1. Introduction
According to the Centers for Disease Control and Prevention, more than 32,000 older adults (age 65+) die from falls every year [
1]. In the United States, healthcare spending on older adult falls has been approximately USD 50 billion annually [
2]. Moreover, the elderly population has a higher risk of stroke [
3]. Nearly half of the 6.5 million individuals who survive a stroke will have moderate to severe neurological deficits, 30% will be unable to walk unassisted and over 25% will need assistance in their daily activities [
4]. These impairments lead to activity limitation, decreased independence, falls and fear of falling [
5].
Sensory integration refers to a mechanism that combines orientation information (often represented as a weighted combination of sensory inputs) to serve as a basis for generating corrective actions that facilitate balance stabilization [
6]. Mahoney et al. reported that magnitude of multisensory integration can predict balance performance [
7]. Multisensory integration impairment is found to be present in people with chronic stroke [
8], older adults [
9], and individuals with Parkinson’s Disease [
10]. The deficits in attentional control and a larger time window of integration may explain why the impaired integration leads to postural instability and even falls in elderly people [
9]. When the elderly and other people with neurologic deficits try to maintain balance and simultaneously perform another task, the activity of the brain region associated with balance is significantly reduced while the brain regions of unrelated modalities are more active, especially in fall-prone individuals [
11].
It is posited that sensorimotor training (SMT), which includes sensory conflict conditions (varying inputs to the sensory systems), will be efficient in facilitating multisensory integration and thus, in enhancing the balance of survivors of stroke and older adults [
12]. Sensory conflicts in training can be achieved by distorting somatosensory inputs using soft surfaces on the ground or by restricting visual input by closing or covering the eyes, or by challenging vestibular inputs during walking backwards or in conditions where the vision and somatosensory inputs are limited or unreliable. A previous review on SMT studies highlighted Janda’s theory that the proprioception serves the critical role in balance and coordinated movement, and that people progress by performing exercises in different postures, bases of support, and challenges to their center of gravity through three stages of SMT: static, dynamic and functional [
13].
Ideally, the design of SMT should aim to facilitate not only the sensory input, but also integration of information in the central nervous system and motor output. In particular, a few functions and systems need to be intact in order for a person to have normal gait. These functions and systems include the locomotor function, which initiates and sustains rhythmic gait, postural reflex, sensory function, sensory integration, the musculoskeletal system and cardiopulmonary functions [
14]. The tasks in our SMT focus on the proprioceptive stimulations, locomotor function, and the musculoskeletal system by asking participants to perform the standing, step-up, and walking tasks on different surfaces with stable posture through three stages. In addition, few SMT studies had developed the details of tasks that aim to improve attentional control and reduce sensory integration time. To develop these tasks is rather difficult, as heightened multisensory integration due to aging can lead to performance decrements when sensory inputs are in conflict [
15]. To address this difficulty, our SMT tasks are designed to train elderly people in a safe environment to become adaptive to using reliable sensory cues, when exposed to different sensory conflicts among visual, vestibular and somatosensory systems.
