Effect of the Addition of Soy Beverage and Propionic Bacteria on Selected Quality Characteristics of Cow’s Milk Yoghurt Products
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Milk, Milk–Soya, and Soya Yoghurt Products
2.2. Analysis of Fermented Milk, Milk–Soya, and Soya Yoghurt Products
2.2.1. pH Measurement
2.2.2. Texture Determination—Hardness and Adhesion
2.2.3. Water-Holding Capacity (WHC) Determination
2.2.4. Determination of the Microflora Population
2.2.5. Determination of Selected Carbohydrates
2.3. Statistical Analysis
3. Results and Discussion
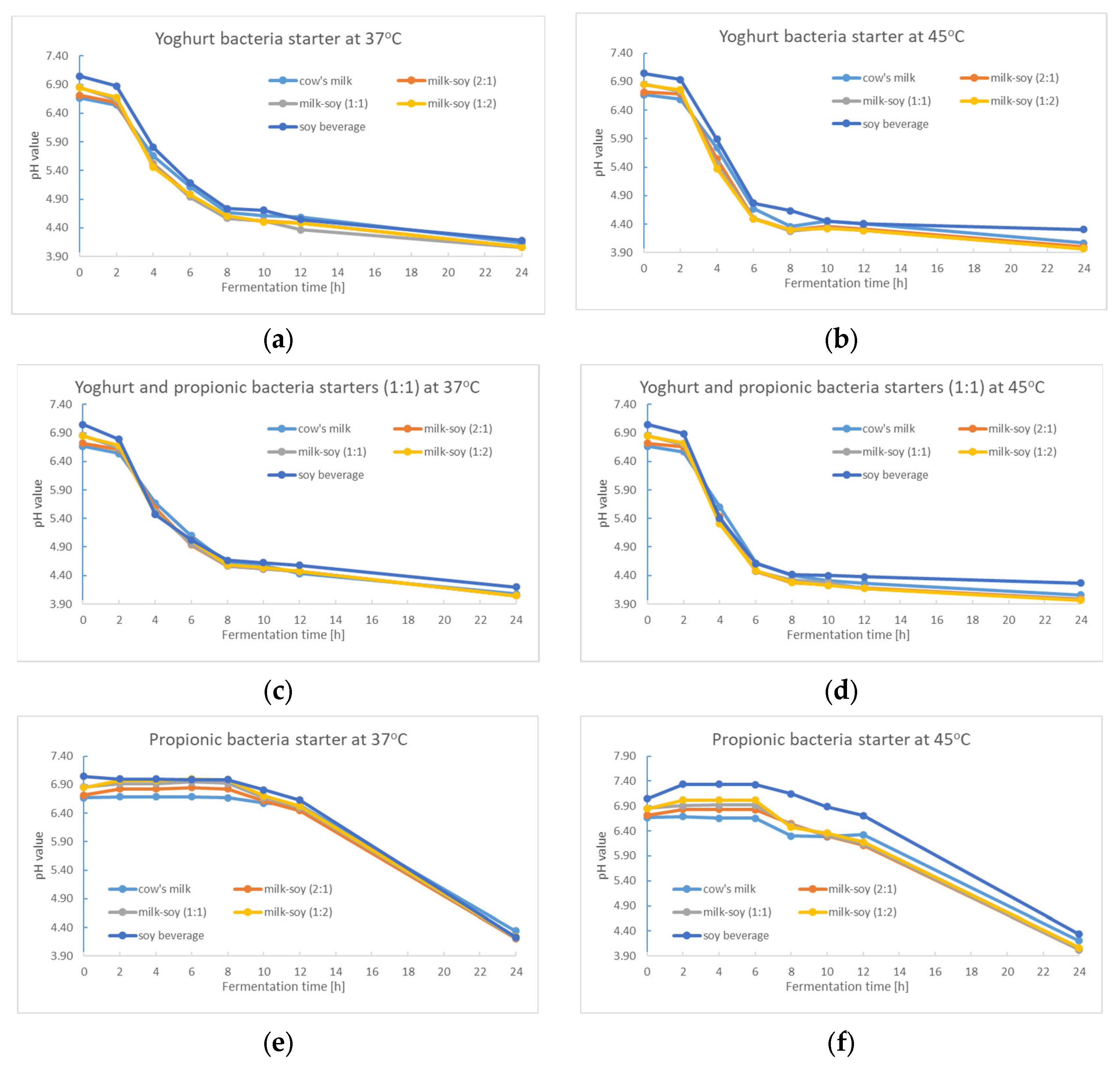
3.1. The Fermentation Kinetic of Milk, Milk–Soya, and Soya Beverages
3.2. Analysis of Fermented Milk, Milk–Soya, and Soya Yoghurt Products
3.2.1. pH Value
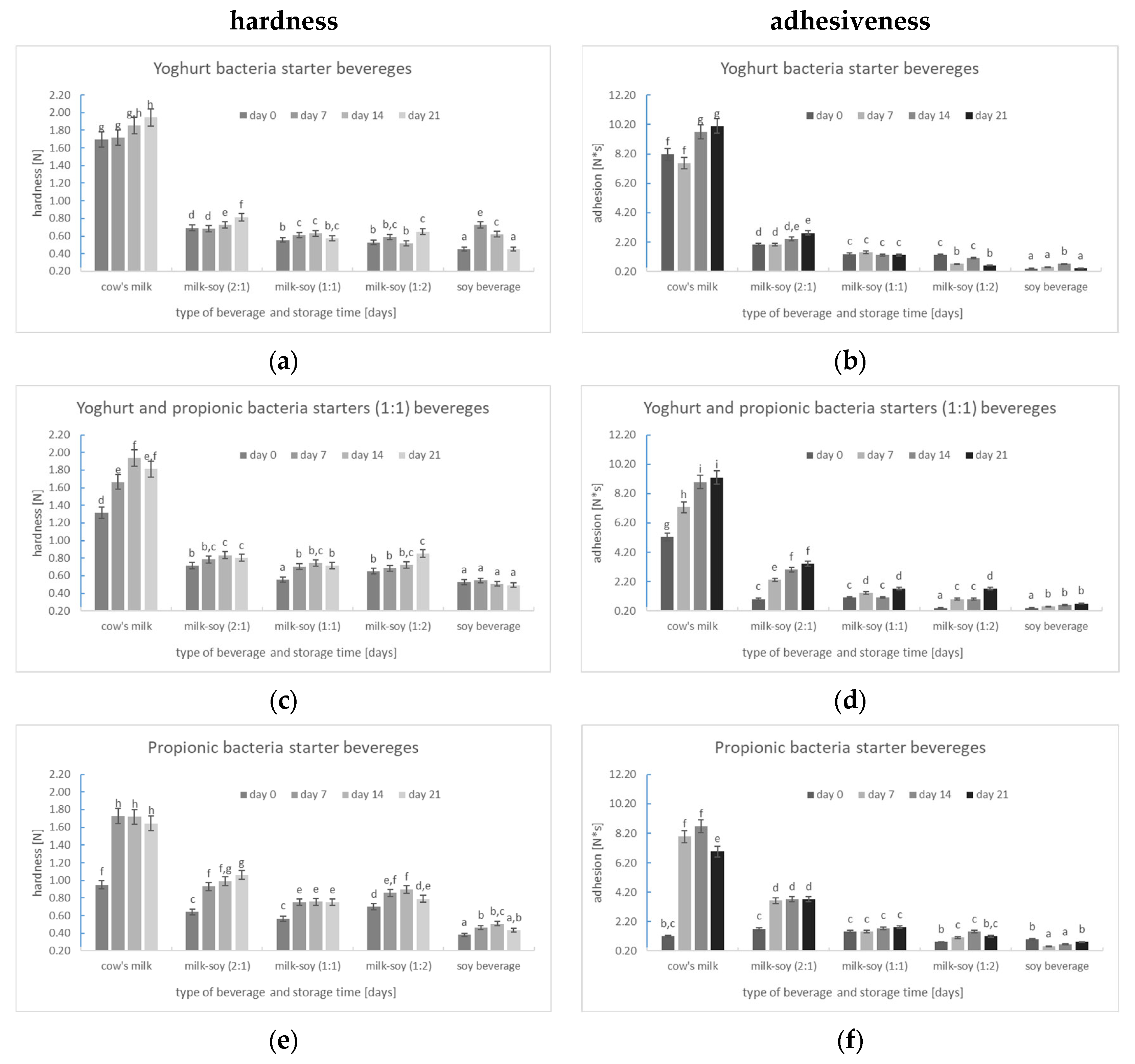
3.2.2. Texture
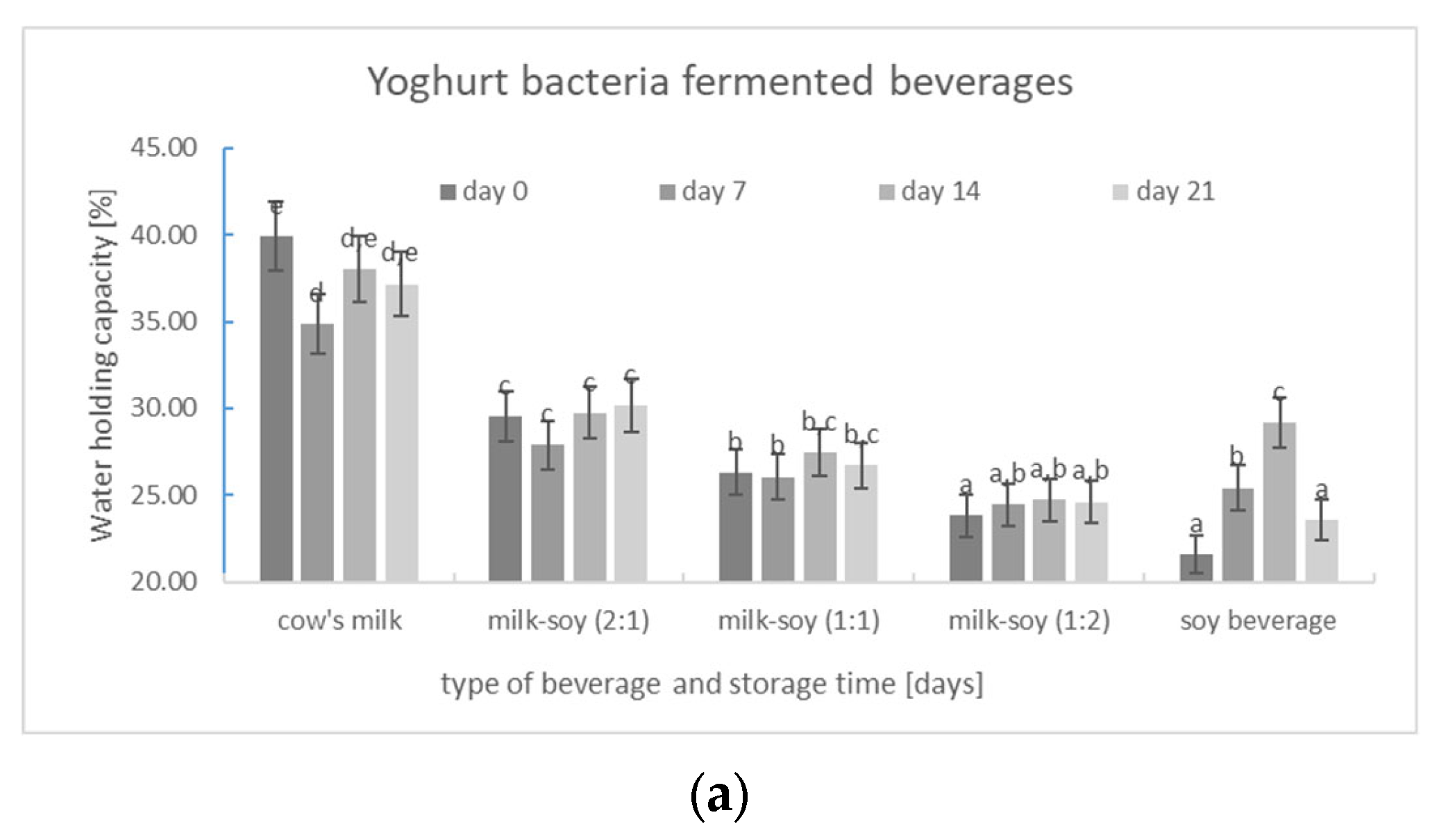
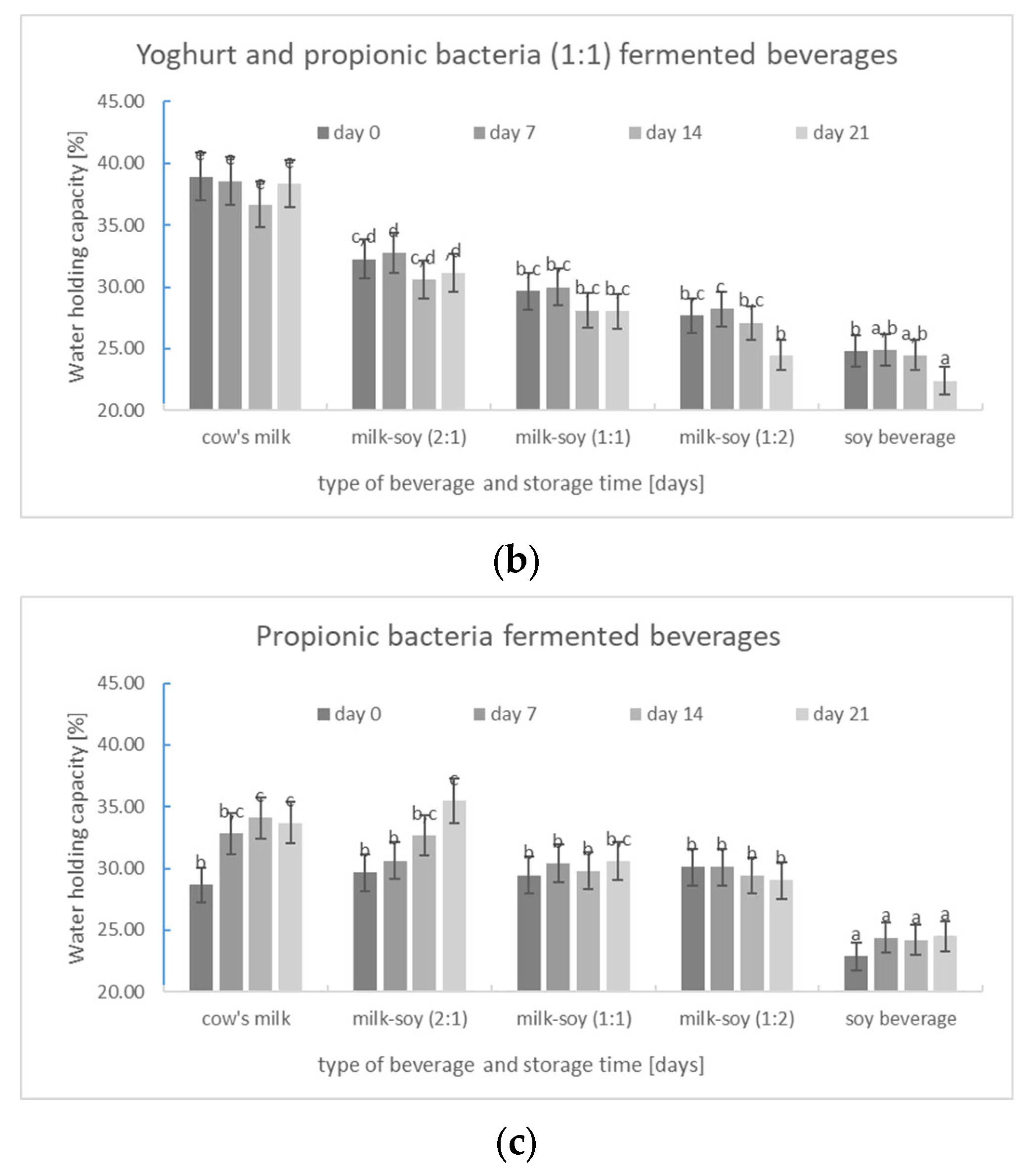
3.2.3. Water-Holding Capacity (WHC)
3.2.4. Microflora Population
3.2.5. Selected Carbohydrates Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, A.M.; Malcata, F.X. Bifidobacterium spp. and Lactobacillus acidophilus: Biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci. Technol. 1999, 10, 139–157. [Google Scholar] [CrossRef]
- Rohit, S.; Bhuwan, B.; Bhagwan, S.S.; Gulab, S.T.; Pallavi, J.; Nitin, Y.; Anjana, S.; Prakash, S.B. Probiotic Efficacy and Potential of Streptococcus thermophilus modulating human health: A synoptic review. J. Pharm. Biol. Sci. 2014, 9, 52–58. [Google Scholar]
- Popović, N.; Brdarić, E.; Ðokić, J.; Dinić, M.; Veljović, K.; Golić, N.; Terzić–Vidojević, A. Yogurt Produced by Novel Natural Starter Cultures Improves Gut Epithelial Barrier in Vitro. Microorganisms 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. CODEX Standard for Fermented Milks 242–2003. Adopted in 2003. Revision 2008. 2010. Available online: www.codexalimentarius.net/download/standards/400/CXS_243e.pdf (accessed on 17 November 2022).
- Wichrowska, D.; Wojdyła, T. Sensory and physicochemical evaluation of selected natural and ecological yogurts. Inż. Ap. Chem. 2014, 53, 421–423. [Google Scholar]
- Kowalska, E.; Pikul, J.; Oziemkowski, P. Physical and chemical properties of plain yoghurt made from milk Concentrated using the ultrafiltration and traditional methods. Żywn 2000, 3, 78–88. [Google Scholar]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non–dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Salehi, G.; Diaz, E.M.; Redondo, R. Consumers switching to vegan, vegetarian, and plant–based (vegan) diets: A systematic review of literature. In Proceedings of the 19th International Congress on Public and Nonprofit Marketing Sustainability: New challenges for marketing and socioeconomic development, Online, 2–4 July 2020; pp. 1–6. [Google Scholar]
