Distinctive Handwriting Signs in Early Parkinson’s Disease
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Clinical Assessments
2.1.2. Demographic and Clinical Features
2.2. Procedure
2.3. Analyzed Features
2.4. Data Analysis
3. Results
3.1. Pen Speed
3.1.1. Free vs. Guided Conditions
3.1.2. Simple vs. Complex Shape Conditions
3.1.3. Habitual vs. Novel Motor Plan
3.1.4. Normal vs. Twice as Large Handwriting Size
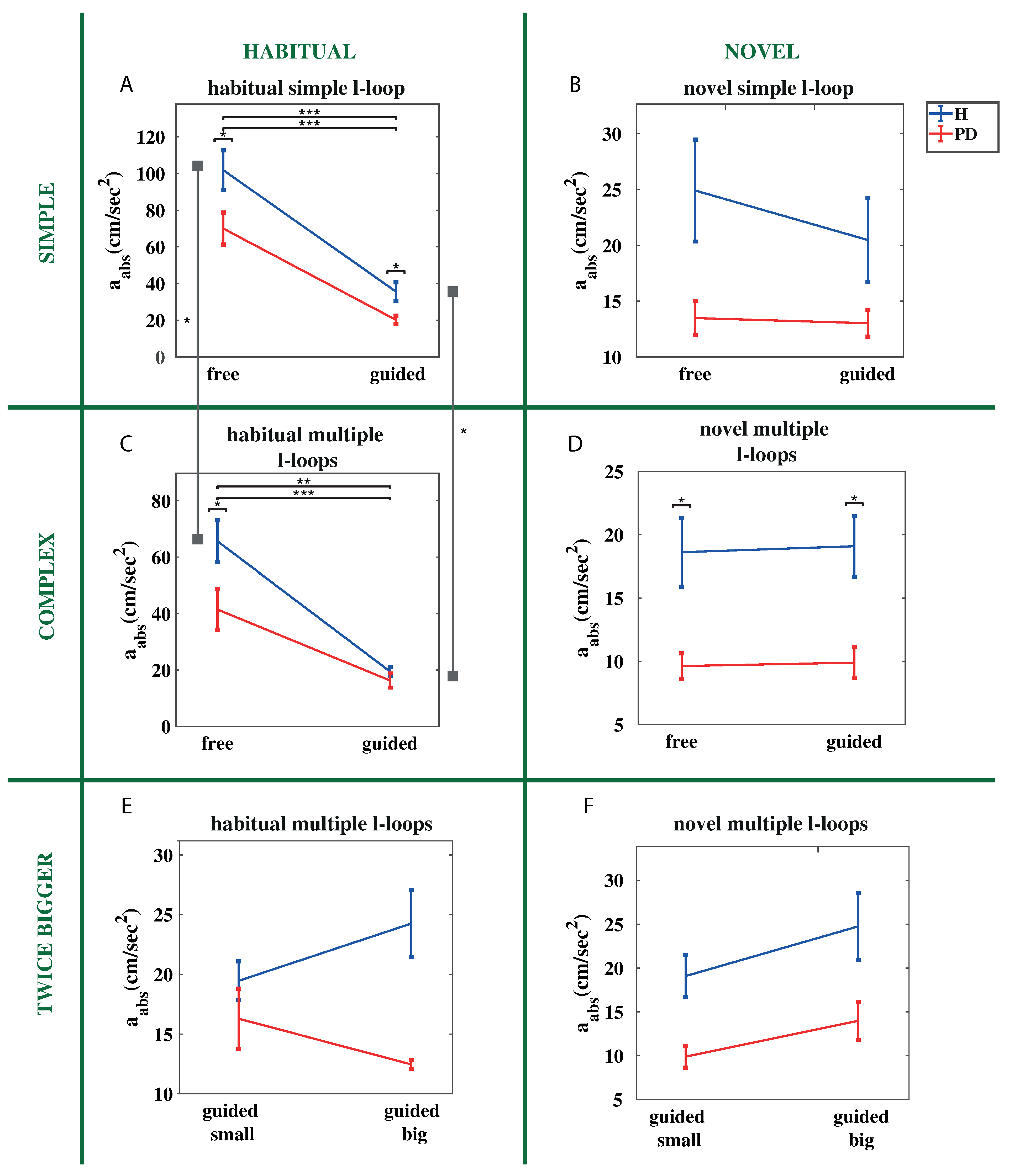
3.2. Acceleration
3.2.1. Free vs. Guided Conditions
3.2.2. Simple vs. Complex Shape Conditions
3.2.3. Habitual vs. Novel Motor Plan
3.2.4. Increased Handwriting Size
3.2.5. Acceleration at Novel Segmentation Points
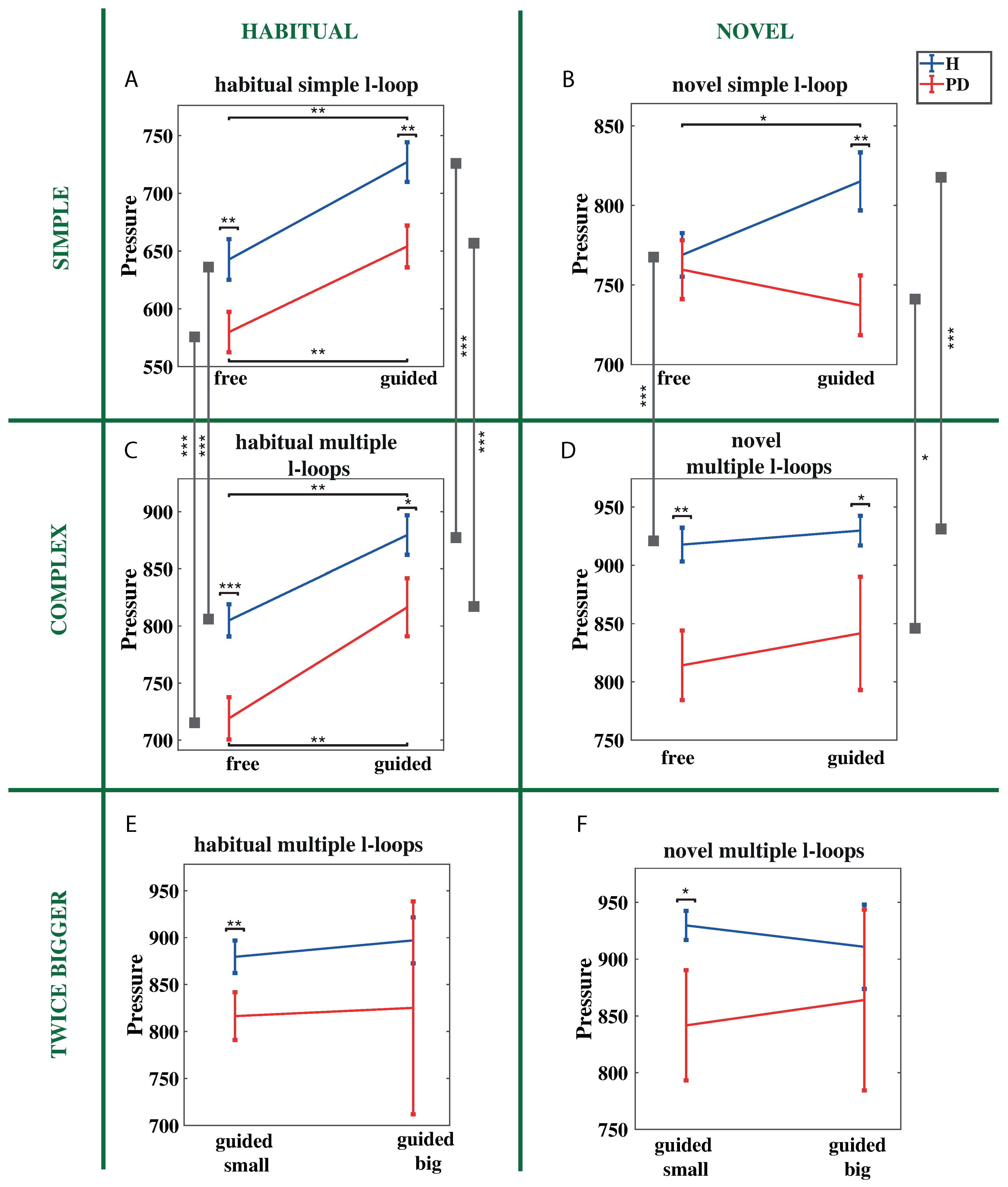
3.3. Pressure
3.3.1. Free vs. Guided Conditions
3.3.2. Simple vs. Complex Shape Conditions
3.3.3. Habitual vs. Novel Motor Plan
3.3.4. Increased Handwriting Size
3.4. Normalized Jerk
3.4.1. Free vs. Guided Conditions
3.4.2. Simple vs. Complex Shape Conditions
3.4.3. Habitual vs. Novel Motor Plan
3.4.4. Increased Handwriting Size
3.5. Stroke Size
3.5.1. Free vs. Guided Conditions
3.5.2. Simple vs. Complex Shape Conditions
3.5.3. Habitual vs. Novel Motor Plan
3.5.4. Increased Handwriting Size
3.6. Reaction Time
3.6.1. Free vs. Guided Conditions
3.6.2. Simple vs. Complex Shape Conditions
3.6.3. Habitual vs. Novel Motor Plan
3.6.4. Increased Handwriting Size
3.7. In-Air and On-Paper Parameters
4. Discussion
4.1. PD Patients Rely More on Visual Feedback with Increasing Shape Complexity
- For simple tasks, the performance difference between the H and PD groups observed in the free condition does not become smaller in the guided condition. In contrast, the discrepancy between groups in terms of handwriting fluency (normalized jerk) increases in the guided condition.