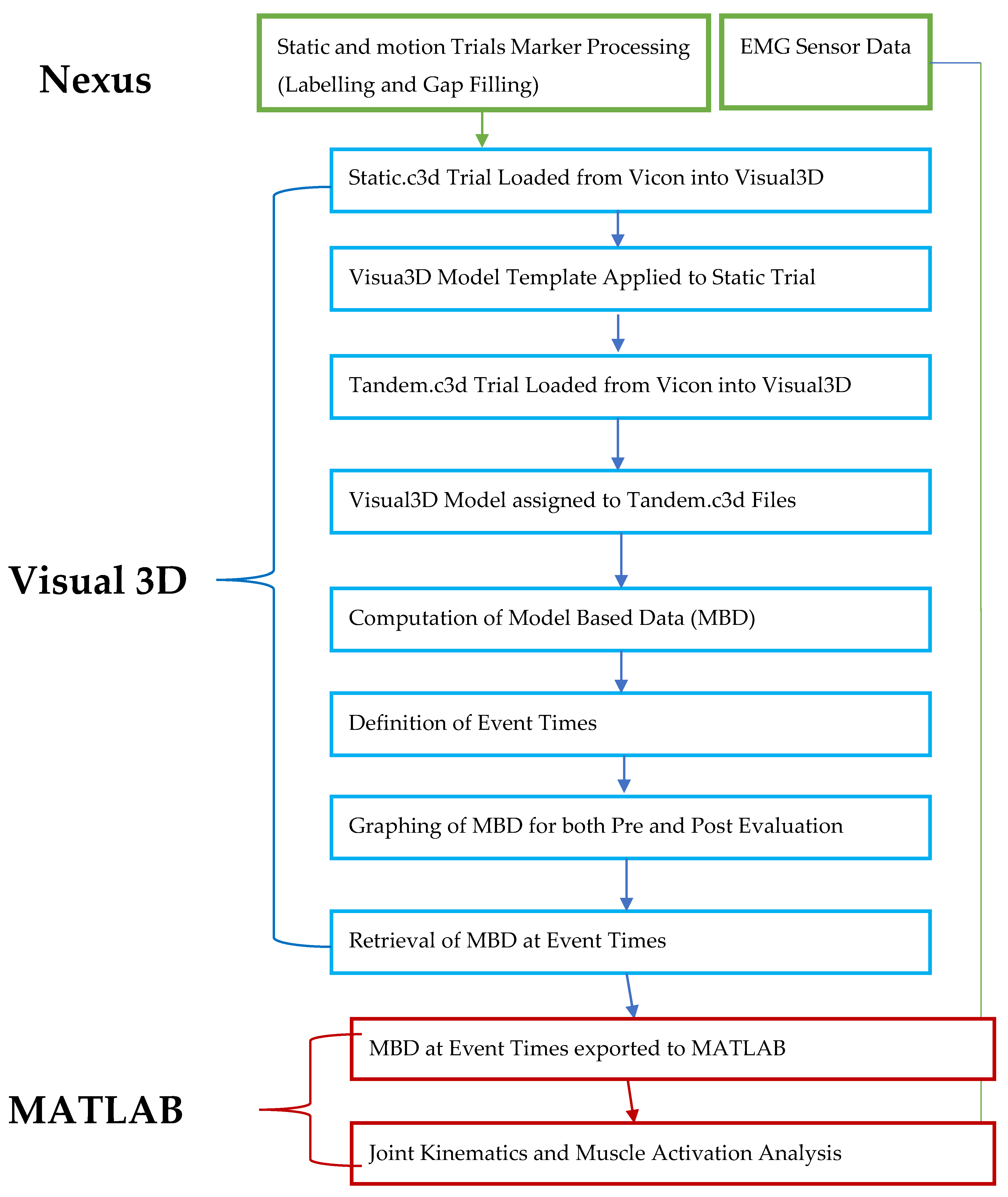
The scientific question for our assessment study is the following: can our 6-week-long SMT improve the gait and balance of people with weakened sensory systems? Biomechanical analysis is critical to the question we proposed. A motion capture-based assessment pipeline was thus developed to evaluate the joint angles, muscle activities and spatiotemporal parameters. We expect that kinematic and surface EMG data are more sensitive in detecting the changes of dynamic balancing between pre and post training than commonly used clinical scales
This manuscript aims to explain our assessment protocol and, as a case study, to describe motion analysis that investigates the effects of our sensorimotor training on the mobility of two elderly participants (one with chronic stroke). Our research hypothesis is that the training would improve the gait and balance by increasing the joint range of motion and extensor muscle activities in lower extremities and spatiotemporal measures.
