Abstract
Herein, we disclosed a method of preparing a nanocatalyst containing nickel-cobalt by impregnation in a hydrochloric acid medium. Optimal conditions were established for all stages of nanocatalyst preparation using the method of probabilistic–deterministic experiment planning: hydrochloric acid concentration—1 mol/L, ratio of HCl concentration to nickel concentration—20, ratio of HCl concentration to cobalt concentration—20. The method of planning the experiment of preparing a nanocatalyst allows varying all the factors simultaneously and obtaining quantitative estimates of the main effects and effects of interaction, as well as establishing the dominant factors affecting the activity and selectivity of the nanocatalyst during the hydrogenation of benzothiophene. The multifactorial equation was obtained, which allowed us to calculate the optimal manufacturing parameters of the nanocatalyst, providing high activity and selectivity during the hydrogenation of benzothiophene. In the proposed nanocatalyst, a readily available natural chrysotile–asbestos with a nanotube diameter of 60–75 nm was used as a carrier for the benzothiophene hydrogenation process.
1. Introduction
The role of the hydrogenation processes has increased in modern oil refining. The main interest in the conversion of sulfur compounds is largely determined not so much by the problems of oil refining, coal chemistry, and synthetic liquid fuel, but by the rapid development of hydrotreating processes of petroleum fuel from sulfur compounds. The need to develop these processes, in turn, was due to the fact that it was necessary to prevent the release of sulfur oxides when burning fuels in engines and boiler plants, as well as the fact that the presence of sulfur compounds in motor fuels accelerates corrosion and wear of engines and reduces their power. In addition, sulfurous compounds accelerate precipitation during the storage of petroleum fuels, which depends on the amount of sulfur bonding with aliphatic carbon [1,2,3,4].
Increasing the demand for motor fuels, tightening the requirements imposed on them, and involving raw materials of various compositions in the reprocessing procedure require new approaches to the synthesis of new catalysts. In the hydrogenation process of the heavy hydrocarbon feedstock, a critical role is played by the catalyst support, since it serves a significant purpose in the activity and stability of the catalyst, and therefore the selection of a suitable, inexpensive, and readily available supporter is crucial for the active catalyst preparation. Catalytic activity can be regulated by controlling the particle size, composition, shape, and nature of metal interaction with the supports such as Al2O3, MgO, TiO2, zeolite, silica, carbon, and mesoporous materials [5]. With the development of material science, clay mineral materials, which play a significant role in scientific research, have gained universal recognition, thanks to which they could possibly be used as cheap substitutes for expensive carbon nanotubes (CNT) or other nanomaterials [6,7]. Chrysotile is one of most widely used nanostructured silicates and can be easily found in nature. The use of chrysotile asbestos as a carrier for the hydrogenation of heavy oil residues, oil sludge, and coal tar to produce motor fuel is of important theoretical and practical significance. The chrysotile nanotubes have a purely surface structure, which allows them to be considered as the most suitable object for physical sorption. Literature analysis has shown that chrysotile is not used as a support for heterogeneous catalysts in the hydrogenation of polycyclic aromatic hydrocarbons. However, ref. [8] showed the hydrogenation of 4-nitrophenol in the presence of a AgNPs/SiO2NWs nanocatalyst, where leached chrysotile acts as a support. Thanks to the developed surface and oriented packaging of nanotubes with hydrophilic properties, the chrysotile has a high sorption ability [9,10,11].
In oil refining, special focus is given to the conversion of organosulfur compounds, which are part of heavy hydrocarbon raw materials, which reduce the quality of obtained motor fuels [12]. The oil stock contains the organosulfur compounds of various reactivities in catalytic processes. Hydrogenation and hydrogenolysis constitute one of the primary directions of the conversion of organosulfur compounds in the hydrocatalytic process [13,14].
In this study, experiments were performed on the hydrogenation of an individual compound—benzothiophene, which allows the simulation of the direction and nature of transformations in hydrocatalytic processes (hydrotreating, hydrocracking) of components of heavy oil fractions and the assessment of the activity and selectivity of chrysotile-based catalysts with supported active metals (Ni, Co).
The purpose of the work was to establish optimal conditions for nanocatalyst preparation based on chrysotile with supported metals (Ni, Co) for the hydrogenation of benzothiophene.
2. Materials and Methods
Among of the main factors affecting hydrogenation of hydrocarbon feedstocks is the nature of the catalyst. Optimal catalyst compositions would allow the process to be carried out under milder conditions and improve the organic conversion of the feedstock and the desired products’ quality.