- For complex tasks, the difference of performance (in terms of velocity and acceleration) between groups in the guided condition is reduced compared to free condition. Indeed, we observed that, in the guided condition, the performance of H subjects has a larger decay compared to that of PD patients, and that the performance of patients becomes not significantly different from that of healthy subjects.
4.2. Increasing Task Shape Complexity Affects the Performance of Healthy Participants More than the Performance of PD Patients
4.3. Isochrony Is Preserved in the Early Stage of PD
4.4. Handwriting Fluency in PD Patients at the Early Stage Is Slightly Affected by the Disease
4.5. Performance of PD Patients Improves in the Execution of Novel Motor Plan Tasks in Terms of Reaction Time and Exerted Handwriting Pressure
4.6. Micrographia in Early PD Is More Associated to Shape Complexity than Motor Plan Complexity
4.7. Criteria for the Early Diagnosis of the Disease through the Analysis of Handwriting
- Contain tasks characterized by different level of shape complexity, as those analyzed in this work. Indeed, reduced differences of performance (in terms of writing speed and acceleration) between simple and complex shape tasks would indicate the presence of the disease.
- Consider task performed with different writing conditions, such as freely and guided performed tasks, since the performance decay observed from free to guided conditions is informative for the early diagnosis. Here, we observed that, for patients with PD, performance decay between free and guided condition is greater for simple shape tasks than for complex shape tasks. For healthy subjects we observed the opposite, that is performance decay is greater for complex shape tasks than for simple shape tasks.
- Contain tasks aimed at testing the ability of patients in increasing handwriting size through the use of guiding dots. However, since we have observed that isochrony is preserved in the early stage of PD, the analysis has to be focused more on the percentage of stroke size increase, rather than other parameters, such as velocity and pressure, for identifying the signs of the disease in the early stage.
- Take into account that handwriting fluency is slightly affected by the disease in the early stage, and that normalized jerk values falling into a “healthy range” do not exclude the presence of the disease.
- Contain tasks involving the use of novel motor plan for drawing a simple known shape in different writing conditions, as those analyzed in this work. Indeed, since PD patients benefits from the execution of novel motor plan tasks in terms of response time and exerted handwriting pressure, a higher increase of exerted pressure when performing simple novel tasks in free condition would suggest the presence of the disease. Similarly, response time decrease or similar response time between free and guided conditions when drawing simple shapes by using a novel motor plan would suggest the presence of the disease.
