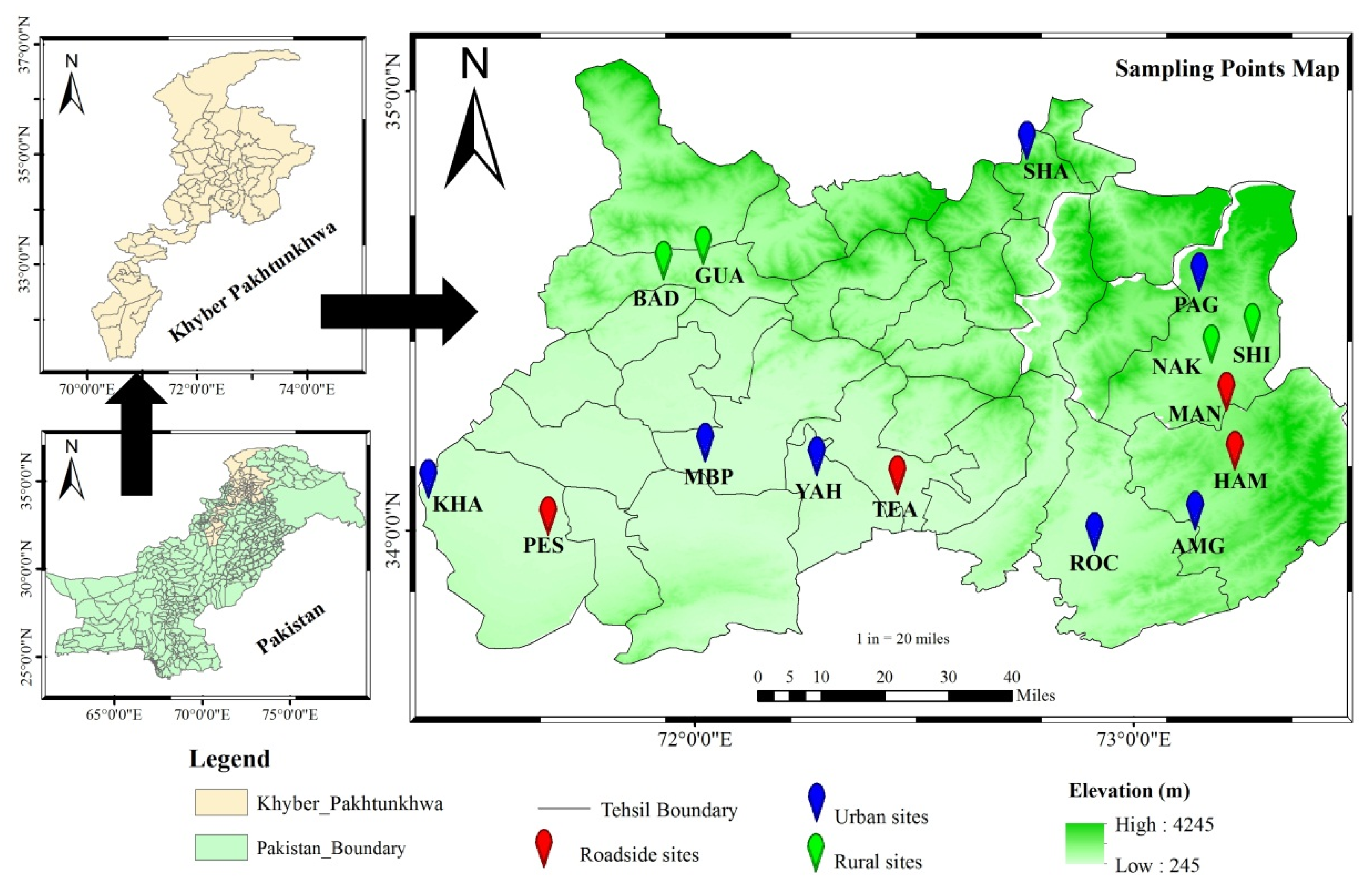
1. Introduction
Heavy metal (HM) pollution has spread across the biosphere, making it a serious worldwide concern because of its risk to humans and animals [
1,
2]. Various anthropogenic activities are mostly related to urban–rural gradients and industrialization, such as mining, application of fertilizers, smelting and industrial manufacturing processes, which can lead to the release of the HMs into the environment [
3,
4,
5]. HMs are a serious matter of concern as these elements are non-biodegradable and persist in the soil for a much longer time than in other parts of the environment [
6,
7]. High concentrations of toxic metals, including Zn, Cu, and Cd are mostly due to human activities [
8,
9]. Metal contamination in the environment would affect the diversity, microbial properties, and functional diversity of soils [
10,
11]. Moreover, increased concentration of metal in the soils can be transferred and deposited into plants and likewise to human beings, producing numerous serious health disorders [
6,
12].
