Sunlight Bleaching of Subporphyrazine Dye Films
Abstract
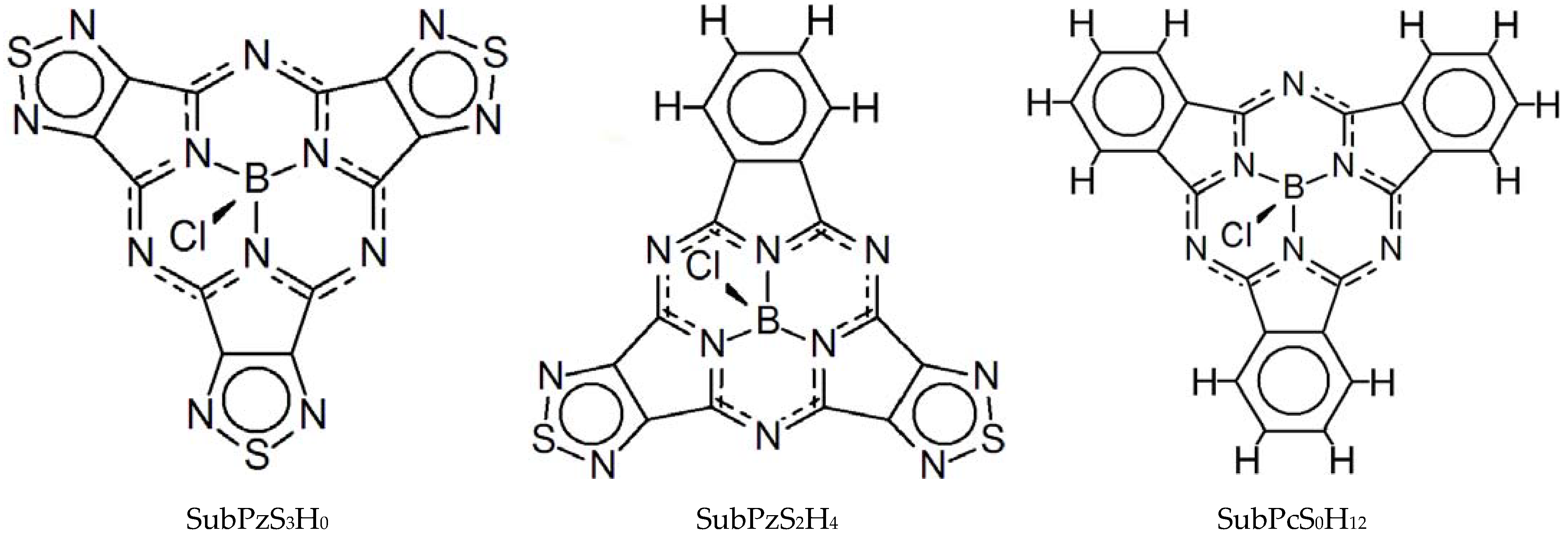
1. Introduction
2. Experimental
3. Results and Discussion
3.1. Temperature Measurements
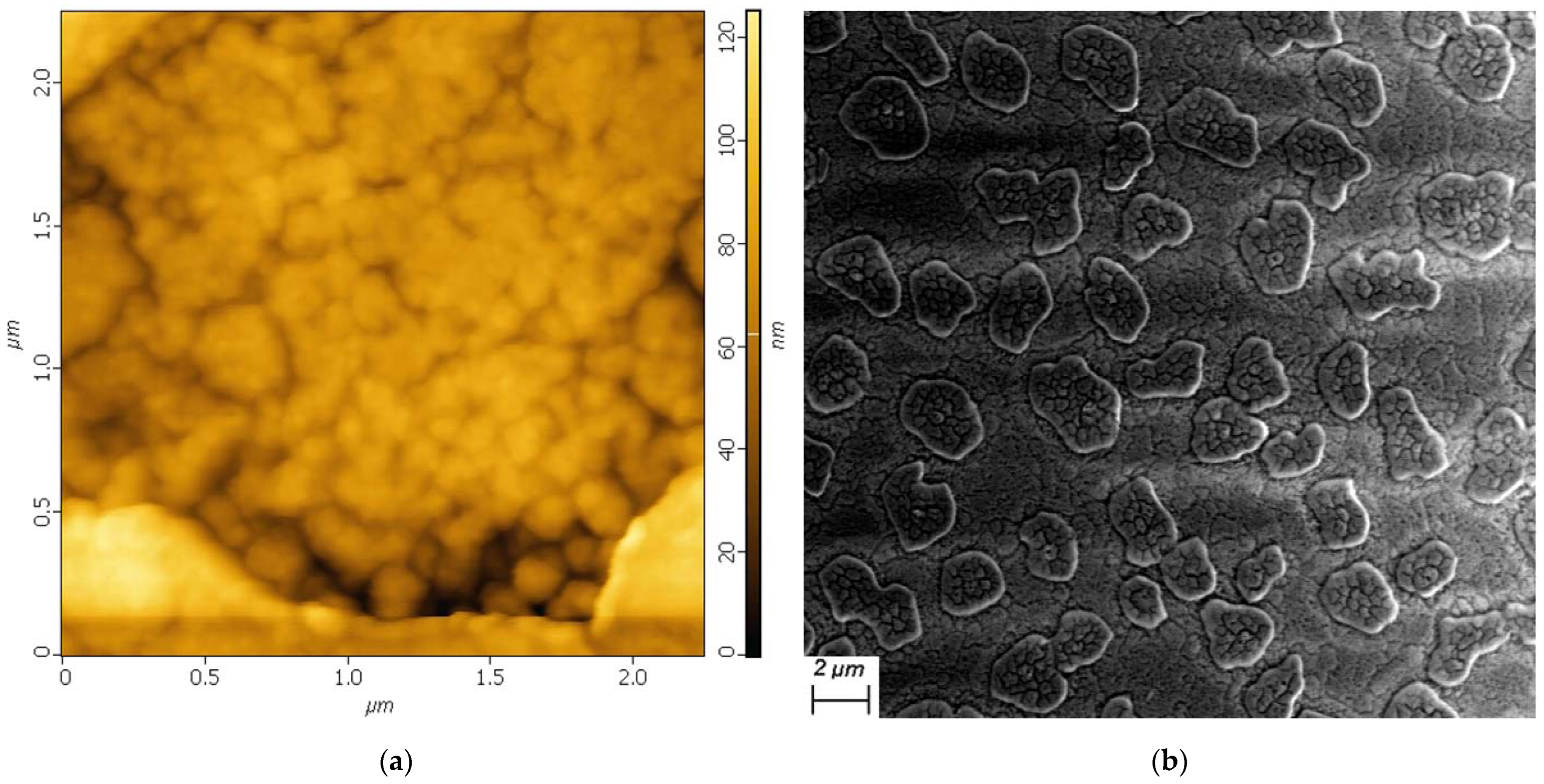
3.2. Film Morphology
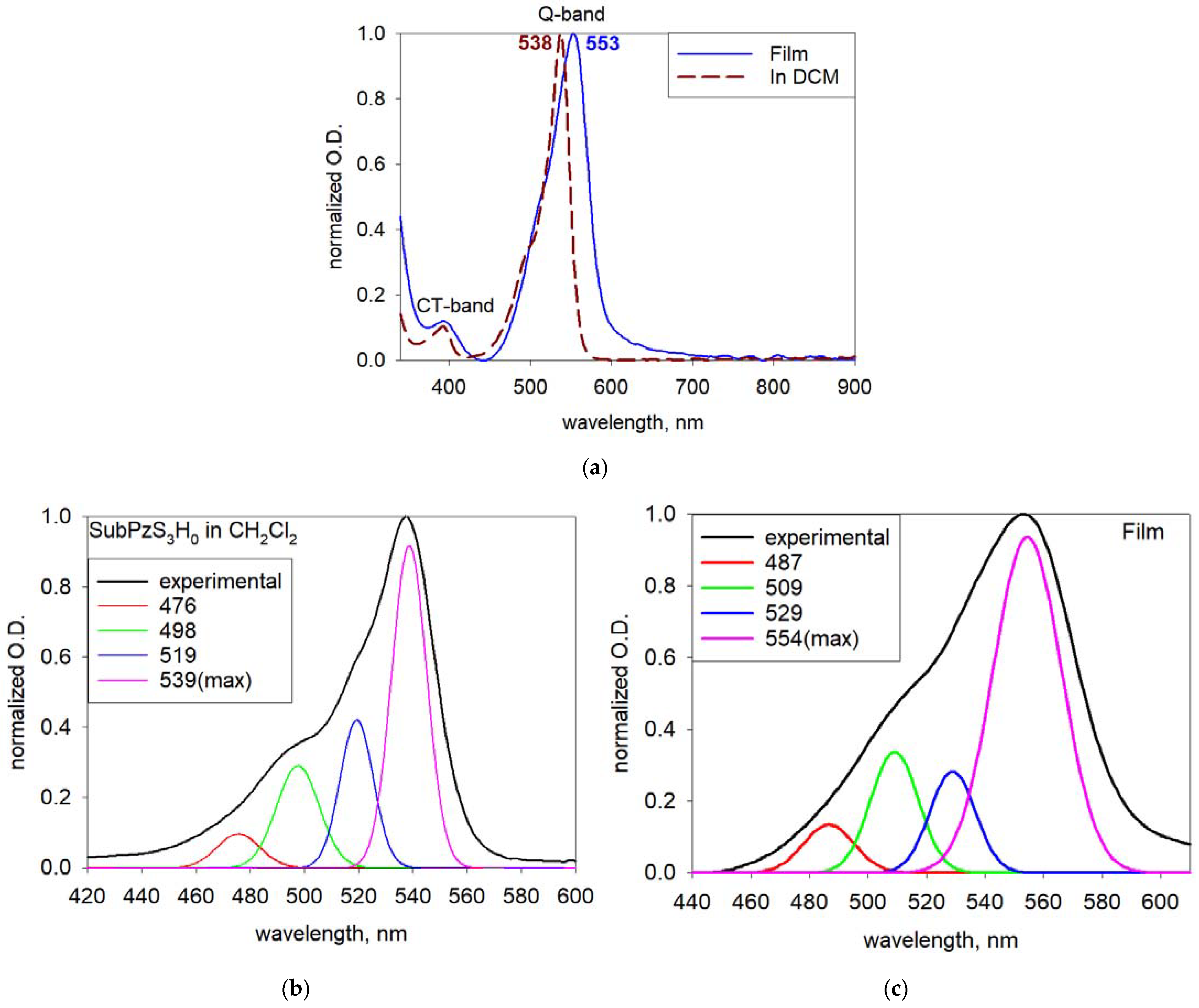
3.3. Absorption Spectra
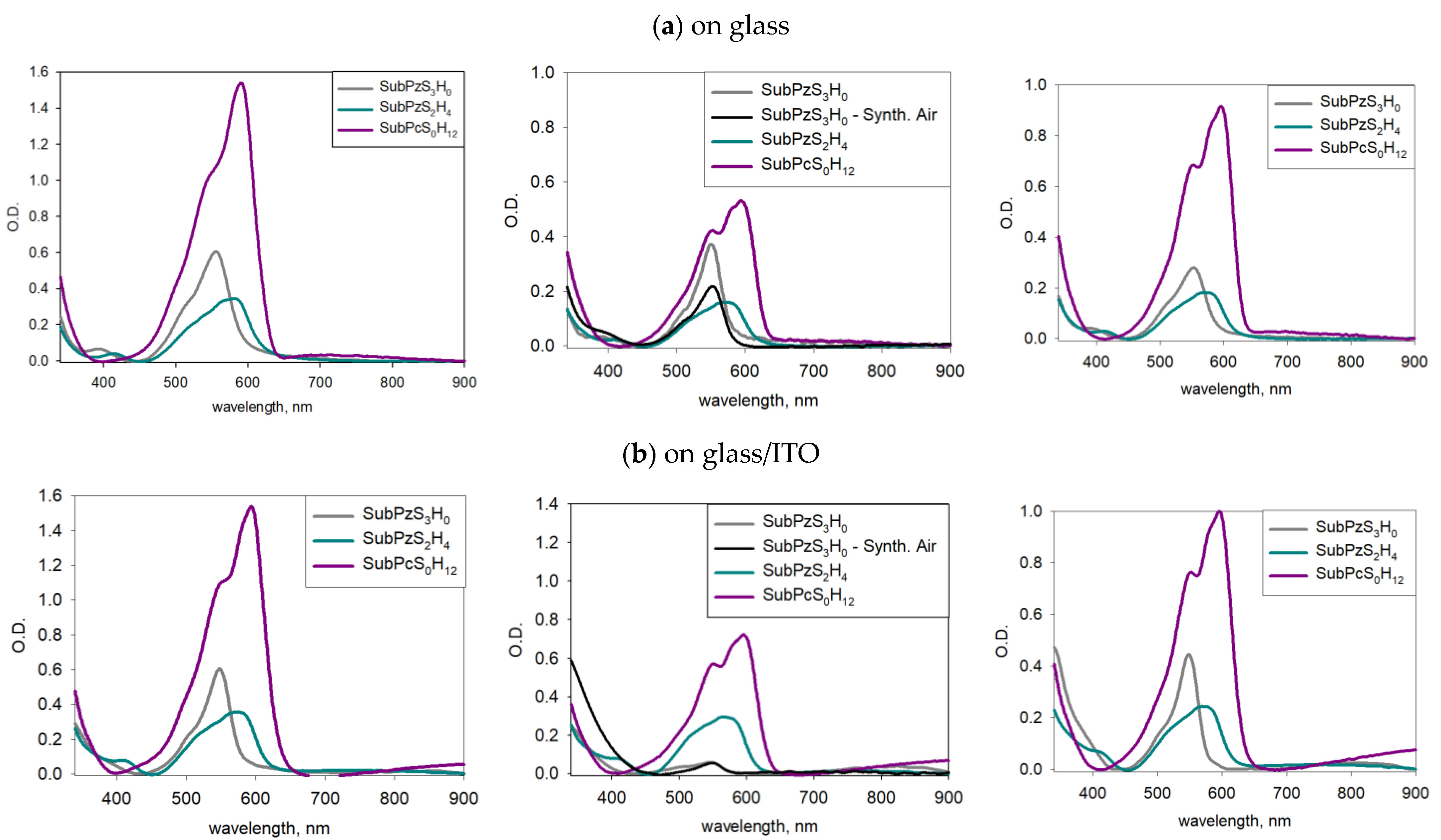
3.4. Photobleaching
3.4.1. On Bare Glass Substrate
3.4.2. On Transparent Conductive Oxides (Glass/ITO, PET/ITO, Glass/FTO)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavarda, G.; Labella, J.; Victoria Martinez-Diaz, M.; Salome Rodriguez-Morgade, M.; Osuka, A.; Torres, T. Recent advances in subphthalocyanines and related subporphyrinoids. Chem. Soc. Rev. 2022, 51, 9482–9619. [Google Scholar] [CrossRef]
- Dowds, M.; Nielsen, M.B. Controlling the optical properties of boron subphthalocyanines and their analogues. Mol. Syst. Des. Eng. 2021, 6, 6–24. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Yang, K.; Chen, P.; Guo, X. Additive-free non-fullerene organic solar cells. ChemElectroChem. 2019, 6, 5547–5562. [Google Scholar] [CrossRef]
- Hildebrand, M.; Holst, D.; Bender, T.; Kronik, L. Electronic structure, bonding, and stability of boron subphthalocyanine halides and pseudohalides. Adv. Theory Simul. 2022, 5, 2100400. [Google Scholar] [CrossRef]