Classic catalysts for hydrogenation processes for processing hydrocarbons are made on the basis of nickel and cobalt salts [15,16]. This study proposed a binary composition of the hydrogenation catalyst based on Co and Ni, where the silicate chrysotile–asbestos was used as the support. The support has a developed specific surface area: it consists of the thinnest flexible fibers and has a porous structure—this allows it to adsorb and tightly hold atoms inside the pores, and provides high activity in hydrogenation reactions.
The catalyst was prepared as follows: the support was impregnated with solutions of active metal chlorides. The salts were prepared by dissolving metal oxides in various concentrations of hydrochloric acid (0.2 M, 0.6 M, and 1 M). Metal oxides dissolve well in hydrochloric acid. The resulting solutions of CoCl3 and NiCl2 salts were then impregnated with chrysotile. The mixture was heated in a water bath for 2 h and then washed with water until neutral. The separated precipitate was dried in a drying oven at 105 °C and then calcined in a muffle furnace at 600 °C for 60 min. During the treatment of the support with acid, magnesium was washed out (to replace it with metals) and washed from various impurities, which positively affects the activity of the catalyst. Previously, ref. [17] showed the preparation of a nanocatalyst by depositing solutions of cobalt nitrate and nickel nitrate on chrysotile. In this work, metals were simultaneously applied to the surface of chrysotile and magnesium leaching from chrysotile, while in the previous paper [17] the process of obtaining a nanocatalyst was carried out in two stages: in the first stage, magnesium was leached from chrysotile; in the second stage, metal salt solutions were applied on the chrysotile’s surface. Photospectrometric examination of the filtrate showed almost complete adsorption of metals on the support surface, 83–89%.
Salts of Co and Ni applied on the silicate chrysotile–asbestos were used as a catalytic additive. The procedure for preparation and characteristics of the catalysts are given in ref. [17]. The catalysts thus prepared contain metals dispersed on the support surface in the form of small crystals of various sizes which are very stable and have high activity. Catalysts were tested in the hydrogenation reaction of a heteroaromatic compound (benzothiophene), modeling the composition of a heavy hydrocarbon feedstock.
The number of elements on the nanocatalyst surface was determined by X-ray fluorescence analysis obtained in air at 600 μA in a FOKUS-2M spectrometer equipped with a molybdenum anode X-ray tube at 30 kV. The results of the elemental chemical composition of the nanocatalyst are presented in Table 1.

Table 1.
Elemental chemical composition of the nanocatalyst.
The use of the X-ray fluorescence analysis method to quantify metals in a nanocatalyst showed that the concentration of metals in the sample was set only locally, not in the whole sample. This is due to the fact that the electron beam does not capture the entire area of the sample. Therefore, to determine the quantitative composition of metals, we used the method of atomic emission spectroscopy. The composition of nickel, cobalt, and silicon oxide was determined on a Profile Plus inductively coupled plasma atomic emission spectrometer at a temperature of 21 °C and an atmospheric pressure of 727 mmHg. The quantitative composition of nickel and cobalt in the NiCo/chrysotile nanocatalyst is shown in Table 2.

Table 2.
Amount of nickel and cobalt supported on chrysotile.
Micrographs of the surface morphology of leached chrysotile nanotubes and nickel and the cobalt-supported nanocatalyst were obtained using a scanning electron microscope MIRA3 TESCAN.
Micrographs of surface morphology of leached chrysotile and the prepared NiCo/chrysotile nanocatalyst are shown in Figure 1a,b.
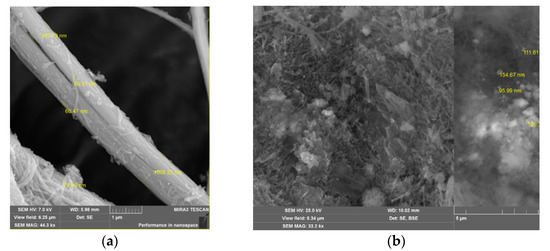
Figure 1.
Microphotography of SEM of leached chrysotile and the NiCo/chrysotile nanocatalyst: (a) leached chrysotile; (b) NiCo/Chrysotile.
Figure 1a shows that the chrysotile nanotubes were wound on top of each other and formed a fibrous structure. Micrograph studies showed that the diameter of chrysotile nanotubes was 60–75 nm, and the diameter of the fiber consisting of long-twisted and combined nanotubes was about 1 μm.