Numerous tools for remediation of contaminated soils have been undertaken, such as chemical/physical treatment and excavation of contaminated material. However, significant efforts have been made lately to identify cost-effective solutions for remediation of HMs-contaminated soil [
13,
14,
15]. Recently, these cost-effective approaches, such as phytoremediation, have become a subject of public and scientific interest to alleviate HM pollutants from the soil, water, and air [
16,
17,
18]. Phytoremediation is the use of plants to remove contamination from the soil, sediments, and water [
19]. Metal hyperaccumulator plant species have been reported, but they are slow-growing, less abundant, and produce little biomass [
20,
21]. Phytoremediation treatments may be more successful if native plant species can overcome these hurdles. Native plants are frequently efficient in growth, reproduction, and survival when faced with challenging circumstances [
22,
23]. Therefore, the search for native plants with phytoremediation potential is important and continues [
24,
25].
A few natural, well-adapted plants have been utilized extensively for HM phytoremediation applications, including lemongrass (vetiver), Siam weed, wild grasses, Crotalaria, Avena, and Sesbania species [
26,
27,
28].
C. procera, a traditional medicinal plant that may accumulate considerable amounts of HMs [
29,
30], is a dominating species in Khyber Pakhtunkhwa, and has been evaluated for its phytoremediation potential in this study. Furthermore,
C. procera was chosen as a HMs pollution biomonitor for a variety of reasons: it may be found on the roadside, in urban and rural regions; it has a wide geographical range and ecological distribution; and sampling, identification, and cultivation are simple and inexpensive [
29,
31].
The World Health Organization (WHO) and the United States Environmental Protection Agency (USEPA) have recognized strict criteria for maximum acceptable limits for accumulation of HMs in soil. Toxicant levels in urban soils have been closely monitored and extensively recorded in developed nations, such as North America and Europe [
32,
33], Still, HM pollution and remediation data are critically deficient in underdeveloped countries. The lack of funds for other treatments makes phytoremediation especially well-suited to these developing nations, where labour, knowledge, and cost-cutting measures are crucial [
31]. This study was carried out to fill the gap by presenting a thorough picture of HM contamination in the semi-arid region of Khyber Pakhtunkhwa, as well as identifying prospective candidate species for effective, practical phytoremediation. The present study aims to investigate the toxic levels of Zn, Pb, Cu, and Cd using
C. procera L. as a bioindicator; and to evaluate the extent of metal accumulation potential of
C. procera in Khyber Pakhtunkhwa, Pakistan.
3. Results
The phytoaccumulation potential of
C. procera with soil physiochemical properties showed a significant variation among the studied habitats (