- Bregnhøj, M.; Prete, M.; Turkovic, V.; Petersen, A.U.; Nielsen, M.B.; Madsen, M.; Ogilby, P.R. Oxygen-dependent photophysics and photochemistry of prototypical compounds for organic photovoltaics: Inhibiting degradation initiated by singlet oxygen at a molecular level. Methods Appl. Fluoresc. 2011, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Martynov, I.V.; Inasaridze, L.N.; Troshin, P.A. Resist or oxidize: Identifying molecular structure–photostability relationships for conjugated polymers used in organic solar cells. ChemSusChem. 2022, 15, e202101336. [Google Scholar] [CrossRef]
- Pakhomov, G.L.; Travkin, V.V.; Drozdov, M.N.; Sachkov, Y.I.; Yunin, P.A. Small-molecule heterojunctions: Stability to ageing under sunlight. Appl. Surf. Sci. 2022, 578, 152084. [Google Scholar] [CrossRef]
- Burlingame, Q.; Tong, X.; Hankett, J.; Slootsky, M.; Chen, Z.; Forrest, S.R. Photochemical origins of burn-in degradation in small molecular weight organic photovoltaic cells. Energy Environ. Sci. 2015, 8, 1005–1010. [Google Scholar] [CrossRef]
- Grant, T.M.; Josey, D.S.; Sampson, K.L.; Mudigonda, T.; Bender, T.P.; Lessard, B.H. Boron subphthalocyanines and silicon phthalocyanines for use as active materials in organic photovoltaics. Chem. Rec. 2019, 19, 1093–1112. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Ding, K.; Sheriff, H.K.M.; Ye, L.; Liu, H.; Li, C.-Z.; Ade, H.; Forrest, S.R. Non-fullerene acceptor organic photovoltaics with intrinsic operational lifetimes over 30 years. Nat. Commun. 2021, 12, 5419. [Google Scholar] [CrossRef]
- Travkin, V.V.; Koptyaev, A.I.; Pakhomov, G.L.; Volkov, P.V.; Semikov, D.A.; Luk’yanov, A.Y. Experimental study of heat transfer in thin-film perovskite-based structures using a low-coherent tandem interferometry. Tech. Phys. Lett. 2021, 23, 31. [Google Scholar] [CrossRef]