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant–Based Alternatives to Yogurt: State–of–the–Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziębicka, A.; Ziarno, M. Properties of Rice–Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium. Molecules 2022, 27, 2558. [Google Scholar] [CrossRef]
- Zaręba, D.; Ziarno, M. Alternatywne probiotyczne napoje warzywne i owocowe. Brom. Chem. Toksykol. 2011, 2, 160–168. [Google Scholar]
- Fructuoso, I.; Romão, B.; Han, H.; Raposo, A.; Ariza-Montes, A.; Araya-Castillo, L.; Zandonadi, R.P. An Overview on Nutritional Aspects of Plant–Based Beverages Used as Substitutes for Cow’s Milk. Nutrients 2021, 13, 2650. [Google Scholar] [CrossRef]
- Dissanayake, M.D.R.; Janak, K.V.; Ramon, S.R.; Celso, F.B.; Adriano, G.C.; Anderson, S.S.; Chaminda, S.R. Plant–based milk substitutes as emerging probiotic carriers. Cur. Opin. Food Sci. 2021, 38, 8–20. [Google Scholar]
- Guillon, F.; Champ, M.M.-J. Carbohydrate fractions of legumes: Uses in human nutrition and potential for health. Br. J. Nutr. 2002, 88, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and Edible Uses of Soy Protein Products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Martinez, A.P.C.; Martinesz, P.C.C.; Souza, M.C.; Canniatti–Brazaca, S.G. Chemical change in soybean grains with germination. Food Sci. Technol. 2011, 31, 23–30. [Google Scholar] [CrossRef]
- Ikya, J.K.; Gernah, D.I.; Ojobo, H.E.; Oni, O.K. Effect of cooking temperature on some quality characteristics of soy milk. Adv. J. Food Sci. Technol. 2013, 5, 543–546. [Google Scholar] [CrossRef]
- Kant, R.; Broadway, A.A. The Benefits of Consuming Soya Milk. Rev. Trends Biosci. 2015, 8, 1159–1162. [Google Scholar]
- Mazumder, M.A.R.; Begum, A.A. Soymilk as source of nutrient for malnourished population of developing country: A review. Int. J. Adv. Sci. Technol. Res. 2016, 6, 192–203. [Google Scholar]
- Ziarno, M.; Zaręba, D.; Maciejak, M.; Veber, A.L. The impact of dairy starter cultures on selected qualitative properties of functional fermented beverage prepared from germinated White Kidney Beans. J. Food Nutr. Res. 2019, 2, 167–176. [Google Scholar]