Table 1 and
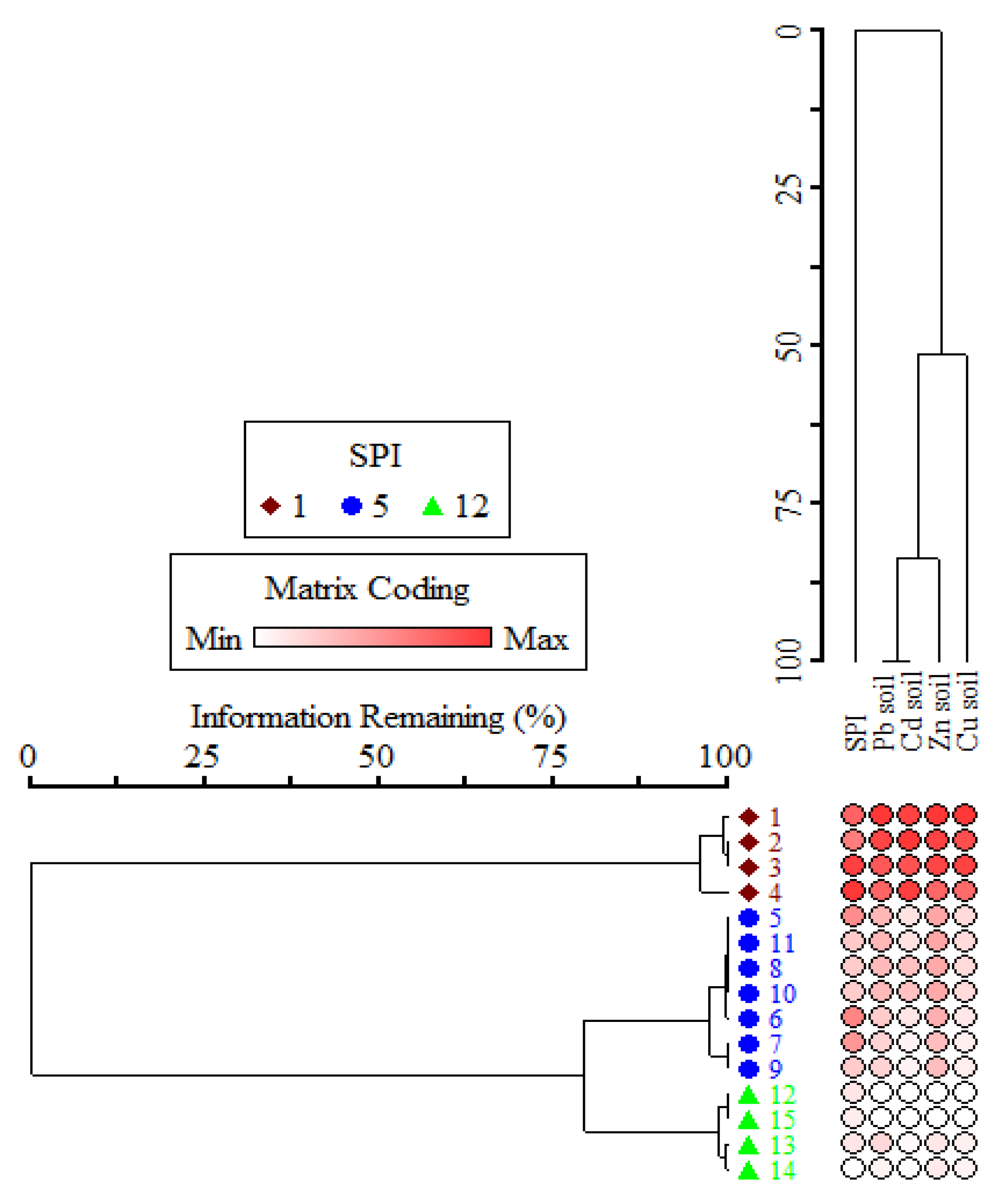
Table 2). The two-way cluster analysis segregated the studied sites into three distinct habitats (roadside, urban, and rural) based on the soil pollution index (
Figure 2), i.e., the roadside areas had four stands (PES, MBP, TEA, and HAM) with high cumulative percentage (30.42), and the urban industrial sites (MAN, AMG, ROC, YAH, KHA, SHA, and PAG) had comparatively low cumulative value followed by the rural areas (NAK, SHI, GUA, BAD) with the lowest cumulative variance i.e., 29.71.
The sites showed a significant variation in soil pollution index (SPI) summarized in
Table 1. The ANOVA results showed significant difference (
F-value = 6.82;
p < 0.001), with marked differences between roadsides and urban areas and less variation between urban and rural areas. SPI at roadside and residential areas were comparatively higher than rural sites and ranged between 1.83 ± 0.2–1.12 ± 0.14 mg kg
−1 (
Table 1).
Soil physiochemical characteristics and diversity indices correlated with the HM contaminated sites are presented in
Table 2. The soil texture shows a sandy texture with higher percentage of silt and clay. The pH for soil samples was slightly acidic to neutral ranging from 6.60 ± 0.18 to 6.925 ± 0.110. Organic matter content was quite low (roadside = 0.710 ± 0.12; urban = 1.188 ± 0.19; rural = 0.719 ± 0.149) in all sites tested as the plant prefers to grow in desert type soils having high sand contents. The sample soils of roadside sites had a higher lime concentration (8.96 ± 0.77), followed by rural and urban areas. The lowest mean value of total bioavailable nitrogen was found in rural areas (0.060 ± 0.02), while the highest mean value of total nitrogen was identified in urban areas (0.142 ± 0.03). The results demonstrate that the nitrogen content in all habitats is higher, except rural areas, which has a low nitrogen concentration. Phosphorous content (mg kg
−1) of soil in all the habitats was low. Potassium content was 97.72 ± 11.89 (mg kg
−1) in the soil of rural areas, which is higher than the soil of all other habitats.
Table 2 shows diversity indices, origin, and sampling sites of
C. procera reported from 15 different sites of Khyber Pakhtunkhwa, Pakistan. The plant was found in patches with lower species diversity and evenness.
Carthamus lanatus,
Cynodon dactylon,
Xanthium stramonium, and
Silybum marianum were the dominant species of the plant communities in the investigated areas. The Shannon diversity index was found to be highest for roadsides (2.13 ± 0.06). The lowest Shannon diversity index was found for rural areas (1.38 ± 0.00). Species diversity (H′), richness (Rs), and evenness (Eh) indices showed significant differences between the three habitats. Diversity within communities decreased with the increase in metal concentration in the soil, which is directly linked with species richness and Shannon diversity index. The diversity indices follow the order of roadside > urban areas > rural areas as presented in
Table 2.