- Burlafinger, K.; Strohm, S.; Joisten, C.; Woiton, M.; Classen, A.; Hepp, J.; Heumüller, T.; Brabec, C.J.; Vetter, A. Accelerated lifetime testing of thin-film solar cells at high irradiances and controlled temperatures. Prog. Photovolt. Res. Appl 2022, 30, 518–527. [Google Scholar] [CrossRef]
- Travkin, V.; Koptyaev, A.; Hamdoush, M.; Pakhomov, G. Molecular optical filtering in perovskite solar cells. J Mater. Sci. Mater. Electron. 2022, 33, 7728–7737. [Google Scholar] [CrossRef]
- Pakhomov, G.L.; Travkin, V.V.; Khamdoush, M.; Zhabanov, Y.A.; Stuzhin, P.A. Thiadiazole fused subporphyrazines as acceptors in organic photovoltaic cells. Macroheterocycles 2017, 10, 548–551. [Google Scholar] [CrossRef]
- Skvortsov, I.A.; Kovkova, U.P.; Zhabanov, Y.A.; Khodov, I.A.; Somov, N.V.; Pakhomov, G.L.; Stuzhin, P.A. Subphthalocyanine-type dye with enhanced electron affinity: Effect of combined azasubstitution and peripheral chlorination. Dyes Pigm. 2021, 185, 108944. [Google Scholar] [CrossRef]
- Hamdoush, M.; Ivanova, S.S.; Pakhomov, G.L.; Stuzhin, P.A. Heterocyclic subphthalocyanine analogue—Boron(III) subporphyrazine with fused 1,2,5-thiadiazole rings. Macroheterocycles 2016, 9, 230–233. [Google Scholar] [CrossRef]
- Afre, R.A.; Sharma, N.; Sharon, M.; Sharon, M. Transparent conducting oxide films for various applications: A review. Rev. Adv. Mater. Sci. 2018, 53, 79–89. [Google Scholar] [CrossRef]