- Babashahi, M.; Mirlohi, M.; Ghiasvand, R.; Azadbakht, L. Comparison of soymilk and probiotic soymilk effects on serum high–density lipoprotein cholesterol and low–density lipoprotein cholesterol in diabetic Wistar rats. ARYA Atheroscler. 2015, 11, 88–93. [Google Scholar]
- Ewe, J.A.; Yeo, S.K. Fermented soymilk as a nutraceutical. In Beneficial Microorganisms in Food and Nutraceuticals; Liong, M.T., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 133–159. [Google Scholar] [CrossRef]
- Atia, M.; Wenshui, X.; Guonong, Z. Effect of soy protein supplementation on the quality of ripening Cheddar-type cheese. Int. J. Dairy Technol. 2004, 57, 209–214. [Google Scholar] [CrossRef]
- Tahis, R.; Baú, S.; Garcia, E.; Iouko, I. Evaluation of a functional soy product with addition of soy fiber and fermented with probiotic kefir culture. Braz. Arch. Biol. Technol. Food Sci. Technol. 2014, 57, 402–409. [Google Scholar] [CrossRef]
- Ashna, T.; Abdulqader, R.; Qader, S.; Sebo, N.H. The Effect of Soymilk Addition on Chemical, Physical and Sensorial Properties of Cow Milk Yogurt. Polytechnic. J. 2022, 12, 30–39. [Google Scholar]
- Tarnaud, F.; Gaucher, F.; Rosa do Carmo, F.L.; Illikoud, N.; Jardin, J.; Briard–Bion, V.; Guyomarc’h, F.; Gagnaire, V.; Jan, G. Differential Adaptation of Propionibacterium freudenreichii CIRM–BIA129 to Cow’s Milk Versus Soymilk Environments Modulates Its Stress Tolerance and Proteome. Front. Microbiol. 2020, 11, 549027. [Google Scholar] [CrossRef]
- Yerlikaya, O.; Akpinar, A.; Saygili, D.; Karagozlu, N. Incorporation of Propionibacterium shermanii subsp. freudenreichii in probiotic dairy drink production: Physicochemical, rheological, microbiological and sensorial properties. Int. J. Dairy Technol. 2019, 73, 392–402. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Rutten, G.; Bruinenberg, P.; Sesma, F.; de Giori, G.S.; Smid, E.J. A novel dairy product fermented with Propionibacterium freudenreichii improves the riboflavin status of deficient rats. Nutrition 2006, 22, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.J.; Jouan–Lanhouet, S.; Théret, N.; Brenner, C.; Jouan, E.; Moigne–Muller, G.L.; Dimanche-Boitrel, M.; Jan, G. The probiotic Propionibacterium freudenreichii as a new adjuvant for TRAIL–based therapy in colorectal cancer. Oncotarget 2016, 7, 7161–7178. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, B.F.; Oliveira, E.R.; Silva, D.H.S.; Savassi, B.M.; Acurcio, L.B.; Lemos, L.; Alves, J.L.; Assis, H.C.; Vieira, A.T.; Faria, A.M.C.; et al. Whey protein isolate–supplemented beverage, fermented by Lactobacillus casei BL23 and Propionibacterium freudenreichii 138, in the prevention of mucositis in mice. Front. Microbiol. 2018, 9, 2035. [Google Scholar] [CrossRef]