4.8. PD Patients Perform Handwriting as They Got Stuck on the Early Stages of the Learning Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Physiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Flash, T.; Inzelberg, R.; Schechtman, E.; Korczyn, A.D. Kinematic analysis of upper limb trajectories in Parkinson’s disease. Exp. Neurol. 1992, 118, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.D. Slowness of movement in Parkinson’s disease. Mov. Disord. 1989, 4, S26–S37. [Google Scholar] [CrossRef] [PubMed]
- McLennan, J.E.; Nakano, K.; Tyler, H.R.; Schwab, R.S. Micrographia in Parkinson’s disease. J. Neurol. Sci. 1972, 15, 141–152. [Google Scholar] [CrossRef]
- Sheridan, M.R.; Flowers, K.A.; Hurrell, J. Programming and execution of movement in parkinson’s disease. Brain 1987, 110, 1247–1271. [Google Scholar] [CrossRef]
- Teulings, H.L.; Stelmach, G.E. Control of stroke size, peak acceleration, and stroke duration in Parkinsonian handwriting. Hum. Mov. Sci. 1991, 10, 315–334. [Google Scholar] [CrossRef]
- Gemmert Van, A.W.A.; Teulings, H.L.; Contreras-Vidal, J.L.; Stelmach, G.E. Parkinson’s disease and the control of size and speed in handwriting. Neuropsychologia 1999, 37, 685–694. [Google Scholar] [CrossRef]
- Teulings, H.; Contreras-Vidal, J.; Stelmach, G.; Adler, C. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Exp. Neurol. 1997, 146, 159–170. [Google Scholar] [CrossRef]
- Van Gemmert, A.; Adler, C.H.; Stelmach, G.E. Parkinson’s disease patients undershoot target size in handwriting and similar tasks. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1502–1508. [Google Scholar] [CrossRef]
- Stelmach, G.E.; Teasdale, N.; Phillips, J.; Worringham, C.J. Force production characteristics in Parkinson’s disease. Exp. Brain Res. 1989, 76, 165–172. [Google Scholar] [CrossRef]
- Stelmach, G.E.; Worringham, C.J. The preparation and production of isometric force in Parkinson’s disease. Neuropsychologia 1988, 26, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Broderick, M.P.; Van Gemmert, A.W.A.; Shill, H.A.; Stelmach, G.E. Hypometria and bradykinesia during drawing movements in individuals with Parkinson’s disease. Exp. Brain Res. 2009, 197, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Vessio, G. Dynamic Handwriting Analysis for Neurodegenerative Disease Assessment: A Literary Review. Appl. Sci. 2019, 9, 4666. [Google Scholar] [CrossRef]
- Senatore, R.; Della Cioppa, A.; Marcelli, A. Automatic Diagnosis of Neurodegenerative Diseases: An evolutionary approach for facing the interpretability problem. Information 2019, 10, 30. [Google Scholar] [CrossRef]
- Senatore, R.; Della Cioppa, A.; Marcelli, A. Automatic diagnosis of Parkinson disease through handwriting analysis: A cartesian genetic programming approach. In Proceedings of the IEEE Symposium on Computer-Based Medical Systems, Cordoba, Spain, 5–7 June 2019; Volume 2019, pp. 312–317. [Google Scholar] [CrossRef]
- Senatore, R.; Marcelli, A. A paradigm for emulating the early learning stage of handwriting: Performance comparison between healthy controls and Parkinson’s disease patients in drawing loop shapes. Hum. Mov. Sci. 2019, 65, 89–101. [Google Scholar] [CrossRef]
- Teulings, H.L.; Contreras-Vidal, J.L.; Stelmach, G.E.; Adler, C.H. Adaptation of handwriting size under distorted visual feedback in patients with Parkinson’s disease and elderly and young controls. J. Neurol. Neurosurg. Psychiatry 2002, 72, 315–324. [Google Scholar] [CrossRef]
- Parisi, R.; Parziale, A.; Marcelli, A. Some observations on lognormality and motor control in handwriting. In Proceedings of the 1st International Conference on Pattern Recognition and Artificial Intelligence, Kolkata, India, 20–22 December 2013; pp. 732–736. [Google Scholar]
- Smits, E.J.; Tolonen, A.J.; Cluitmans, L.; Van Gils, M.; Conway, B.A.; Zietsma, R.C.; Leenders, K.L.; Maurits, N.M. Standardized handwriting to assess bradykinesia, micrographia and tremor in Parkinson’s disease. PLoS ONE 2014, 9, e97614. [Google Scholar] [CrossRef]
- Van Gemmert, A.W.; Teulings, H.L.; Stelmach, G.E. Parkinsonian patients reduce their stroke size with increased processing demands. Brain Cogn. 2001, 47, 504–512. [Google Scholar] [CrossRef]
- Nackaerts, E.; Vervoort, G.; Heremans, E.; Smits-Engelsman, B.C.M.; Swinnen, S.P.; Nieuwboer, A. Relearning of writing skills in Parkinson’s disease: A literature review on influential factors and optimal strategies. Neurosci. Biobehav. Rev. 2013, 37, 349–357. [Google Scholar] [CrossRef]
- Viviani, P.; Schneider, R. A Developmental Study of the Relationship Between Geometry and Kinematics in Drawing Movements. J. Exp. Psychol. Hum. Percept. Perform. 1991, 17, 198–218. [Google Scholar] [CrossRef]
- Gibb, W.R.; Lees, A.J. A comparison of clinical and pathological features of young- and old-onset parkinson’s disease. Neurology 1988, 38, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 2001, 57, 318. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef]
- Fahn, S.; Elton, R. Unified Parkinsons Disease Rating Scale. In Recent Developments in Parkinson’s Disease; Macmillan Health Care Information: Florham Park, NJ, USA, 1987; pp. 153–163. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Maeland, A.F.; Karlsdottir, R. Development of reading, spelling, and writing skills from third to sixth grade in normal and dysgraphic school children. In Development of Graphic Skills: Research Perspectives and Educational Implications; Academic Press: Cambridge, MA, USA, 1991; pp. 179–189. [Google Scholar]
- Marr, D.; Cermak, S. Consistency of handwriting in early elementary students. Am. J. Occup. Ther. 2003, 57, 161–167. [Google Scholar] [CrossRef][Green Version]
- Teulings, H. MovAlyzeR. 2010. Available online: www.neuroscript.net (accessed on 1 January 2022).