- Hamdoush, M.; Nikitin, K.; Skvortsov, I.; Somov, N.; Zhabanov, Y.; Stuzhin, P.A. Influence of heteroatom substitution in benzene rings on structural features and spectral properties of subphthalocyanine dyes. Dyes Pigm. 2019, 170, 107584. [Google Scholar] [CrossRef]
- Volkov, P.; Semikov, D.; Goryunov, A.; Luk’yanov, A.; Tertyshnik, A.; Vopilkin, E.; Krayev, S. Miniature fiber-optic sensor based on Si microresonator for absolute temperature measurements. Sens. Actuator A Phys. 2020, 316, 112385. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Zhao, X.; Yang, L. Boron subphthalocyanine chloride crystalline thin film with a long range exciton diffusion length grown assisted by negative surface charges. Thin Solid Films 2017, 636, 527–531. [Google Scholar] [CrossRef]
- Wang, N.; Tong, X.; Burlingame, Q.; Yu, J.; Forrest, S.R. Photodegradation of small-molecule organic photovoltaics. Sol. Energy Mater. Sol. Cells 2014, 125, 170–175. [Google Scholar] [CrossRef]
- Son, Y.H.; Kim, G.W.; Jeon, W.S.; Pode, R.; Kwon, J.H. Thermal annealing effect of subphthalocyanine (SubPc) donor material in organic solar cells. Mol. Cryst. Liq. Cryst. 2012, 565, 8–13. [Google Scholar] [CrossRef]
- Karan, S.; Mallik, B. Templating effects and optical characterization of copper (II) phthalocyanine nanocrystallites thin film: nanoparticles, nanoflowers, nanocabbages, and nanoribbons. J. Phys. Chem. C 2007, 111, 7352–7365. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, S. Inferring the molecular arrangements of boron subphthalocyanine chloride in thin film from a DFT/TDDFT study of molecular clusters and experimental electronic absorption spectra. Org. Electron. 