- do Carmo, F.L.R.; Rabah, H.; Cordeiro, B.F.; da Silva, S.H.; Pessoa, R.M.; Fernandes, S.O.A.; Cardoso, V.N.; Gagnaire, V.; Deplanche, M.; Savassi, B.; et al. Probiotic Propionibacterium freudenreichii requires SlpB protein to mitigate mucositis induced by chemotherapy. Oncotarget 2019, 10, 7198–7219. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; ASM Press: Washington, DC, USA, 2010; p. 1429. [Google Scholar]
- Baer, A. Influence of casein proteolysis by starter bacteria, rennet and plasmin on the growth of propionibacteria in Swiss–type cheese. Lait 1995, 75, 391–400. [Google Scholar] [CrossRef]
- Gagnaire, V.; Lortal, S.; Leonil, J. Free active peptidases are detected in Emmental juice extracted before ripening in the warm room. J. Dairy Res. 1998, 65, 119–128. [Google Scholar] [CrossRef]
- Gagnaire, V.; Molle, D.; Herrouin, M.; Leonil, J. Peptides identified during Emmental cheese ripening: Origin and proteolytic systems involved. J. Agric. Food Chem. 2001, 49, 4402–4413. [Google Scholar] [CrossRef] [PubMed]
- Gagnaire, V.; Thierry, A.; Leonil, J. Propionibacteria and facultatively heterofermentative lactobacilli weakly contribute to secondary proteolysis of Emmental cheese. Lait 2001, 81, 339–353. [Google Scholar] [CrossRef]
- Thierry, A.; Salvat–Brunaud, D.; Madec, M.N.; Michel, F.; Maubois, J.L. Swiss cheese ripening: Dynamics of bacterial populations and evolution of the aqueous phase composition for three industrial cheeses. Lait 1998, 78, 521–542. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic–treated and in caesarean–born infants. Microbiome 2018, 6, 182. [Google Scholar] [CrossRef]
- Mojka, K. Characteristics of fermented milk drinks. Probl. Hig. Epidemiol. 2013, 94, 722–729. [Google Scholar]
- Zárate, G. Dairy Propionibacteria: Less Conventional Probiotics to Improve the Human and Animal Health. W: Probiotic in Animals; Rigobelo, E.C., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Riaz, M.N. Soya Applications in Foods, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 27–51. [Google Scholar]
- Domagała, J. Sensory evaluation and rheological properties of yoghurts prepared from goat, cow and sheep milk. EJPAU 2008, 11, #04. Available online: http://www.ejpau.media.pl/volume11/issue3/art–04.html (accessed on 21 November 2022).