- Teulings, H.; Maarse, F. Digital recording and processing of handwriting movements. Hum. Mov. Sci. 1984, 3, 193–217. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Rosenblum, S.; Livneh-Zirinski, M. Handwriting process and product characteristics of children diagnosed with developmental coordination disorder. Hum. Mov. Sci. 2008, 27, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Viviani, P.; Burkhard, P.R.; Chiuvé, S.C.; Dell’Acqua, C.C.; Vindras, P. Velocity control in Parkinson’s disease: A quantitative analysis of isochrony in scribbling movements. Exp. Brain Res. 2009, 194, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, S.; Samuel, M.; Zlotnik, S.; Erikh, I.; Schlesinger, I. Handwriting as an objective tool for Parkinson’s disease diagnosis. J. Neurol. 2013, 260, 2357–2361. [Google Scholar] [CrossRef]
- Bloxham, C.A.; Mindel, T.A.; Frith, C.D. Initiation and execution of predictable and unpredictable movements in parkinson’s disease. Brain 1984, 107, 371–384. [Google Scholar] [CrossRef]
- Hallett, M. Clinical neurophysiology of akinesia. Rev. Neurol. 1990, 146, 585–590. [Google Scholar]
- Doyon, J.; Ungerleider, L.G. Functional anatomy of motor skill learning. In Neuropsychology of Memory, 3rd ed.; The Guilford Press: New York, NY, USA, 2002; pp. 225–238. [Google Scholar]
- Krebs, H.; Hogan, N.; Hening, W.; Adamovich, S.; Poizner, H. Procedural motor learning in parkinson’s disease. Exp. Brain Res. 2001, 141, 425–437. [Google Scholar] [CrossRef]