It was observed that the deposition of metal nanoparticles on the surface of leached chrysotile by wet mixing led to the opening of the fibrous structure (Figure 1b). It was noticed that the diameter of the deposited particles was 70–120 nm.
According to the general rules of catalyst theory, catalyst activity is determined by specific surface area. The high specific surface area depends on the micro-, meso-, and macropores’ presence in the catalyst structure.
The Brunauer–Emmett–Teller (BET) method was employed to characterize the porous structure of the leached chrysotile and NiCo/chrysotile nanocatalyst samples. Using the BET method based on low-temperature (77 K) nitrogen adsorption isotherms (Figure 2a,b) obtained with SoftSorbi-II ver.1.0 No. 843, the specific surface area and pore volume of the samples were calculated.
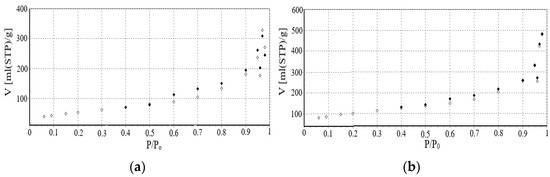
Figure 2.
Isotherms of leached chrysotile adsorption and NiCo/chrysotile nanocatalyst: (a) leached chrysotile; (b) NiCo Nano catalyst/chrysotile.
From the nitrogen adsorption isotherm of leached chrysotile and NiCo/chrysotile nanocatalyst obtained in the range of relative partial pressures P/P0 = 0.05−1, it is difficult to determine the nature of the pores. However, according to de Boer’s classification, it resembles a hysteresis loop of type H3 [18], which means that the pores are considered mainly hole-like. The catalyst support (chrysotile) is an adsorbent structured material since the pores are nanotubes, channels, and cavities in the solid matrix. Table 3 shows the specific surface area and the specific pore volume of the leached chrysotile and the NiCo/chrysotile nanocatalyst.

Table 3.
Porosity characteristics of the leached chrysotile and the NiCo/chrysotile nanocatalyst.
The porosity of the leached chrysotile was also relatively high. High porosity is influenced by the presence of nanoscale tubes in chrysotile. Wet deposition of nickel and cobalt on the surface of chrysotile leads to a decrease in its specific surface area. The decrease in the volume of pores in the nanocatalyst from 0.121 to 0.074 cm3/g is associated with the adsorption of metals on the surface of chrysotile.
Experiments on the hydrogenation of the model compound were carried out in a high-pressure reactor (autoclave, maximum operating pressure of the reactor is 20 MPa), with the capacity of 0.05 L, with the internal stirrer manufactured by PRC. A mixture of 1 g benzothiophene and 0.01 g nanocatalyst was premixed and then placed in the high-pressure reactor. The autoclave was closed, purged with hydrogen, and given the overpressure of hydrogen (3.0 MPa). The mixture was heated to the desired temperature (400 °C) and held for 60 min. After the experiment, the autoclave was cooled to 25 °C. The individual and quantitative chemical composition of the hydrogenates was determined by of chromato-mass spectrometry using HP 5890/5972 MSD from Aguilent (USA). Identification of substances was carried out using the mass spectral database of NIST98. Chromatographic conditions: column: DB-5, 30 m × 0.25 mm × 0.5 μm. Gas: helium, 0.8 mL/min.
3. Results
To plan the experiment and determine the optimal conditions for preparing the catalysts, the method of probabilistic–deterministic planning of the experiment (PDPE) relying on the use of Latin squares was applied [19]. The use of Latin squares allows combining each of the levels of each of the factors with the rest once and only once. This ensures the equivalence of the contribution of all factors to the obtained mathematical model, and, as a consequence, the statistical reliability of the results of using the experimental plan [19].
To determine the optimal conditions of all stages of the catalyst preparation, studies were carried out at three levels.
The following factors were selected as studied: HCl concentration (mol/L) with the range of 0.2–1; the ratio of HCl concentration to nickel concentration —5–20; the ratio of HCl concentration to cobalt concentration —5–20; Vvf—1–3 (vacant factor). The variables and their levels of the variation are shown in Table 4. The criterion for evaluating the activity and selectivity of the prepared nanocatalyst is the total conversion of benzothiophene to 2,3-dihydrobenzothiophene and ethylbenzene.

Table 4.
Levels of factors studied and ranges of variation.
The experiments were carried out using the method of mathematical planning and static processing of results with the conclusion of the generalized Protodyakonov equation. The conditions of the matrix experiment are shown in Table 5.

Table 5.
Factor planning matrix at three levels.