The HMs content of soil and
C. procera are presented in
Table 3, which shows the roadside areas having the highest concentrations of HMs followed by urban site compared to rural sites. The Zn and Pb concentrations in soil were within the range of permissible levels. However, the average content of Cd in soil ranged from 3.69 ± 0.05 to 0.90 ± 0.02 which was two times higher than the permissible value (
Table 3). The highest concentration of Zn in plant (103.70 ± 3.19) was observed in roadside areas having significant differences from urban and rural site (
p < 0.001). The average Cu concentration ranged from 23.45 ± 0.60 to 4.23 ± 0.66; Zn from 103.70 ± 3.19 to 26.15 ± 1.85; Pb from 19.45 ± 0.68 to 3.29 ± 0.50; and Cd from 2.275 ± 0.15 to 1.46 ± 0.08, respectively. The statistical analysis results in
Table 3 shows that there is road to rural gradients and that the concentration differences are significant and influence the HMs concentrations, especially for Cu, Zn, and Cd.
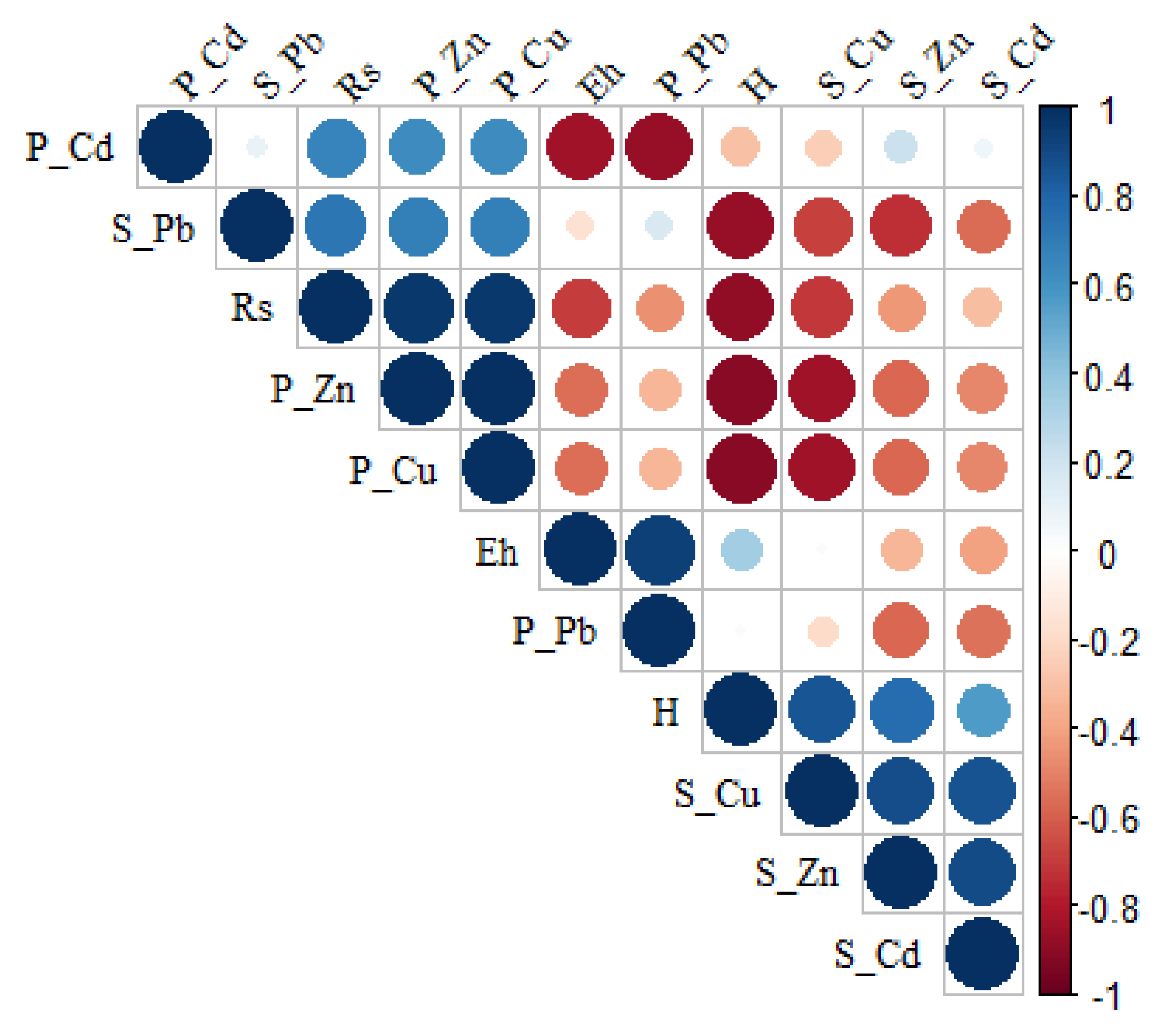
A correlation plot (
Figure 3) was used for studying the association between species diversity indices and HM accumulation in soil and plant. The colour represents the degree of pairwise correlation with regards to the Pearson correlation coefficient. Diversity indices exhibited substantial negative associations with HMs in soil and plants, according to the Pearson correlation coefficient (
Figure 3). Evenness index was negatively associated with Cu and Zn content in soil, but species richness was positively correlated with Cu and Zn content in the soil. The accumulation of HMs has an impact on the plant density in the community. Plant species diversity demonstrated a substantial positive correlation with metals.