- Svejstil, R.; Musilová, S.; Rada, V. Raffinose–series oligosaccharides in soybean products. Sci. Agric. Bohem. 2015, 46, 73–77. [Google Scholar] [CrossRef]
- Beal, C.; Skokanova, J.; Latrille, E.; Martin, N.; Corrieu, G. Combined Effects of Culture Conditions and Storage Time on Acidification and Viscosity of Stirred Yogurt. J. Dairy Sci. 1999, 82, 673–681. [Google Scholar] [CrossRef]
- Mituniewicz–Małek, A.; Ziarno, M.; Dmytrów, I.; Tuma, P.; Witczak, A.; Vovk, S. Properties of drinking yogurt from cow’s and goat’s organic milk fermented by traditional jogurt cultures. Infr. Ecol. Rural Areas 2017, 3, 1755–1771. [Google Scholar]
- Bonczar, G.; Wszołek, M. Charakterystyka jogurtów z mleka owczego o normalizowanej zawartości tłuszczu. Żywn 2002, 1, 109–115. [Google Scholar]
- Salvador, A.; Fiszman, S.M. Textural and sensory characteristics of whole and skimmed flavored set–type yogurt during long storage. J. Dairy Sci. 2004, 87, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Mituniewicz-Małek, A.; Dmytrów, I.; Nowak, Z. Quality characteristics of yoghurt made from refrigerated goat’s milk. Przegl. Mlecz. 2009, 7, 4–8. [Google Scholar]
- Osman, M.M.D.; Razigm, K.A.A. Quality attributes of soy–yoghurt during storage period. Pak. J. Nutr. 2010, 9, 1088–1093. [Google Scholar] [CrossRef]
- Božanić, R.; Lovković, S.; Jeličić, I. Optimizing fermentation of soymilk with probiotic bacteria. Czech J. Food Sci. 2011, 29, 51–56. [Google Scholar] [CrossRef]
- Gomes, J.J.L.; Duarte, A.M.; Batista, A.S.M.; de Figueiredo, R.M.F.; de Sousa, E.P.; de Souza, E.L.; Queiroga, R.C.R.E. Physicochemical and sensory properties of fermented dairy beverages made with goat’s milk, cow’s milk and a mixture of the two milks. LWT 2013, 54, 18–24. [Google Scholar] [CrossRef]
- Vanegas–Azuero, A.M.; Gutiérrez, L.F. Physicochemical and sensory properties of yogurts containing sacha inchi (Plukenetia volubilis L.) seeds and β–glucans from Ganoderma lucidum. J. Dairy Sci. 2018, 101, 1020–1033. [Google Scholar] [CrossRef]
- El–Sayed, E.; El–Gawad, A.; Murad, I.H.; Salah, S. Utilization of laboratory–produced xanthan gum in the manufacture of yogurt and soya yogurt. Eur. Food Res. Technol. 2002, 215, 298–304. [Google Scholar] [CrossRef]
- Chanasattru, W.; Corradinim, G.; Peleg, M. Determination of practically significant differences in the sensorily perceived consistency of semiliquid foods. J. Text Stud. 2002, 33, 445–460. [Google Scholar] [CrossRef]
- Tratnik, L.; Božanić, R.; Herceg, Z.; Drgalić, I. The quality of plain and supplemented kefir from goat’s and cow’s milk. Int. J. Dairy Technol. 2006, 59, 40–46. [Google Scholar] [CrossRef]
- Herrero, A.M.; Requena, T. The effect of supplementing goats milk with whey protein concentrate on textural properties of set–type yogurt. Int. J. Food Sci. Technol. 2006, 41, 87–92. [Google Scholar] [CrossRef]
- Andiç, S.; Boran, G.; Tunçtürk, Y. Effects of carboxyl methyl cellulose and edible cow gelatin on physico–chemical, textural and sensory properties of yoghurt. Int. J. Agricult. Biol. 2013, 15, 245–251. [Google Scholar]
- Mituniewicz–Małek, A.; Dmytrów, I.; Balejko, J.; Ziarno, M. Commercial Probiotic Lactobacillus Sp. Cultures (Lb. Paracasei, Lb. Casei And Lb. Acidophilus) in Fermented Drinks Made from Goat’s Milk. Żywn 2013, 3, 99–110. [Google Scholar] [CrossRef]
- Szajnar, K.; Znamirowska, A.; Pawlos, M.; Kalicka, D. Physicochemical and textural properties of calcium citrate-enriched yogurts. Brom. Chem. Toksykol. 2014, 4, 946–952. [Google Scholar]
- Mituniewicz–Małek, A.; Ziarno, M.; Dmytrów, I. Application of Frozen Goat’s Milk to Production of Potentially Probiotic Fermented Drink. Żywn 2015, 6, 140–149. [Google Scholar] [CrossRef]
- Miocinovic, J.; Miloradovic, Z.; Josipovic, M.; Nedeljkovic, A.; Radovanovic, M.; Pudja, P. Rheological and textural properties of goat and cow milk set type yogurts. Int. Dairy J. 2016, 58, 43–45. [Google Scholar] [CrossRef]
- Yan, C.; Fu, D.; McClements, D.J.; Xu, P.; Zou, L.; Zhu, Y.; Cheng, C.; Liu, W. Rheological and microstructural properties of cold–set emulsion gels fabricated from mixed proteins: Whey protein and lactoferrin. Food Res. Int. 2019, 119, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mao, L.K.; Zheng, H.X.; Chen, H.Q.; Gao, Y.X. Characterization of beta–carotene loaded emulsion gels containing denatured and native whey protein. Food Hydrocoll. 2020, 102, 105600. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Prosello, W.; Molinari, F.; Ghiberto, D.; Reinheimer, J.A. Growth of Lactobacillus paracasei A 13 in Argentinian probiotic cheese and its impact on the characteristic of the product. Int. J. Food Microbiol. 2009, 135, 171–174. [Google Scholar] [CrossRef]
- Zhang, L.; Folkenberg, D.M.; Amigo, J.M.; Ipsen, R. Effect of exopolysaccharide–producing starter cultures and post–fermentation mechanical treatment on textural properties and microstructure of low fat yogurt. Int. Dairy J. 2016, 53, 10–19. [Google Scholar] [CrossRef]
- Znamirowska, A.; Buniowska, M.; Rożek, P.; Kalicka, D.; Pawlos, M. Evaluation of the quality of thermostatic yoghurts with spelt fibre and inulin. Nauka Przyr. Technol. 2018, 12, 103–112. [Google Scholar]
- Domagała, J. Texture of yogurts and bio–yogurts from goat’s milk depending on starter culture type. Milchwissenschaft 2005, 60, 289–292. [Google Scholar]
- Domagała, J.; Wszołek, M. Effect of Concentration Method and Starter Culture Type on the Texture and Susceptibility to Syneresis of Yoghurt and Bio-Yoghurts Made of Goat’s Milk. Żywn 2008, 6, 118–126. [Google Scholar]
- Tang, C.H.; Luo, L.J.; Liu, F.; Chen, Z. Transglutaminase-set soya globulin–stabilized emulsion gels: Influence of soya β−conglycinin/glycinin ratio on properties, microstructure and gelling mechanism. Food Res. Int. 2013, 51, 804–812. [Google Scholar] [CrossRef]