After the experiment in accordance with the matrix (Table 5), sampling was conducted at the level and the selection of partial dependencies by the method of sequential approximation. The obtained data were approximated by the equation of the line. If the model turned out to be adequate (with a significant correlation coefficient (R > 0.66)), then it remained; in case of inadequacy, we switched to the models of higher orders of the magnitude. The studied dependence was given physical meaning.
Results from the matrix experiments were used to construct the dot plots (Table 5). The dot plots and approximation curves to determine the optimal conditions for preparing the nanocatalyst for the benzothiophene hydrogenation process are shown in Figure 3.
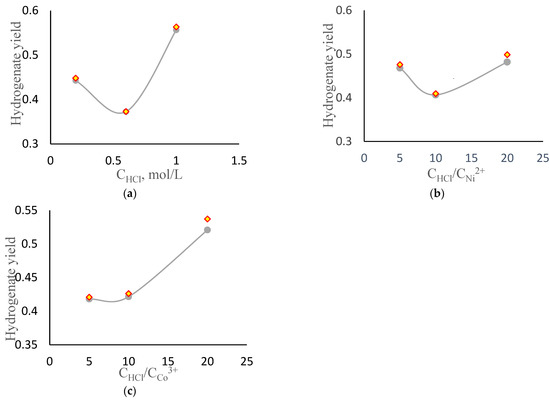
Figure 3.
Dot plots and approximation curves. Dependence of the yield of benzothiophene hydrogenation products on: (a)—HCl concentration, mol/L; (b) is the ratio of HCl concentration to nickel concentration; (c)—ratio of HCl concentration to cobalt concentration.
Each of the particular functions (Table 6) was tested for significance using the nonlinear multiple correlation coefficient (R);
and its significance for the 5% level
where N is the number of described points; K is the number of factors in effect.

Table 6.
Calculated values of the partial functions for YP.
Figure 3a–c shows that the acid concentration increases and the amount of cobalt in the composition increases, the conversion of the benzothiophene increases. This may be due to the substitution of magnesium ion by hydrogen ion, as well as an increase in the acid centers of the support. Literature analysis showed that there is no data on the effect of acid concentration on chrysotile activity during benzothiophene hydrogenation.
After the selection of partial dependencies, their calculated values at matrix levels were combined into the separate table (Table 6).
Having determined the significance of particular functions, the generalized Protodyakonov equation was used for each optimization parameter:
where —generalized function, —private function, product of all private functions, —total average of all considered values.
Using the generalized Protodyakonov Equation (4), the theoretical data were calculated, which were checked for significance by comparing the calculated results with the experimental data:
Table 7 shows the comparison of the calculated hydrogenate yield values with the actual experimental values.

Table 7.
Experimental and design values of hydrogenate yield.
Acording to the data captured in Table 7, the correlation coefficient R = 0.9849 and the significance tR = 73.48 were found using Formulas (1) and (2).
From Equation (4), the optimal catalyst preparation parameters for the highest catalyst activity were calculated:
- -
- the hydrochloric acid concentration equal to 1 M;
- -
- the ratio of HCl concentration to nickel concentration —20 (nickel weight (in terms of pure nickel) equal to 21.96% of the support weight);
- -
- the ratio of HCl concentration to cobalt concentration —20 (cobalt weight (in terms of pure cobalt) equal to 29.47% of the support weight).
The results of the reaction of the hydrogenation of benzothiophene with the catalyst prepared according to the above conditions are as follows:
- -
- hydrogenate yield (benzothiophene conversion)—69.01%;
- -
- 2,3-dihydrobenzothiophene yield—3.52%;
- -
- ethylbenzene yield—63.52%.
An increase in temperature above 400 °C led to an increase in the yield of by-products (mercaptan, diphenyl) [12]. It was experimentally found that an increase in nickel concentration did not result in an increase in ethylbenzene yield. In this case, the yield of ethylbenzene was 63.52%, which shows high activity and selectivity of the prepared nanocatalyst.
The most active catalyst for hydrogenation of benzothiophene is molybdenum catalyst [12,20]. The yields of 2,3-dihydrobenzothiophene and ethylbenzene presented in the articles are relatively comparable to our results of hydrogenation of benzothiophene in the presence of a nanocatalyst based on nanoparticles on chrysotile (Table 7).
Chromato-mass spectrometry was used to specify the individual chemical composition of the hydrogenates. The results of studying the component composition of the hydrogenate are given in Table 8.

Table 8.
Individual chemical compositions of the hydrogenates obtained under the various conditions of the benzothiophene hydrogenation process.