Phytoremediation potential of plants for Pb, Zn, Cu, and Cd was indicated by a bioaccumulation factor (BAF) from soil to plant, and translocation factor (TF) from soil to plant organs presented in
Table 4 and
Table 5. The BAF in roadside areas was higher in root than the whole plant indicating that metal is retained in the root compared to its aerial parts. Simultaneously, the reverse is true for urban and control areas, which may be due to low metal concentration allowing for plant growth and favouring the transfer of metal to aerial portions, thus increasing the BAF. The BAF value for the root of plants growing on the roadside sites was more than 1, indicating its hyper accumulation ability and can be used efficiently in phytoremediation operations particularly for Zn and Cu. The BAF for Pb was found to be more than 1 in roadside areas for root, which suggested that
C. procera can absorb and accumulate Pb (
Table 4).
The translocation factor (TF) is an important indicator for the transportation of metal in living tissues, from root to stem (R-S), stem to leaves (S-L), and from leaves to flowers (L-F) of a plant body.
Table 5 presents the TF of Zn, Pb, Cu, and Cd in different habitats. Translocation factors for roadside areas and urban areas show a similar pattern in all the studied sites. The TF value was less than 1.00 for R-S, and greater than 1.00 from S-L. Pb TF values for R-S and S-L were found to be higher than 1 in roadside areas. The TF values in most of the sites was more than 1, indicating the plant’s efficient ability to transport metal in the upper parts of the plant. TF for Zn is R-S ≥ to S-L > L-F. Zn translocation is decreased in floral parts so that floral parts may receive a lower quantity of Zn compared to Pb and Cu and Cd. The highest Cu translocation was from R-S followed by the S-L, and less translocation is for L-F. Cd was found to be less mobile compared to Pb, Zn and Cu. Cd translocation was found to be higher for L-F. The ANOVA result was significant, indicating significant differences in translocation among sites and plant parts. In many cases, the translocation factor was more than 1, indicating the ability of
C. procera to accumulate and translocate high quantities of metal and potentially be used in phytoremediation practices.
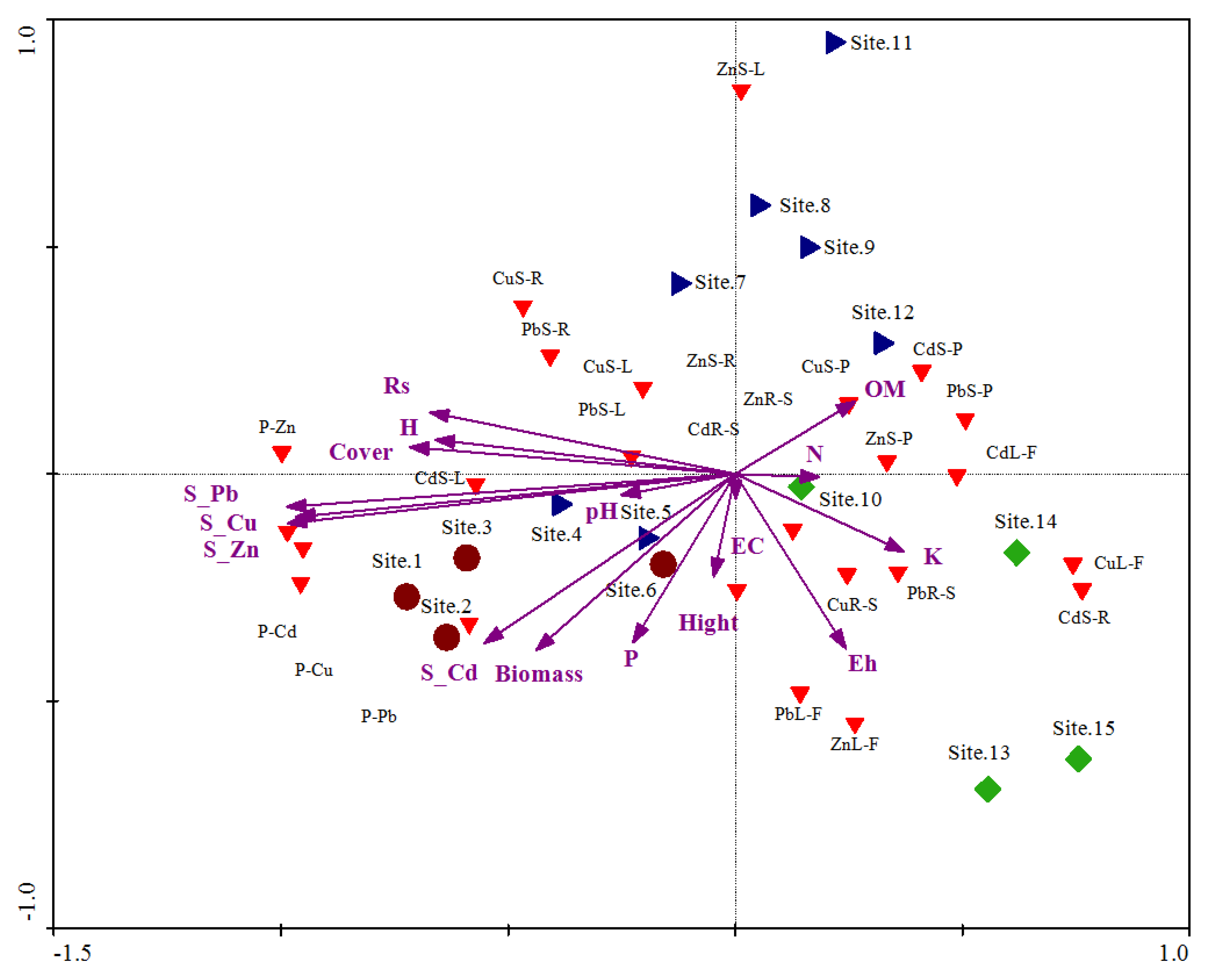
Principal component analysis (PCA) shows the relationship of plant–soil HM concentration, bioaccumulation, and translocation with soil physiochemical properties (
Figure 4). The PCA biplot of samples and environmental data show a distinct scattering that was found to be impacted mostly by metal concentration of soil (Zn, Pb, and Cu), soil texture (clay, silt, and sand), organic matter, electrical conductivity, biomass, cover, and available water. Roadside sites are present at the bottom left side of plot and rural areas lie at the bottom right side, while urban areas are present between roadside areas and rural areas.
The first quadrant (on bottom left) of the PCA biplot indicated that roadside areas were mostly gathered under the influence of metal concentrations of soil, biomass, phosphorous, pH, and height of plants, while in the second quadrants (on bottom right), most of the environmental variables are clustered around electrical conductivity, potassium, and evenness index. Furthermore, the third and fourth quadrants of the PCA biplot elaborated that urban areas were assembled under the influence of diversity indices, nitrogen, organic matter, and cover of plants (