- Ghosh, D.; Chattoraj, D.K.; Chattopadhyay, P. Studies on changes in microstructure and proteolysis in cow and soya milk curd during fermentation using lactic cultures for improving protein bioavailability. J. Food Sci. Technol. 2013, 50, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Hsia, S.Y.; Hsiao, Y.H.; Li, W.T.; Hsieh, J.F. Aggregation of soya protein isoflavone complexes and gel formation induced by gluconolactone in soymilk. Sci. Rep. 2016, 6, 35718. [Google Scholar] [CrossRef] [PubMed]
- Gumus, C.E.; Gharibzahedi, S.M.T. Yogurts supplemented with lipid emulsions rich in omega-3 fatty acids: New insights into the fortification, microencapsulation, quality properties, and health-promoting effects. Trends Food Sci. Technol. 2021, 110, 267–279. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, J.G.; Shin, Y.W.; Kim, S.H.; Whang, K.Y. Effect of dietary inclusion of Lactobacillus acidophilus ATCC 43121 on cholesterol metabolism in rats. J. Microbiol. Biotechnol. 2007, 17, 655–662. [Google Scholar]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef]
- Malaki Nik, A.; Tosh, S.; Poysa, V.; Woodrow, L.; Corredig, M. Physicochemical characterization of soymilk after step-wise centrifugation. Food Res. Int. 2008, 41, 286–294. [Google Scholar] [CrossRef]
- Zaręba, D.; Ziarno, M.; Obiedziński, M. Viability of yogurt bacteria and probiotic strains in models of fermented and non–fermented milk. Med. Weter. 2008, 64, 1007–1011. [Google Scholar]
- Sady, M.; Domagała, J.; Grega, T.; Kalicka, D. Effect of the Storing Period on Micro-Flora in Yoghurts Containing Amaranth Seeds and Oat Grains Added. Żywn 2007, 6, 242–250. [Google Scholar]
- Kycia, K.; Krysiński, C. Microbiological and hygienic quality of commercial goat milk yogurts in context of their therapeutic properties. Probl. Hig. Epidemiol. 2014, 95, 186–191. [Google Scholar]
- Shori, A.B.; Baba, A.S. Viability of lactic acid bacteria and sensory evaluation in Cinnamomum verum and Allium sativum–bio–yogurts made from camel and cow milk. J. Assoc. Arab Univ. Basic Appl. Sci. 2012, 11, 50–55. [Google Scholar]
- Vinderola, C.G.; Bailo, N.; Reinheimer, J.A. Survival of probiotic microflora in Argentinian yoghurts during refrigerated storage. Food Res. Int. 2000, 33, 97–102. [Google Scholar] [CrossRef]
- Li, S.; Walsh, H.; Gokavi, S.; Guo, M. Interactions between Lactobacillus acidophilus strains and the starter cultures, Lactobacillus bulgaricus and Streptococcus thermophilus during fermentation of goats’ milk. Afr. J. Biotechnol. 2012, 11, 11271–11279. [Google Scholar]
- Hassan, A.N.; Frank, J.F.; Farmer, M.A.; Schmidt, K.A.; Shalabi, S.I. Formation of yogurt microstructure and three–dimensional visualization as determined by confocal scanning laser microscopy. J. Dairy Sci. 1995, 78, 2629–2636. [Google Scholar] [CrossRef]
- Hassan, A.N.; Frank, J.F.; Farmer, M.A.; Schmidt, K.A.; Shalabi, S.I. Observation of encapsulated lactic acid bacteria using confocal scanning laser microscopy. J. Dairy Sci. 1995, 78, 2624–2628. [Google Scholar] [CrossRef]
- Cerning, J.; Bouillanne, C.; Landon, M.; Desmazeaud, M. Isolation and characterization of exocellular polysaccharide produced by Lactobacillus bulgaricus. Biotechnol. Lett. 1986, 8, 625–628. [Google Scholar] [CrossRef]
- Low, D.; Ahlgren, J.A.; Horne, D.; McMahon, D.J.; Oberg, C.J.; Broadbent, J.R. Influence of Streptococcus thermophilus MR–1C capsular exopolysaccharide on cheese moisture level. Appl. Environ. Microbiol. 1998, 64, 2147–2151. [Google Scholar] [CrossRef]
- Graumann, P.; Marahiel, M.A. Some like it cold: Response of microorganisms to cold shock. Arch. Microbiol. 1996, 166, 293–300. [Google Scholar] [CrossRef]
- Thieringer, A.H.; Jones, P.; Inouye, M. Cold shock and adaptation. BioEssays 1998, 20, 49–57. [Google Scholar] [CrossRef]
- Hebraud, M.; Potier, P. Cold shock response and low temperature adaptation in psychrotrophic bacteria. J. Mol. Microbiol. Biotechnol. 1999, 1, 211–219. [Google Scholar] [PubMed]
- Neuhaus, K.; Rapposch, S.; Francis, K.P.; Scherer, S. Restart of exponential growth of cold–shocked Yersinia enterocolitica occurs after down–regulation ofcspA1/A2 mRNA. J. Bacteriol. 2000, 182, 3285–3288. [Google Scholar] [CrossRef]
- Marahiel, A.M.; Weber, M.H.W. Bacterial cold shock responses. Sci. Prog. 2003, 86, 9–75. [Google Scholar]
- Loux, V.; Mariadassou, M.; Almeida, S.; Chiapello, H.; Hammani, A.; Buratti, J.; Gendrault, A.; Barbe, V.; Aury, J.M.; Deutsch, S.M.; et al. Mutations and genomic islands can explain the strain dependency of sugar utilization in 21 strains of Propionibacterium freudenreichii. BMC Genom. 2015, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- Ziarno, M.; Zaręba, D. The use of propionic acid bacteria in dairy production. Przem Spoż 2019, 73, 13–15. [Google Scholar]
- Hassanzadeh–Rostami, Z.; Mazloomi, S.M.; Rahmdel, S.; Kazemi, A. Mixtures of soy–and cow’s milk as potential probiotic food carriers. J. Biol. Today’s World 2014, 4, 29–33. [Google Scholar] [CrossRef]
| Beverages Received | Variants 1: Yoghurt Bacteria Fermented Beverages | Variants 2: Yoghurt and Propionic Bacteria (1:1) Fermented Beverages | Variants 3: Propionic Bacteria Fermented Beverages |
|---|---|---|---|
| cow’s milk | x | x | x |
| milk–soya (2:1) | x | x | x |
| milk–soya (1:1) | x | x | x |
| milk–soya (1:2) | x | x | x |
| soya beverage | x | x | x |
| Type of Beverage | Storage Time [Days] | 0 | 7 | 14 | 21 |
|---|---|---|---|---|---|
| Yoghurt Bacteria Fermented Beverages | |||||
| cow’s milk | 4.05 b,c ± 0.05 | 4.01 b ± 0.05 | 4.02 b ± 0.05 | 4.01 b ± 0.05 | |
| milk–soya (2:1) | 3.94 a ± 0.04 | 3.93 a ± 0.04 | 3.92 a ± 0.04 | 3.92 a ± 0.04 | |
| milk–soya (1:1) | 3.93 a ± 0.04 | 3.90 a ± 0.04 | 3.91 a ± 0.04 | 3.90 a ± 0.04 | |
| milk–soya (1:2) | 3.92 a ± 0.04 | 3.92 a ± 0.04 | 3.90 a ± 0.04 | 3.90 a ± 0.04 | |
| soya beverage | 4.11 c ± 0.05 | 4.38 f ± 0.04 | 4.21 d,e ± 0.05 | 4.13 c,d ± 0.05 | |
| Yoghurt and Propionic Bacteria (1:1) Fermented Beverages | |||||
| cow’s milk | 4.01 b ± 0.05 | 4.00 b ± 0.04 | 3.97 a,b ± 0.04 | 3.99 b ± 0.04 | |