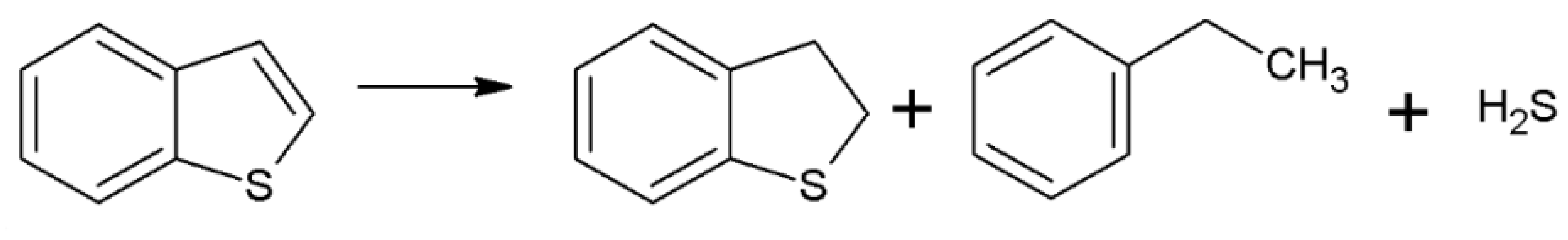
All the hydrogenates identified benzothiophene, 2,3-dihydrothiophene, ethylbenzene, fentantrene, and deep hydrogenolysis products—octane, decane, and undecane in various amounts.
Analysis of the component composition of the hydrogenates shows that for all types of the catalytic additives, the mechanism of the destructive hydrogenation was identical, with some difference in the flow rates of individual stages and directions of conversion of intermediates.
From the literature analysis [8], it is known that the mechanism of the benzothiophene dusulfurization can proceed with preliminary saturation of the aromatic structure:
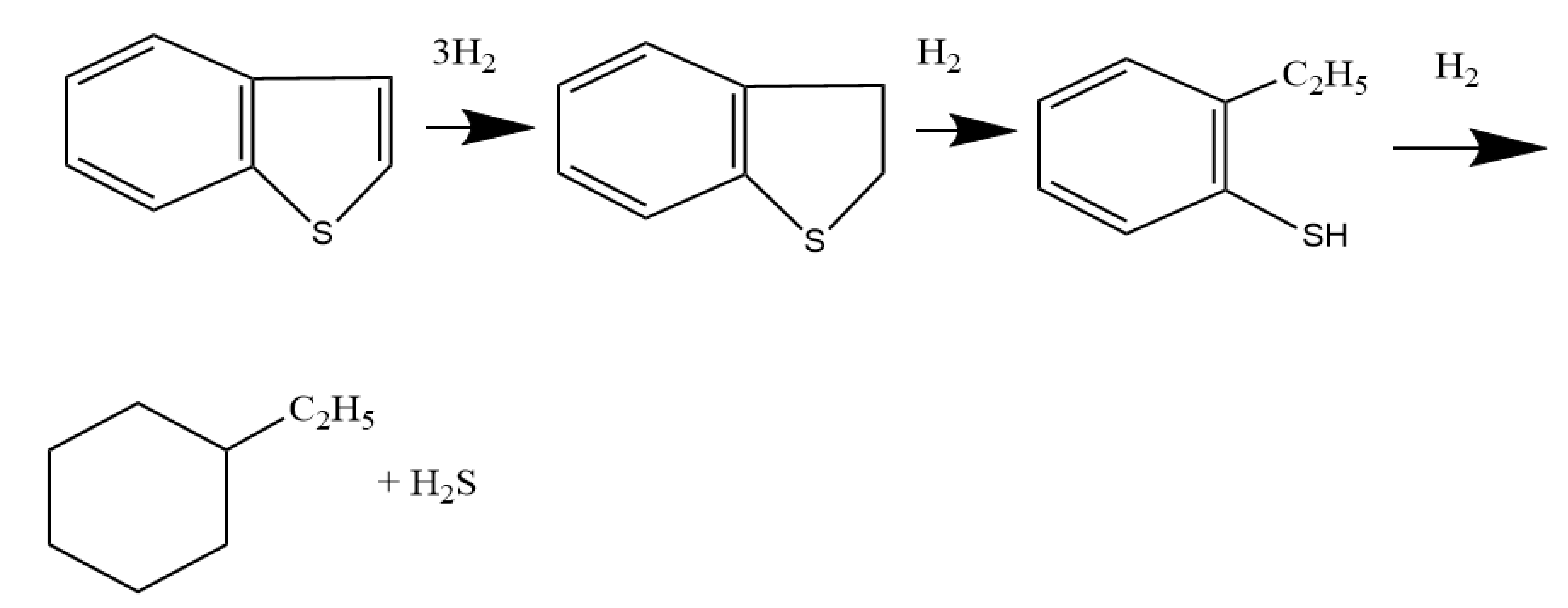
or:
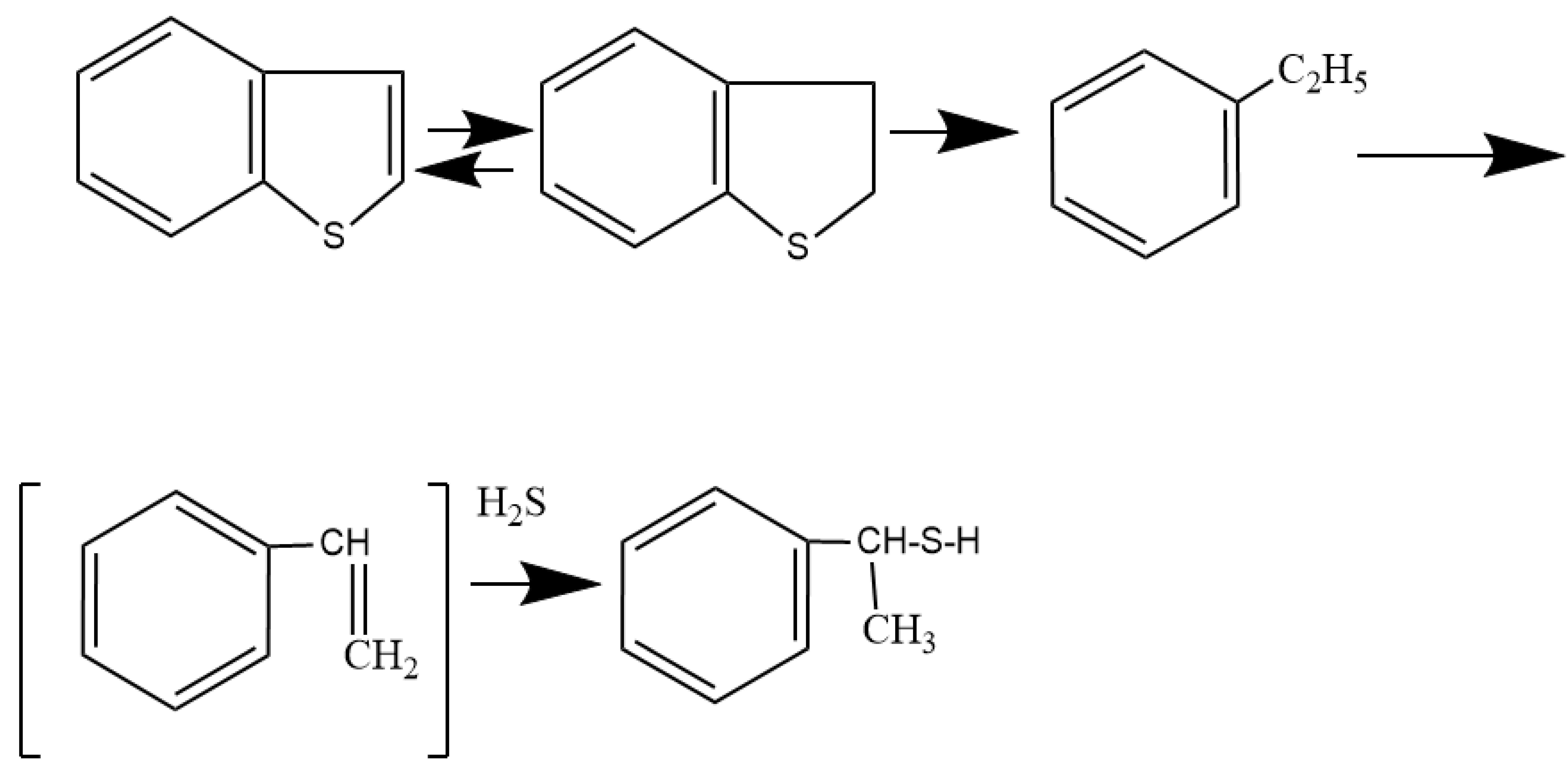
In hydrogenation processes, sulfur compounds are highly reactive. The catalyst activity was evaluated using the residual amount of the non-converted benzothiophene feedstock. The largest observation of the residual benzothiophene 63.96% was observed for the following test conditions: —0,6 mol/L, —5, —5. The minimum residual benzothiophene value of 30.99% was observed for the following test conditions: —1 mol/L, —20, —20, i.e., these conditions of the experience correspond to the maximum conversion of raw materials.