- Packard, M.G.; Knowlton, B.J. Learning and Memory Functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef]
- Horvitz, J.C.; Won, Y.C.; Morvan, C.; Eyny, Y.; Balsam, P.D. A “good parent” function of dopamine: Transient modulation of learning and performance during early stages of training. Ann. N. Y. Acad. Sci. 2007, 1104, 270–288. [Google Scholar] [CrossRef]
- Senatore, R.; Marcelli, A. A Neural Scheme for Procedural Motor Learning of Handwriting. In Proceedings of the 2012 International Conference on Frontiers in Handwriting Recognition, Bari, Italy, 18–20 September 2012; pp. 659–664. [Google Scholar] [CrossRef]
- Smith-Roe, S.L.; Kelley, A.E. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 7737–7742. [Google Scholar] [CrossRef]
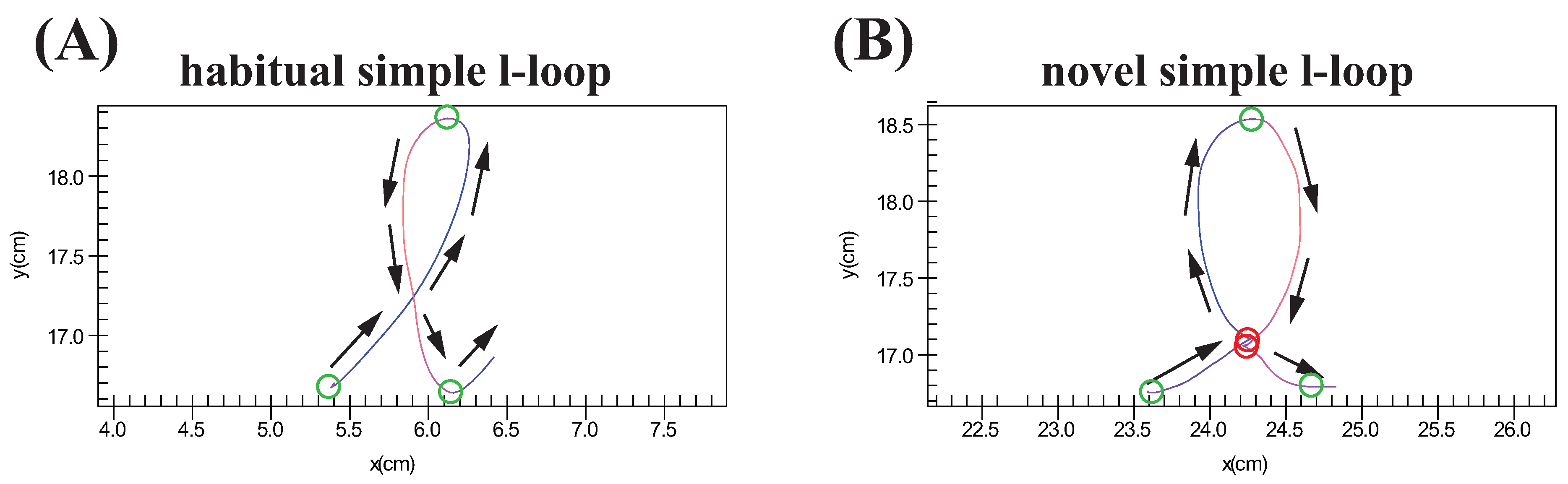



| PD N = 13 | Healthy N = 14 | Two-Tailed t-Test df = 25 | |
|---|---|---|---|
| Gender | 7 men and 6 women | 8 men and 6 women | p > 0.05 |
| Age (years) | 64.4 ± 1.5 | 60 ± 1.9 | p > 0.05 |
| Education (years) | 9.4 ± 1.2 | 11.3 ± 1.3 | p > 0.3 |
| Handedness score | 76.7 ± 12.2 | 69.4 ± 12.1 | p > 0.6 |
| MoCA corrected | 21.6 ± 0.7 | 22.1 ± 0.8 | p > 0.6 |
| BDI II score | 3.2 ± 0.7 | 2.5 ± 0.8 | p > 0.5 |
| Disease Duration (months) | 44.1 ± 5.6 | ||
| Modified Hoehn & Yahr score | 1.5 ± 0.1 | ||
| UPDRS III | 20.6 ± 2.6 |
| Task | Instructions | Example |
|---|---|---|
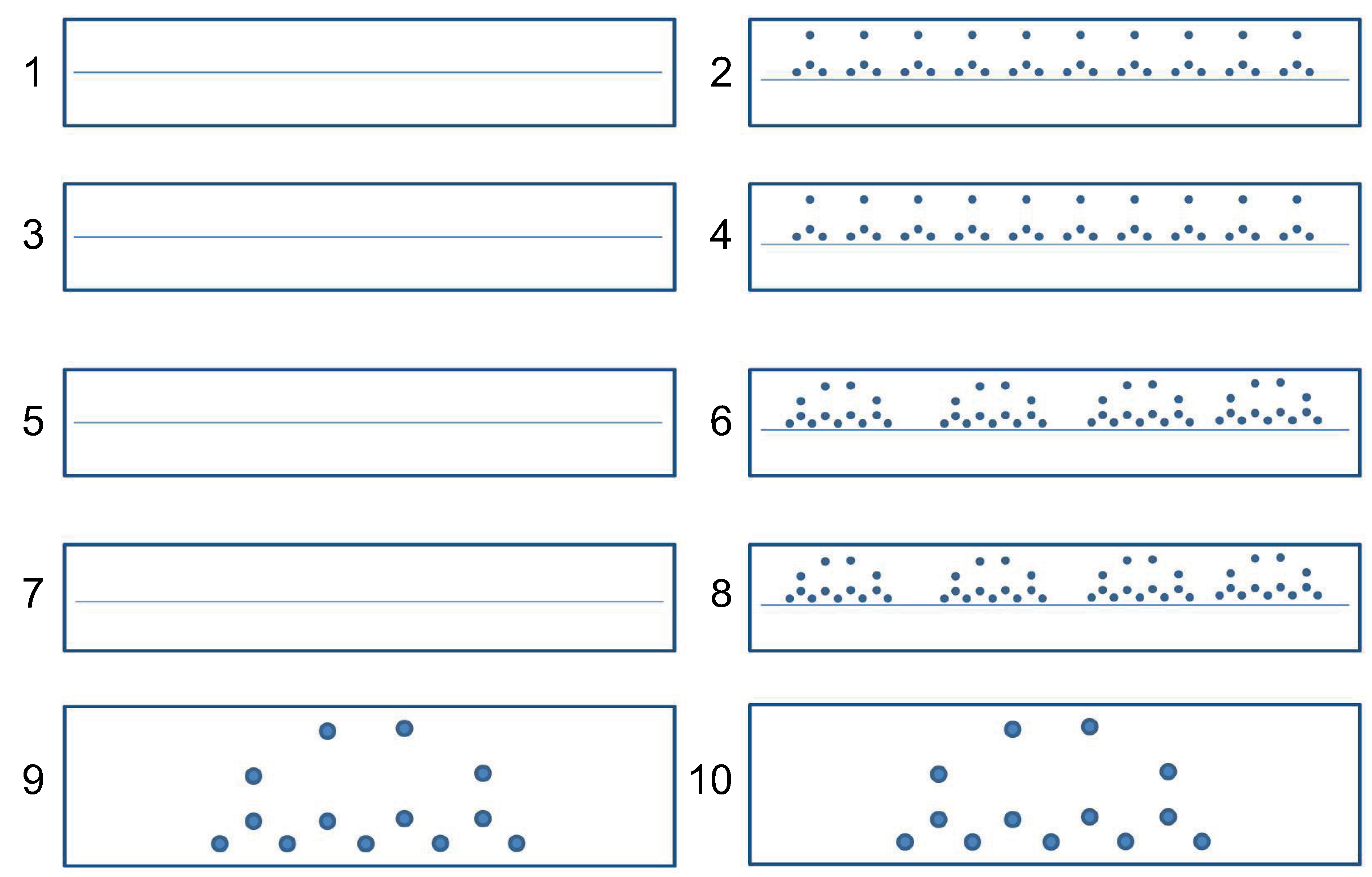
| 1 | Write a sequence of separated l-loops. |  |
| 2 | Write a sequence of 10 separated l-loops following the dots |  |
| 3 | Write a sequence of clockwise separated l-loops. |  |