2018, 62, 667–675. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Y.-C.; Liang, W.Z.; Zhao, Y. Vibrationally resolved absorption spectra and exciton dynamics in zinc phthalocyanine aggregates: Effects of aggregation lengths and remote exciton transfer. J. Phys. Chem. A 2021, 125, 2932–2943. [Google Scholar] [CrossRef]
- Virdo, J.D.; Crandall, L.; Dang, J.D.; Fulford, M.V.; Lough, A.J.; Durfee, W.S.; Bender, T.P. The influence of strong and weak hydrogen bonds on the solid state arrangement of hydroxy-containing boron subphthalocyanines. CrystEngComm 2013, 15, 8578–8586. [Google Scholar] [CrossRef]
- Mack, J.; Stillman, M.J. Assignment of the optical spectra of metal phthalocyanines through spectral band deconvolution analysis and ZINDO calculations. Coord. Chem. Rev. 2001, 219–221, 993–1032. [Google Scholar] [CrossRef]
- Muckley, E.S.; Miller, N.; Jacobs, C.B.; Gredig, T.; Ivanov, I.N. Morphology-defined interaction of copper phthalocyanine with O2/H2O. J. Photon. Energy 2016, 6, 045501. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Pakhomov, G.L.; Drozdov, M.N.; Travkin, V.V.; Lopatin, M.A.; Shashkin, V.I. Reversal of rectification in fullerene-based devices. Synth. Met. 2014, 195, 91–96. [Google Scholar] [CrossRef]
- Lincke, G. Molecular stacks as a common characteristic in the crystal lattice of organic pigment dyes A contribution to the ‘‘soluble–insoluble’’ dichotomy of dyes and pigments from the technological point of view. Dyes Pigm. 2003, 59, 1–24. [Google Scholar] [CrossRef]