Figure 4). The percent cumulative variance from the PCA shows 100% of the variance.
Table 6 shows the variance extracted from the first 14 Axis where the Axis 1 shows 31.375% of the total variance, which gradually decreased to 0.332 for Axis 14.
HM accumulation in the soils and plant were compared with other studies conducted in Pakistan and other countries with guideline values. Cu and Cd concentrations in soil (15.24, 3.69) of roadside areas were higher due to vehicular emissions as compared to urban and rural areas. The result of the present work in comparison with Egypt [
29] except the railway station, the thermal power plant area in India [
46], and Riyadh and Ghaza [
9], showing higher concentrations of HMs in soil and
C. procera. Compared to Pakistan [
47], Qatar [
30], and India [
31], all HM concentrations in addition to Pb and Zn (6.08, 32.18) were higher in the roadside areas. Similarly, Cd concentrations were higher compared to those found in roadside soil of India. Cu concentrations of the present study were less than those of the study conducted in Qatar, India and the study conducted in Egypt (
Table 7), indicating that soils in Pakistan were less contaminated by Cu as compared to other countries but highly contaminated as compared to the WHO reference range.
The high concentration of these metals reported in Egyptian railways may be due to wastes generated during train maintenance. This explains why the concentrations of metals analyzed were somewhat higher than those observed in prior research from Egypt and other countries. This is because serious metal pollution has been recorded from several metal processing sections i.e., battery manufacturing and smelting sectors. The mean Pb and Zn contents of the current study (6.08 and 32.18, respectively) were lower than the permissible level (
Table 7).
4. Discussion
The soil pollution index (SPI) is considered a key determinant of HMS contamination [
53,
54], and its value > 1 indicates a higher health risk [
55]. Our results revealed that SPIs of roadside areas and residential areas range between 1.83 ± 0.27 and 1.12 ± 0.14 (
Table 1), showing that the site was highly polluted. Similarly, Bose et al. (2008) and Alyemeni and Almohisen (2014) [
56,
57] reported HM pollution in industrial and residential areas, and densely populated commercial districts. Likewise, elevated Pb, Cd, Zn, and Cu were identified in soil and
C. procera vegetation along roadside areas and urban areas in the current investigation, which might be attributed to traffic congestion and fast urbanization. Many researchers, for example, Apeagyei et al. [
58], Modrzewska and Wyszkowski [
59], Soltani et al. [
60], and Trujillo-Gonzalez et al. [
61], documented increasing HM contamination near roadways and urban areas. The increasing HMs pollution along roadways originates from various causes, including wear and tear of automotive tires, metals in catalysts, deterioration of different parts, and paint erosion [
62,
63]. Furthermore, uncontrolled migration from rural to urban regions has resulted in a rapid increase in industrial and home effluents, including HMs [
62].