Ethylbenzene was found in all products, resulting from the deeper transformations that proceed through the reaction:
It should be noted that the ethylbenzene is the main product in the hydrogenate. It was present in an amount of 10.74–63.52% An intermediate to the formation of the ethylbenzene is 2,3-dihydrobenzothiophene, which was identified in a smaller amount in the hydrogenate products, 3.52–19.61%. The presence of this product indicates the progress of the desulfurization through preliminary hydrogenation, followed by the break of hydrogenated bonds, that is, the elimination of sulfur without preliminary hydrogenation did not occur.
The degree of the benzothiophene conversion was evaluated by the yield of the desired product (ethylbenzene) in the hydrogenate. The greatest degree of benzothiophene conversion was observed for Run 7 conditions: —1 mol/L; —20; —20. The degradation of non-aromatic sulphur compounds is easier than aromatic sulphur compounds with C–S bond breaks. The composition of the products showed that rings containing a heteroatom were hydrogenated on the catalysts, followed by a bond break.
Hydrogen sulfide generated by the hydrogenolysis also activates the hydrogenation of the benzothiophene, forming active radicals (), which are donors of active hydrogen:
Thus, as the result of the studies, the optimal conditions for the hydrogenation of benzothiophene simulating the conversion of oil and coal feedstock in the hydrotreating and the hydrocracking processes were determined. We disclosed a new composition of the catalyst system and a new catalyst with cobalt and nickel metals deposited on the silicate chrysotile–asbestos. The individual chemical composition of the hydrogenates consisting of the starting substance the benzothiophene, 2,3-dihydrobenzothiophene, and the ethylbenzene was studied.
4. Conclusions
The subject material provides the process for preparing a catalyst system comprising the following steps:
- -
- cleaning and trimming chrysotile asbestos from magnesium and other contaminants;
- -
- preparation of the initial oxide nickel–cobalt system by impregnation in hydrochloric acid medium.
The effect of the acid solution concentration on the final activity of the catalyst was determined and the optimal concentration was shown to be 1 mol/L. Optimal conditions of all stages of nanocatalyst preparation were determined: —1 mol/L; —20; —20. According to the final equation, it is possible to calculate the optimal preparation parameters of the nanocatalyst, which provides the greatest activity and selectivity of the catalyst during the hydrogenation of benzothiophene. Using photospectrometric analysis, data on adsorption of cobalt and nickel on the surface of chrysotile–asbestos (83–89%) during impregnation were obtained. The final equation of influence of all factors taken into account when planning the experiment was derived. The calculated values of the statistical indicators for the final equation were as follows: R = 0.9849, tR = 73.48. The results of the hydrogenation of the benzothiophene with the catalyst obtained under the optimal conditions were as follows: the conversion of benzothiophene was 69.04%, the yield of 2,3-dihydrobenzothiophene was 3.52%, and ethylbenzene was 63.52%.