- Szkutnik, P.D.; Roussel, H.; Lahootun, V.; Mescot, X.; Weiss, F.; Jiménez, C. Study of the functional properties of ITO grown by metalorganic chemical vapor deposition from different indium and tin precursors. J. Alloys Compd. 2014, 603, 268–273. [Google Scholar] [CrossRef]
- Donzello, M.P.; Agostinetto, R.; Ivanova, S.S.; Fujimori, M.; Suzuki, Y.; Yoshikawa, H.; Shen, J.; Awaga, K.; Ercolani, C.; Kadish, K.M.; et al. Tetrakis(thiadiazole)porphyrazines. 4. Direct template synthesis, structure, general physicochemical behavior, and redox properties of AlIII, GaIII, and InIII complexes. Inorg. Chem. 2005, 44, 8539–8551. [Google Scholar] [CrossRef] [PubMed]
- Guilleme, J.; González-Rodríguez, D.; Torres, T. Triflate-subphthalocyanines: Versatile, reactive intermediates for axial functionalization at the boron atom. Angew. Chem. Int. Ed. 2011, 50, 3506–3509. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Andersson, G.; Lewis, D.A. Role of humidity on indium and tin migration in organic photovoltaic devices. Phys. Chem. Chem. Phys. 2011, 13, 4381–4387. [Google Scholar] [CrossRef]


| Sample | Pristine | Direct Sunlight, Ar | Filtered Sunlight, Ar | Direct Sunlight, Air | |
|---|---|---|---|---|---|
| Scanning area | 0.9 × 0.9 cm2 (WLI) | 5.8 | 2.0 | 4.4 | 4.9 |
| 0.3 × 0.3 cm2 (WLI) | 5.4 | 1.5 | 3.9 | 4.6 | |
| 0.1 × 0.1 cm2 (WLI) | 5.4 | 0.8 | 3.8 | 4.3 | |
| 2.25 × 2.25 µm2 (AFM) | 17.7 | 11.8 | 19.4 | 17.5 |