| milk–soya (2:1) | 3.92 a ± 0.05 | 3.91 a ± 0.05 | 3.89 a ± 0.04 | 3.89 a ± 0.04 | |
| milk–soya (1:1) | 3.91 a ± 0.04 | 3.90 b ± 0.05 | 3.88 a ± 0.04 | 3.88 a ± 0.04 | |
| milk–soya (1:2) | 3.90 a ± 0.04 | 3.88 a ± 0.05 | 3.87 a ± 0.05 | 3.93 a ± 0.04 | |
| soya beverage | 4.13 c,d ± 0.05 | 4.11 c ± 0.04 | 4.11 c ± 0.05 | 4.14 c,d ± 0.05 | |
| Propionic Bacteria Fermented Beverages | |||||
| cow’s milk | 4.31 f ± 0.05 | 4.37 f ± 0.05 | 4.23 e ± 0.05 | 4.36 f ± 0.05 | |
| milk–soya (2:1) | 3.98 b ± 0.05 | 4.02 b ± 0.05 | 4.03 b ± 0.05 | 4.05 b,c ± 0.05 | |
| milk–soya (1:1) | 3.96 a,b ± 0.05 | 4.00 b ± 0.04 | 3.98 b ± 0.04 | 4.00 b ± 0.05 | |
| milk–soya (1:2) | 4.01 b ± 0.04 | 4.03 b ± 0.05 | 4.00 b ± 0.04 | 4.04 b,c ± 0.06 | |
| soya beverage | 4.26 e ± 0.05 | 4.22 d ± 0.05 | 4.29 e ± 0.06 | 4.25 e ± 0.06 | |
| Type of Beverage | Storage Time [days] | 0 | 7 | 14 | 21 |
|---|---|---|---|---|---|
| Yoghurt Bacteria Fermented Beverages | |||||
| S. thermophilus population [log(CFU/g)] | |||||
| cow’s milk | 7.9 a ± 0.1 | 8.2 a ± 0.1 | 8.2 a ± 0.1 | 7.9 a ± 0.1 | |
| milk–soya (2:1) | 7.9 a ± 0.1 | 8.4 a ± 0.1 | 8.4 a ± 0.1 | 7.9 a ± 0.1 | |
| milk–soya (1:1) | 7.9 a ± 0.1 | 8.4 a ± 0.1 | 8.6 a,b ± 0.1 | 7.9 a ± 0.1 | |
| milk–soya (1:2) | 7.7 a ± 0.1 | 8.4 a ± 0.1 | 8.7 a,b ± 0.1 | 7.9 a ± 0.1 | |
| soya beverage | 7.4 a ± 0.1 | 8.5 a ± 0.1 | 8.8 b ± 0.1 | 8.0 a ± 0.1 | |
| Lactobacillus spp. population [log(CFU/g)] | |||||
| cow’s milk | 7.4 a ± 0.1 | 7.7 a ± 0.1 | 7.9 a ± 0.1 | 7.9 a ± 0.1 | |
| milk–soya (2:1) | 7.5 a ± 0.1 | 7.8 a ± 0.1 | 8.0 a ± 0.1 | 7.4 a ± 0.1 | |
| milk–soya (1:1) | 7.6 a ± 0.1 | 7.9 a ± 0.1 | 8.1 a ± 0.1 | 7.1 a,b ± 0.1 | |
| milk–soya (1:2) | 7.6 a ± 0.1 | 8.0 a ± 0.1 | 8.0 a ± 0.2 | 6.8 b ± 0.2 | |
| soya beverage | 7.4 a ± 0.1 | 8.1 a ± 0.1 | 8.1 a ± 0.1 | 6.8 b ± 0.2 | |
| Yoghurt and Propionic Bacteria (1:1) Fermented Beverages | |||||
| S. thermophilus population [log(CFU/g)] | |||||
| cow’s milk | 7.5 a ± 0.1 | 7.8 a ± 0.1 | 7.8 a ± 0.1 | 8.2 a ± 0.1 | |
| milk–soya (2:1) | 7.6 a ± 0.1 | 7.8 a ± 0.1 | 7.7 a ± 0.1 | 8.3 a,b ± 0.1 | |
| milk–soya (1:1) | 7.6 a ± 0.1 | 7.8 a ± 0.1 | 7.8 a ± 0.1 | 8.3 a,b ± 0.1 | |
| milk–soya (1:2) | 7.7 a ± 0.1 | 7.8 a ± 0.1 | 7.8 a ± 0.1 | 8.6 b ± 0.1 | |
| soya beverage | 7.6 a ± 0.1 | 7.6 a ± 0.1 | 7.7 a ± 0.1 | 8.1 a ± 0.1 | |
| Lactobacillus spp. population [log(CFU/g)] | |||||
| cow’s milk | 7.2 a ± 0.4 | 7.3 a ± 0.1 | 7.8 a ± 0.1 | 7.7 a ± 0.1 | |
| milk–soya (2:1) | 7.4 a ± 0.1 | 7.6 a ± 0.1 | 8.0 a ± 0.1 | 8.0 a ± 0.1 | |
| milk–soya (1:1) | 7.7 a ± 0.1 | 7.7 a ± 0.1 | 7.9 a ± 0.1 | 7.9 a ± 0.1 | |
| milk–soya (1:2) | 7.7 a ± 0.1 | 7.9 a ± 0.1 | 7.9 a ± 0.2 | 8.1 a ± 0.1 | |
| soya beverage | 7.8 a ± 0.1 | 7.7 a ± 0.2 | 7.9 a ± 0.1 | 8.0 a ± 0.1 | |
| Propionibacterium spp. population [log(CFU/g)] | |||||
| cow’s milk | 5.7 a ± 0.1 | 5.8 a ± 0.1 | 5.6 a ± 0.1 | 5.4 a ± 0.1 | |
| milk–soya (2:1) | 5.6 a ± 0.1 | 5.7 a ± 0.1 | 5.7 a ± 0.1 | 5.7 a ± 0.1 | |
| milk–soya (1:1) | 5.7 a ± 0.1 | 5.7 a ± 0.1 | 5.7 a ± 0.1 | 5.8 a ± 0.1 | |
| milk–soya (1:2) | 5.8 a ± 0.1 | 5.8 a ± 0.1 | 5.8 a ± 0.1 | 5.6 a ± 0.1 | |
| soya beverage | 5.9 a ± 0.1 | 5.6 a ± 0.1 | 5.8 a ± 0.1 | 5.8 a ± 0.1 | |
| Propionic Bacteria Fermented Beverages | |||||
| Propionibacterium spp. population [log(CFU/g)] | |||||
| cow’s milk | 5.7 a ± 0.1 | 5.3 a ± 0.1 | 5.4 a ± 0.1 | 5.7 a ± 0.1 | |
| milk–soya (2:1) | 5.4 a ± 0.1 | 5.1 a ± 0.1 | 5.5 a ± 0.1 | 5.5 a ± 0.2 | |
| milk–soya (1:1) | 5.7 a ± 0.1 | 5.1 a ± 0.3 | 5.6 a ± 0.1 | 5.6 a ± 0.2 | |
| milk–soya (1:2) | 5.8 a ± 0.1 | 5.6 a ± 0.1 | 5.7 a ± 0.1 | 5.5 a ± 0.1 | |
| soya beverage | 5.8 a ± 0.1 | 5.7 a ± 0.1 | 5.6 a ± 0.2 | 5.3 a ± 0.1 | |
| Type of Carbohydrate | Storage Time [days] | 0 | 7 | 14 | 21 |
|---|---|---|---|---|---|
| Cow’s Milk Yoghurt Products | |||||
| yoghurt bacteria fermented beverages | |||||
| glucose | nd1 | nd | nd | nd | |
| galactose | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.01 | 0.01 a ± 0.01 | |
| lactose | 0.44 b ± 0.03 | 0.43 b ± 0.02 | 0.40 b ± 0.02 | 0.42 b ± 0.03 | |
| yoghurt and propionic bacteria (1:1) fermented beverages | |||||
| glucose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| galactose | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | <0.01 a | |
| lactose | 0.42 b ± 0.03 | 0.45 b ± 0.03 | 0.40 b ± 0.02 | 0.34 b ± 0.03 | |
| propionic bacteria fermented beverages | |||||
| glucose | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | |
| galactose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.49 b ± 0.03 | 0.48 b ± 0.02 | 0.50 b ± 0.03 | 0.47 b ± 0.02 | |
| Milk–Soya (2:1) Yoghurt Products | |||||
| yoghurt bacteria fermented beverages | |||||
| glucose | nd | nd | nd | nd | |
| galactose | 0.01 a ± 0.01 | 0.01 a ± 0.01 | 0.01 a ± 0.01 | 0.01 a ± 0.01 | |
| maltose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.34 b ± 0.02 | 0.33 b ± 0.02 | 0.31 b ± 0.03 | 0.29 b ± 0.02 | |
| sucrose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| raffinose | nd | nd | nd | nd | |
| stachyose | nd | nd | nd | nd | |
| verbascose | nd | nd | nd | nd | |
| yoghurt and propionic bacteria (1:1) fermented beverages | |||||
| glucose | nd | nd | nd | nd | |
| galactose | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.00 | |
| maltose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.25 b ± 0.02 | 0.26 b ± 0.02 | 0.23 b ± 0.02 | 0.27 b ± 0.02 | |
| sucrose | nd | nd | nd | nd | |
| raffinose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| stachyose | nd | nd | nd | nd | |
| verbascose | nd | nd | nd | nd | |
| propionic bacteria fermented beverages | |||||
| glucose | nd | nd | nd | nd | |
| galactose | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | |
| maltose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.27 b ± 0.02 | 0.27 b ± 0.03 | 0.26 b ± 0.03 | 0.26 b ± 0.02 | |
| sucrose | nd | nd | nd | nd | |
| raffinose | nd | nd | nd | nd | |