Author Contributions
Conceptualization, A.B. and N.B.; methodology, M.B.; software, D.A.; validation, M.B., Y.A. and Z.S.K.; formal analysis, A.T.; investigation, B.T.; data curation, T.G.; writing—original draft preparation, N.B.; writing—review and editing, A.B.; visualization, M.B.; supervision, Y.A.; project administration, D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, P. Suvorov, Hydrogenation of petroleum residues with the use of cobalt-molybdenum and molybdenum-manganese catalysts. Solid Fuel Chem. 2007, 41, 342–345. [Google Scholar]
- Liu, Q.; Yang, J.; Zhang, H.; Sun, H.; Wu, S.; Ge, B.; Wang, R.; Yuan, P. Tuning the properties of Ni-based catalyst via La incorporation for efficient hydrogenation of petroleum resin. Chin. J. Chem. Eng. 2022, 45, 41–50. [Google Scholar] [CrossRef]
- Wei, C.Q.; Wang, L.L.; Chen, X.P.; Pan, X.L.; Lin-Lin, S.U.; Wang, Y. Micro-size Eggshell-type 28 Ni/SFC3R Catalyst for C5 Petroleum Resin Hydrogenation Reaction. Fine Chem. 2016, 33, 1061–1068. [Google Scholar]
- Xu, H.M. Production technology and development current situation of hydrogenated petroleum resin. China Adhes. 2014, 23, 47–51. [Google Scholar]
- Maity, S.K.; Ancheyta, J.; Soberanis, L.; Alonso, F. Alumina-silica binary mixed oxide used as support of catalysts for hydrotreating of Maya heavy crude. Appl. Catal. A Gen. 2013, 2, 231–238. [Google Scholar] [CrossRef]
- Liu, M.; Chang, Y.; Yang, J. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.R.; Guan, W.H.; Luo, W.H. Study on synthesis and activity of modified TiO2 coated on modified Chrysotile. New. Chem. Mater. 2013, 41, 109–111. [Google Scholar]
- Zhang, H.; Duan, T.; Zhu, W.; Yao, W.-T. Chrysotile-Based Nanowires Decorated with Monodispersed Ag Nanoparticles as a Highly Active and Reusable Hydrogenation Catalyst. J. Phys. Chem. 2015, 119, 21465–21472. [Google Scholar] [CrossRef]
- Jamanbalin, K.K. Structure and properties of chrysotile-asbestos nanotubes. Sci. Bus. Dev. Paths 2015, 54, 8–13. [Google Scholar]
- Feng, Q.M.; Wang, Q.; Liu, K.; Ou, L.; Zhang, G.; Lu, Y. Adsorption kinetics and thermodynamics of copper (II) on chrysotile. J. Cent. South Univ. 2011, 11, 3225–3231. [Google Scholar]
- Wang, L.J.; Lu, A.H.; Wang, C.Q.; Zheng, X.; Zhao, D.; Liu, R. Nano-fibriform production of silica from natural chrysotile. J. Colloid Interface Sci. 2006, 295, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Kalechits, I.V. Modeling Coal Liquefaction; IVTAN: Moscow, Russia, 1999; p. 229. [Google Scholar]
- Zhang, D.-Q.; Duan, A.-J.; Zhao, Z.; Wang, X.; Jiang, G.; Liu, J.; Wang, C.; Jin, M. Synthesis, characterization and catalytic performance of meso-microporous material Beta-SBA-15-supported NiMo catalysts for hydrodesulfurization of dibenzothiophene. Catal. Today 2011, 175, 477–484. [Google Scholar] [CrossRef]
- Alfredo, G.L.; Alida, E.C.P.; Zeferino, C.V.; Mogica-Betancourt, J.; Alvarez-Hernández, A.; Vrinat, M. Effect of Ni promoter in the oxide precursors of MoS2/MgO–Al2O3 catalysts tested in dibenzothiophene hydrodesulphurization. Catal. Today 2010, 149, 288–294. [Google Scholar]
- Liu, T.-L.; Cao, J.-P.; Zhao, X.-Y.; Wang, J.-X.; Ren, X.-Y.; Fan, X.; Zhao, Y.-P.; Wei, X.-Y. In Situ upgrading of Shengli lignite pyrolysis vapors over metal-loaded HZSM-5 catalyst. Fuel Process. Technol. 2017, 160, 19–26. [Google Scholar] [CrossRef]
- Ren, L.X.; Cao, Z.; Fan, X. Effect of Promoter on Sulfur Resistance of Ni/Al2O3 Catalyst during Hydrogenation Process of Petroleum Resin. Contemp. Chem. Ind. 2010, 39, 321–323. [Google Scholar]
- Baikenov, M.I.; Aitbekova, D.E.; Balpanova, N.Z.; Tusipkhan, A.; Baikenova, G.G.; Aubakirov, Y.A.; Brodskiy, A.R.; Fengyun, M.; Makenov, D.K. Hydrogenation of polyaromatic compounds over NiCo/chrysotile catalyst. Bull. Univ. Karaganda Chem. 2021, 103, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Broeckhoff, J.C.P.; De Boer, J.H. Studies on pore systems in catalysts: XIII. Calculations of pore distributions from the desorption branch of nitrogen sorption isotherms in the case of cylindrical pores. B. Applications. J. Cataisys 1968, 10, 377–390. [Google Scholar] [CrossRef]
- Malyshev, V.P. Probabilistic-deterministic planning of the experiment. Alma-Ata Sci. 1981, 116, 116. [Google Scholar]
- Hao, Z.; Guang-Ren, Q.; Hong, L. Simultaneous hydrodesulfurization of thiophene and benzothiophene over NiMoS catalysts supported on γ-Al2O3 composites containing ZSM-5. Microporous Mesoporous Mater. 2022, 346, 112296. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).