| Pristine | Direct Sunlight in Air | Filtered Sunlight in Air | Direct Sunlight in Ar | Filtered Sunlight in Ar | Direct Sunlight in Synth. Air | |
|---|---|---|---|---|---|---|
| λQmax, nm | 553 | 553 | 553 | 553 | 554 | 555 |
| DQpristine/DQirradiated | - | 1.6 | 1.2 | 2.2 | 1.2 | 1.4 |
| λQmax, nm | DQpristine/DQirradiated | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pristine | Irradiated in Air | in Argon | in Synth.Air | ||||||
| Dye | glass | ITO | Glass | ITO | glass | ITO | Glass | ITO | ITO |
| SubPzS3H0 | 553 | 548 | 553 | 546 | 1.6 | 10.2 | 2.2 | 1.4 | 10.1 |
| SubPzS2H4 | 581 | 570 | 569 | 565 | 2.1 | 1.2 | 2.0 | 1.4 | 1.2 |
| SubPcS0H12 | 590 | 594 | 596 | 596 | 3 | 2.1 | 1.7 | 1.4 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Travkin, V.V.; Semikov, D.A.; Stuzhin, P.A.; Skvortsov, I.A.; Pakhomov, G.L. Sunlight Bleaching of Subporphyrazine Dye Films. Appl. Sci. 2023, 13, 1211. https://doi.org/10.3390/app13021211
Travkin VV, Semikov DA, Stuzhin PA, Skvortsov IA, Pakhomov GL. Sunlight Bleaching of Subporphyrazine Dye Films. Applied Sciences. 2023; 13(2):1211. https://doi.org/10.3390/app13021211
Chicago/Turabian StyleTravkin, Vlad V., Danila A. Semikov, Pavel A. Stuzhin, Ivan A. Skvortsov, and Georgy L. Pakhomov. 2023. "Sunlight Bleaching of Subporphyrazine Dye Films" Applied Sciences 13, no. 2: 1211. https://doi.org/10.3390/app13021211
APA StyleTravkin, V. V., Semikov, D. A., Stuzhin, P. A., Skvortsov, I. A., & Pakhomov, G. L. (2023). Sunlight Bleaching of Subporphyrazine Dye Films. Applied Sciences, 13(2), 1211. https://doi.org/10.3390/app13021211