| stachyose | nd | nd | nd | nd | |
| verbascose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| Milk–Soya (1:1) Yoghurt Products | |||||
| yoghurt bacteria fermented beverages | |||||
| glucose | nd | nd | nd | nd | |
| galactose | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.03 a ± 0.00 | 0.01 a ± 0.00 | |
| maltose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.18 b ± 0.02 | 0.20 b ± 0.02 | 0.21 b ± 0.02 | 0.20 b ± 0.02 | |
| sucrose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| raffinose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| stachyose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| verbascose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| yoghurt and propionic bacteria (1:1) fermented beverages | |||||
| glucose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| galactose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| maltose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.13 b ± 0.02 | 0.21 b ± 0.02 | 0.21 b ± 0.02 | 0.20 b ± 0.02 | |
| sucrose | nd | nd | nd | nd | |
| raffinose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| stachyose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| verbascose | nd | nd | nd | nd | |
| propionic bacteria fermented beverages | |||||
| glucose | nd | nd | nd | nd | |
| galactose | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.01 | 0.01 a ± 0.00 | |
| maltose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.24 b ± 0.02 | 0.18 b ± 0.02 | 0.17 b ± 0.03 | 0.22 b ± 0.02 | |
| sucrose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| raffinose | nd | nd | nd | nd | |
| stachyose | nd | nd | nd | nd | |
| verbascose | nd | nd | nd | nd | |
| Milk–Soya (1:2) Yoghurt Products | |||||
| yoghurt bacteria fermented beverages | |||||
| glucose | nd | nd | nd | nd | |
| galactose | 0.01 ± 0.01 | <0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 | |
| maltose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.12 ± 0.02 | 0.10 ± 0.02 | 0.01 ± 0.01 | 0.11 ± 0.02 | |
| sucrose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| raffinose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| stachyose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| verbascose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| yoghurt and propionic bacteria (1:1) fermented beverages | |||||
| glucose | nd | nd | nd | nd | |
| galactose | 0.01 a ± 0.00 | 0.01 a ± 0.01 | <0.01 a | 0.01 a ± 0.01 | |
| maltose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| lactose | 0.09 b ± 0.01 | 0.12 b ± 0.01 | 0.08 b ± 0.02 | 0.10 b ± 0.02 | |
| sucrose | nd | nd | nd | nd | |
| raffinose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| stachyose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| verbascose | nd | nd | nd | nd | |
| propionic bacteria fermented beverages | |||||
| glucose | nd | nd | nd | nd | |
| galactose | 0.01 a ± 0.01 | <0.01 a | 0.01 a ± 0.00 | 0.01 a ± 0.01 | |
| maltose | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.01 | 0.01 a ± 0.01 | |
| lactose | 0.12 b ± 0.02 | 0.10 b ± 0.02 | 0.12 b ± 0.02 | 0.13 b ± 0.02 | |
| sucrose | nd | nd | nd | nd | |
| raffinose | nd | nd | nd | nd | |
| stachyose | nd | nd | nd | nd | |
| verbascose | nd | nd | nd | nd | |
| Soya Yoghurt Products | |||||
| yoghurt bacteria fermented beverages | |||||
| glucose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| galactose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| maltose | <0.01 a | 0.01 a ± 0.01 | 0.03 a ± 0.01 | 0.01 a ± 0.01 | |
| lactose | nd | nd | nd | nd | |
| sucrose | nd | nd | nd | nd | |
| raffinose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| stachyose | nd | nd | nd | nd | |
| verbascose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| yoghurt and propionic bacteria (1:1) fermented beverages | |||||
| glucose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| galactose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| maltose | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.01 | 0.02 a ± 0.01 | |
| lactose | nd | nd | nd | nd | |
| sucrose | nd | nd | nd | nd | |
| raffinose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| stachyose | nd | nd | nd | nd | |
| verbascose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| propionic bacteria fermented beverages | |||||
| glucose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| galactose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| maltose | 0.01 a ± 0.01 | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.00 | |
| lactose | nd | nd | nd | nd | |
| sucrose | nd | nd | nd | nd | |
| raffinose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
| stachyose | nd | nd | nd | nd | |
| verbascose | <0.01 a | <0.01 a | <0.01 a | <0.01 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziarno, M.; Zaręba, D.; Dryzek, W.; Hassaliu, R.; Florowski, T. Effect of the Addition of Soy Beverage and Propionic Bacteria on Selected Quality Characteristics of Cow’s Milk Yoghurt Products. Appl. Sci. 2022, 12, 12603. https://doi.org/10.3390/app122412603
Ziarno M, Zaręba D, Dryzek W, Hassaliu R, Florowski T. Effect of the Addition of Soy Beverage and Propionic Bacteria on Selected Quality Characteristics of Cow’s Milk Yoghurt Products. Applied Sciences. 2022; 12(24):12603. https://doi.org/10.3390/app122412603
Chicago/Turabian StyleZiarno, Małgorzata, Dorota Zaręba, Wiktoria Dryzek, Rozeta Hassaliu, and Tomasz Florowski. 2022. "Effect of the Addition of Soy Beverage and Propionic Bacteria on Selected Quality Characteristics of Cow’s Milk Yoghurt Products" Applied Sciences 12, no. 24: 12603. https://doi.org/10.3390/app122412603
APA StyleZiarno, M., Zaręba, D., Dryzek, W., Hassaliu, R., & Florowski, T. (2022). Effect of the Addition of Soy Beverage and Propionic Bacteria on Selected Quality Characteristics of Cow’s Milk Yoghurt Products. Applied Sciences, 12(24), 12603. https://doi.org/10.3390/app122412603