| 4 | Write a sequence of 10 clockwise separated l-loops following the dots |  |
| 5 | Write a sequence of words “elle”. |  |
| 6 | Write a sequence of 4 words “elle” following the dots |  |
| 7 | Write a sequence of word “elle” drawing loop clockwise. |  |
| 8 | Write a sequence of 4 words “elle”, drawing loop clockwise and following the dots |  |
| 9 | Write the word “elle” bigger following the dots | 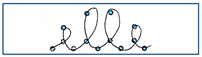 |
| 10 | Write the word “elle” drawing the loop clockwise, following the dots | 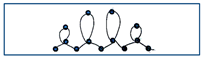 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senatore, R.; Marcelli, A.; De Micco, R.; Tessitore, A.; Teulings, H.-L. Distinctive Handwriting Signs in Early Parkinson’s Disease. Appl. Sci. 2022, 12, 12338. https://doi.org/10.3390/app122312338
Senatore R, Marcelli A, De Micco R, Tessitore A, Teulings H-L. Distinctive Handwriting Signs in Early Parkinson’s Disease. Applied Sciences. 2022; 12(23):12338. https://doi.org/10.3390/app122312338
Chicago/Turabian StyleSenatore, Rosa, Angelo Marcelli, Rosa De Micco, Alessandro Tessitore, and Hans-Leo Teulings. 2022. "Distinctive Handwriting Signs in Early Parkinson’s Disease" Applied Sciences 12, no. 23: 12338. https://doi.org/10.3390/app122312338
APA StyleSenatore, R., Marcelli, A., De Micco, R., Tessitore, A., & Teulings, H.-L. (2022). Distinctive Handwriting Signs in Early Parkinson’s Disease. Applied Sciences, 12(23), 12338. https://doi.org/10.3390/app122312338










