 (0.999) | 3.95 | 0.25 (0.988) | 0.28 (0.999) |
| Photo-Fenton with Hydrogen Peroxide | |||||||
|---|---|---|---|---|---|---|---|
| Catalyst Type | Model Dye(s) | Process Efficiency | Rate Constants k | Iron Amount in Solution | Oxidant Amount | pH | Reference |
| Stainless steel | MB | Discoloration eff.: max. 99.5%, mineralization eff. 74.5% | 0.77–4.05 h−1 | 0.13–0.28 mg/L | 333–1665 μg/L | 6.5 | This study |
| MO | Discoloration eff.: max. 93.5%, mineralization eff. 49.9% | 0.33–1.33 h−1 | 0.15–0.32 mg/L | ||||
| Nano ZVI particles | MB | Discoloration eff.: max. 98% | max 2.34 h−1 | 150 mg/L | 3100 mg/L | 2 | [42] |
| Fe-Ni/SiO2 | MB | Discoloration eff.: max. 99.80% at pH 3 and 83% at pH=9 | n.a. | n.a. | 102 mg/L | 1.5–11 | [33] |
| Au-Fe3O4/graphene | MB | Discoloration eff.: max. 99% | n.a. | max. 0.6 mg/L | 0.2–1 g/L | 7.2 | [43] |
| Fe3O4 and α-Fe2O3 | MB | Discoloration eff.: max. 95% | max. 0.78 h−1 | n.a. | 300–500 mg/L | 6.5 | [44] |
| nitrilotriacetic acid-bonded Fe2+ | MB | Discoloration eff.: max. 97.4% | n.a. | 1–3 mg/L | 40–100 mg/L | 6.5 | [45] |
| Fe-Based Metal–Organic Frameworks | MB | Discoloration eff.: max. 99% | n.a. | 100 mg/L | 735 mg/L | 6.5 | [46] |
| FeCu bimetallic system | MO | mineralization eff. 78% | n.a. | max. 0.32 mg/L | 490 mg/L | 7 | [47] |
| Photo-Fenton with persulfate | |||||||
| Stainless steel | MB | Discoloration eff.: max. 88.4%, mineralization eff. 69.9% | 0.10–1.36 h−1 | 0.94–3.68 mg/L | 333–1665 μg/L | 6.5 | This study |
| MO | Discoloration eff.: max. 75.7%, mineralization eff. 57% | 0.10–0.36 h−1 | 0.98–3.95 mg/L | ||||
| Fe2+ (FeSO4) under UV | MO | Discoloration eff.: max. 99% at pH 3, 2% at pH 7 | ~30 h−1 | 2–20 mg/L | 50 mg/L | 3–7 | [48] |
| ZVI metallic Fe nanoparticles | MO | Discoloration eff.: max. 98% at pH 3, 70% at pH 7 | n.a. | n.a. | 30 mg/L | 3–7 | [49] |
| Sample | Relative Element Concentration (wt.%) | Fe Chemical Species (2p) (rel. %) | |||||
|---|---|---|---|---|---|---|---|
| Fe2p | Cr2p | Ni2p | O1s | C1s | |||
| St-0 | 70.48 | 17.9 | 9.54 | 2.02 | 0.06 | Fe0 Fe2+ Fe3+ | 98.4 - 1.6 |
| St-H2O2 | 68.47 | 17.8 | 9.50 | 4.19 | 0.04 | Fe0 Fe2+ Fe3+ | 90.3 7.3 2.4 |
| St-S2O82− | 67.37 | 17.8 | 9.55 | 5.24 | 0.04 | Fe0 Fe2+ Fe3+ | 89.1 7.8 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croitoru, C.; Roata, I.C.; Machedon-Pisu, T.; Olah, A. Austenitic Stainless Steel as a Catalyst Material for Photo-Fenton Degradation of Organic Dyes. Appl. Sci. 2022, 12, 1008. https://doi.org/10.3390/app12031008
Croitoru C, Roata IC, Machedon-Pisu T, Olah A. Austenitic Stainless Steel as a Catalyst Material for Photo-Fenton Degradation of Organic Dyes. Applied Sciences. 2022; 12(3):1008. https://doi.org/10.3390/app12031008
Chicago/Turabian StyleCroitoru, Catalin, Ionut Claudiu Roata, Teodor Machedon-Pisu, and Arthur Olah. 2022. "Austenitic Stainless Steel as a Catalyst Material for Photo-Fenton Degradation of Organic Dyes" Applied Sciences 12, no. 3: 1008. https://doi.org/10.3390/app12031008
APA StyleCroitoru, C., Roata, I. C., Machedon-Pisu, T., & Olah, A. (2022). Austenitic Stainless Steel as a Catalyst Material for Photo-Fenton Degradation of Organic Dyes. Applied Sciences, 12(3), 1008. https://doi.org/10.3390/app12031008