In the current investigation, HM pollution was identified to spread from various sources, e.g., in District Peshawar, the industrial zones at various sample sites added pollutants. Moreover, toy manufacturers and sugar mills are the principal HM pollutant sources in District Mardan. Furthermore, one of the primary sources of HMs in the Malakand region is dust particles produced from crushed plant industries. Our findings revealed substantial changes in Pb, Zn, Cu, and Cd contents in plant components from different habitats. The order of metal contents being root > stem > leaves > flower. Cd and Cu concentrations were found to be above the [
64] permissible limit in all soil samples, but Pb and Zn concentrations were found to be within the acceptable range values.
HMs bioavailability is influenced by external (soil-related) and internal (plant-related) factors and is responsible for the plant’s ability to absorb HMs [
65]. In external factors, soil texture of the studied groups shows a sandy texture, having low organic matter content, favouring bioavailability of HMs [
66]. In addition, HMs may form complexes with organic matter, limiting their bioavailability. Organic matter content is known to impact metal bioavailability [
67]. The pH varied from 6.60 ± 0.18 to 6.92 ± 0.110 for soil samples that were slightly acidic to neutral. The results obtained were compared to previous studies, which indicated that soil pH significantly impacted metal intake and solubility [
68,
69]. The electrical conductivity ranging from 256.85 ± 13.56 to 276.68 ± 35.84 µS/cm indicates a normal availability of metal in soil. These findings contrast to the data reported by Ullah and Khan [
34].
It is reported by Byrne et al. [
70] that HMs concentration negatively affects the diversity and abundance of species in an area. Similarly, soil metal concentration directly impacts species richness and the Shannon diversity index, impacting plant diversity [
11]. The current results revealed that a community’s species richness and Shannon diversity index decreases with increased HM contents and substantially correlates. After long-term exposure of plant communities to HM-contaminated soil, plants may develop a mechanism to prevent or minimize the absorption of HMs [
71]. In contrast, soil Cd and Cu levels revealed a favourable relationship with species diversity. Cu in the soil is crucial in controlling plant community species diversity [
72].
The results in
Table 3 show that the urban to rural gradients could significantly influence the HMs concentrations, especially for Cu, Zn, and Cd. Trends of decreasing HM accumulation with urban to rural gradients were also noted by Pouyat and Mcdonnell [
64] in a study of HM accumulation in forest soil. In the present work, similar trends were observed in the Cu, Cd, Pb, and Zn concentrations, suggesting that these pollutants accumulated highly in roadside areas compared to rural areas. In rural areas, a high concentration of metals is attributed to the fact that these areas become a wasteland where
C. procera grows abundantly. The HMs have been recorded in the order: roadside areas > residential areas > rural areas. In the present study, Zn contents in
C. procera samples ranged from 103.70 mg kg
−1 in roadside areas to 26.15 mg kg
−1 in rural areas. Allen [
73] found Zn concentrations in vascular plants between 15 and 100 mg kg
−1, with 230 mg kg
−1 being toxic. In contrast, Ghaderian and Ravandi [
74] revealed that Zn concentrations in vascular plants ranged from 10 to 200 mg kg
−1, with Zn essential plants [
75]. In the unpolluted region, the zinc level in the upper horizon was 9 mg kg
−1, whereas in the contaminated area, the zinc content averaged 71 mg kg
−1 as reported by Degryse and Smolders [
76]. Zn concentration in leaves of
C. procera was shown to be safe in both rural and urban areas.
Cu is an essential element for photosynthesis and the metabolism of protein and carbohydrates [
77]. The soil Cu concentrations ranged from 15.24 ± 0.96–3.82 ± 0.37 mg kg
−1 at various locations and plant components, whereas the maximum permissible level for Cu concentration is 10 mg kg
−1 in plants [
78]. Consequently, the plants collected from the roadside were regarded as Cu-toxic. For photosynthesis and protein and carbohydrate metabolism, Cu is needed [
77]. There may be harmful implications if copper levels in shoots or leaves exceed 20 mg kg
−1 [
75]. In biochemical methods,
C. procera roots were used as a biosorbent to extract Cu from solutions with known amounts of the metal [
79].
Pb has no benefit in plant nutrition and is found in plant tissues at concentrations of 1–10 mg kg
−1 [
74]. Toy manufacturing, printing and the oil industry produce contaminated water and exhaust gases, which contain Pb. Pb concentrations greater than 5 mg kg
−1 are considered toxic to plants [
8]. Pb levels in
C. procera plants from roadside areas (19.45 ± 0.68) and urban areas (10.36 ± 0.89) were toxic, while rural areas (3.29 ± 0.50) were normal. It might be because Pb is mainly stationary in soil, lowering its bioavailability and subsequent plant absorption [
80].
The concentration of Cd in the soil of different sites and plant parts was found in the range of 3.69 ± 0.05 to 0.90 ± 0.02 mg kg
−1 for road and rural areas and 2.275 ± 0.15–1.46 ± 0.08 mg kg
−1 for roadside and rural areas in plants, respectively. Furthermore, Cd is a hazardous element that occurs naturally, accompanying Zn. Cd is less readily absorbed by soil and organic matter, making it more readily available to plants [
81]. Metal manufacturing, plastics, household wastes, automobile fossil fuels, and sewage are the most significant sources of Cd contamination. Plants in an unpolluted environment contain 0.01–0.30 mg kg
−1 Cd [
73]; hence, Cd concentrations in all tested environments were in the phytotoxic range.
Table 4 and
Table 5 show the phytoremediation potential of Pb, Zn, Cu, and Cd using the BAF from soil to plant and the TF in plant parts. The BAF can assess the plants ability to take HMs from soil; however, it is not a precise estimate of HMs concentrations [
82,
83,
84]. According to Sheoran et al. [
85], if the BAF is <1, plants cannot be used for metal phytoextraction. Plants with a BAF value of <1 are not appropriate for metal phytoextraction [
86]. Cd > Zn > Cu > Pb was the order of BAF for HMs by
C. procera, which was similar to that reported by Al-Farraj and Al-Wabel [
49] and Galal and Shehata [
87] on
Plantago major L. Zu et al. [
88] showed that BAF >1 could suggest plant capacities for metal accumulation. BAF > 1 was recorded in
C. procera plant tissues (
Table 4). Similarly, D’Souza et al. [
31] observed accumulation ratios under natural conditions and showed that
C. procera has a robust phytoextraction potential. Pb and Cd accumulation in leaves was greater than in roots in the
C. procera plant samples, suggesting that metals were more heavily allocated to leaves. Waleed [
89] reported that
C. procera is one of the most useful bioindicators for monitoring pollution levels in Mn, Cr, and Zn polluted areas.
The BAF in roadside areas was higher than 1 in root than the whole plant indicating that metal is retained in roots compared to aerial parts. Significant anthropogenic activities were present in the roadside sample, which may have contributed to the high metal content around the soil and plants. Simultaneously, the opposite is true for residential and control areas, which may be the cause of low metal concentrations, which would allow plant growth and favour metal transfer to aerial portions, thus increasing the transfer factor. The BAF was >1 at roadside areas, which indicates that
C. procera can absorb and accumulate these metals [
74]. Among the studied areas (road, urban, and rural), the Cu content range of BAF (BAF > 1) was significantly higher at roadside areas, which show the phytotoxicity of this metal. It was expected that this metal has a high concentration in soil because Cu is essential for plant growth [
56,
90]. BAF of Pb was less than one in roots, which suggested that Pb’s bioavailability is <1, and the plant can only absorb but not accumulate it. Pb concentration shows a significant accumulation of BAF between the root and the whole plant. The BAF for Cd in the studied areas was found to be less than 1.0 (
Table 4), which suggests that
C. procera can absorb and accumulate Cd but less efficiently than Pb, Zn, and Cu. The results reveal that the roots of plants tend to accumulate more Cd than the stems and leaves as was reported by [
91]. Galal and Shehata [
87] found that roadside plants had significant bioaccumulation of Cd. Cd uptake may have been linked to organic matter [
43,
92] and soil pH [
87].
The TF is important in relocating metal in living tissues, from root to stem, stem to leaf, and from leaf to flower of a plant body. TFs for roadside and residential areas show a similar pattern at all the studied sites. The TF was <1 from root to stem and >1 from stem to leaf [
90]. The results indicate that these metals are transferred marginally to the stem from the root zones but easily mobilize to the leaf if available in the stem [
93]. The Pb TF from root to stem and leaf was >1 at roadside areas. The translocation factor in most of the sites was >1, indicating the plant’s ability to efficiently transport metal in the upper parts of the plant. The Zn TF order is the root to stem ≥ stem to leaf > leaf to flower. Zn translocation decreases in floral parts so that floral parts may receive a lower quantity of Zn than Pb and Cu and more Cd. The highest Cu translocation was from root to stem, followed by the stem to leaves, and less translocation from leaf to flowers. Cd was less mobile compared to Pb, Zn, and Cu. Cd translocation was found to be higher from leaves to flowers. Only plant species with a BAF and TF value of more than one have the potential to be employed for metal phytoextraction, according to Yoon et al. [
23].
Many studies in Pakistan, e.g., [
47] and abroad, e.g., [
9,
29,
48], have shown similar patterns of HMs content in soils and
C. procera plant parts. Compared to these reports, minor changes in mean concentrations were found due to traffic intensity, industry type, urbanization level, and residential sewage. The quantities of HMs in the soil and soil parameters, such as soil texture, pH, and organic matter, influence the concentration of HMs in plants [
94].