4. Discussion
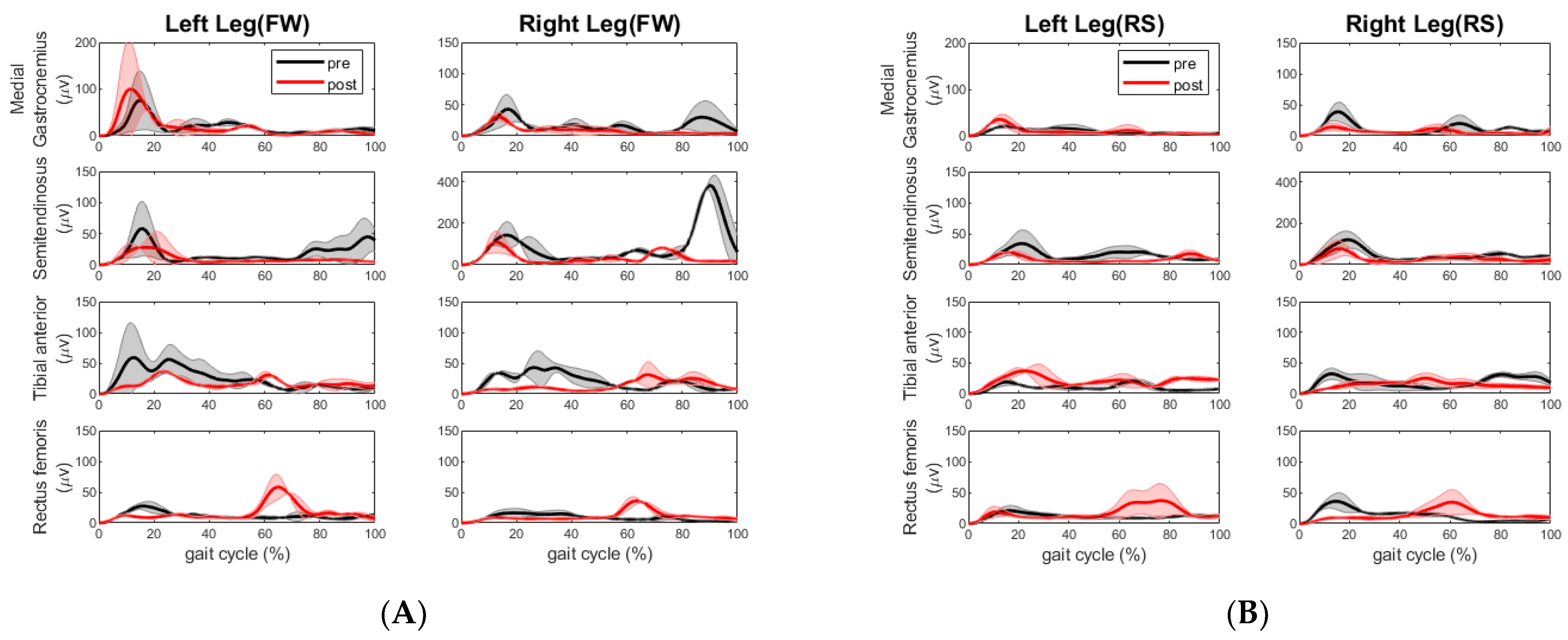
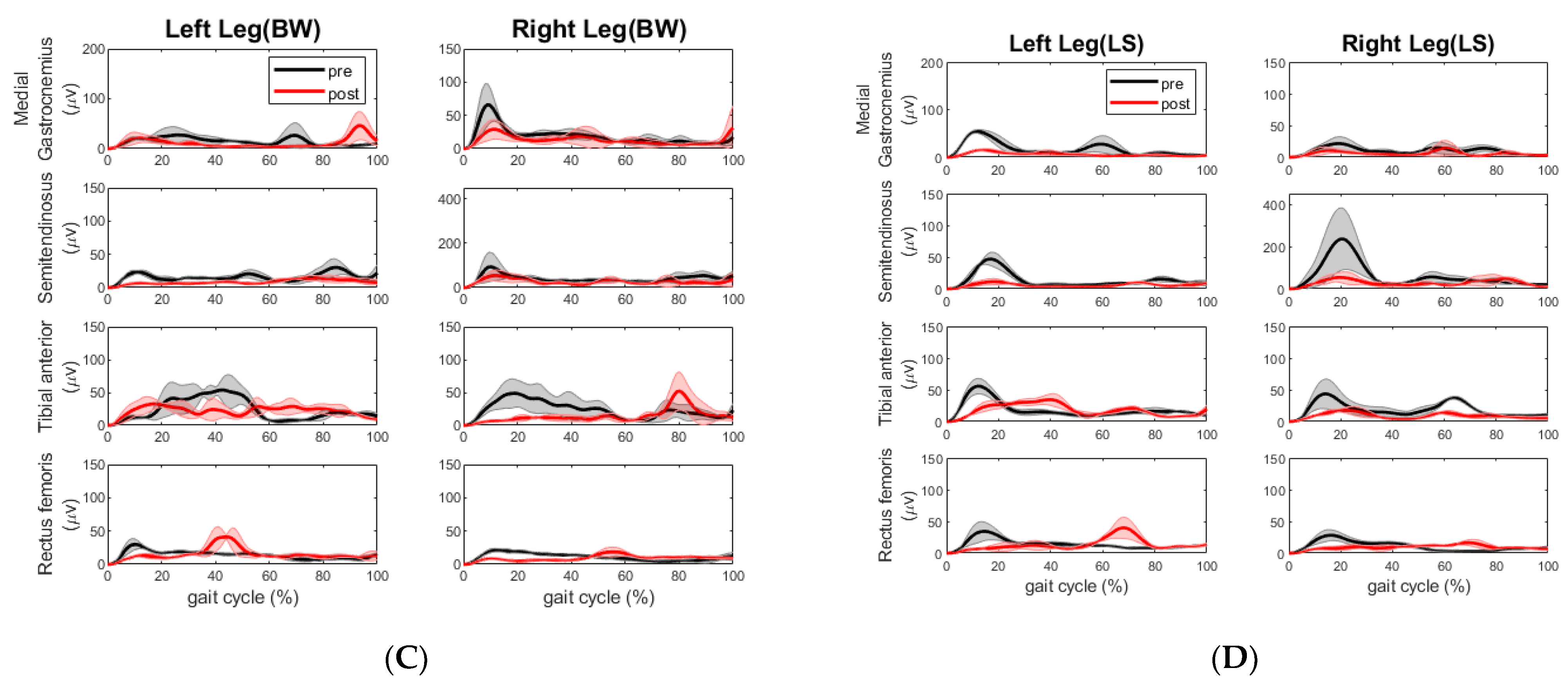
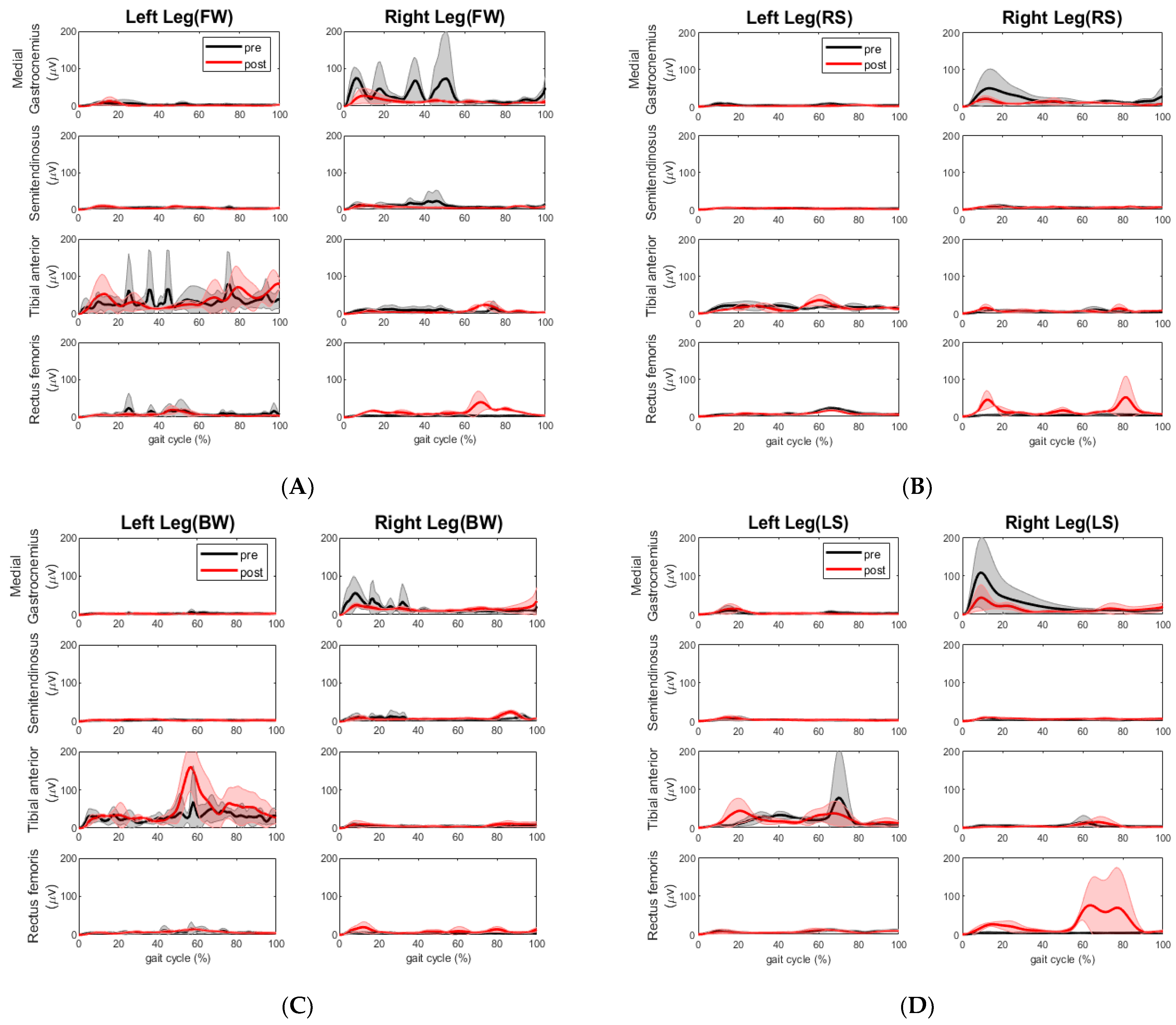
Here, we conducted a case study that utilized motion analysis to investigate the effects of a 6-week multi-sensorimotor training program on two elderly participants, one of whom had suffered a chronic stroke. Following our sensorimotor training, the walking speed of the chronic stroke participant improved across all segments. The overall time it took her to complete the tandem assessment walking pattern reduced significantly by 19 s. We took this to mean a positive result for the stroke participant. We also observed more consistent steps made during post-walking assessment compared to those made during pre-assessment. The impaired left leg improved step length and cadence and the non-impaired right leg improved cadence during forward and backward walking, as shown in
Table 6. The improvement of cadence and step length were also observed by the change of EMG activities. The extensor muscle on the right leg (rectus femoris) increased during forward walking. The extensor muscle activities (rectus femoris) and flexor muscle activities (semitendinosus) on the left leg decreased, whereas the same extensor muscle group on the right leg increased during right side stepping. This muscle coactivation pattern change is desired as the right leg is the leading leg for the right side stepping. During the backward walking, the increased extensor and decreased flexor muscle activities on the right leg may have allowed this stroke participant to have better control of footwork. During the left sidestepping, the increased extensor (rectus femoris) and flexor muscle (semitendinosus) activities on the right leg indicated that this participant relied on the right leg to make strides, although the impaired left leg was the lead leg. However, the decreased flexor muscle (medial gastrocnemius) activities might be a sign of reduced impairment in the left leg. Surely, it would be better if there was increased extensor muscle activity from the left leg and a more coordinated extensor and flexor muscle coactivation pattern during all walking segments.
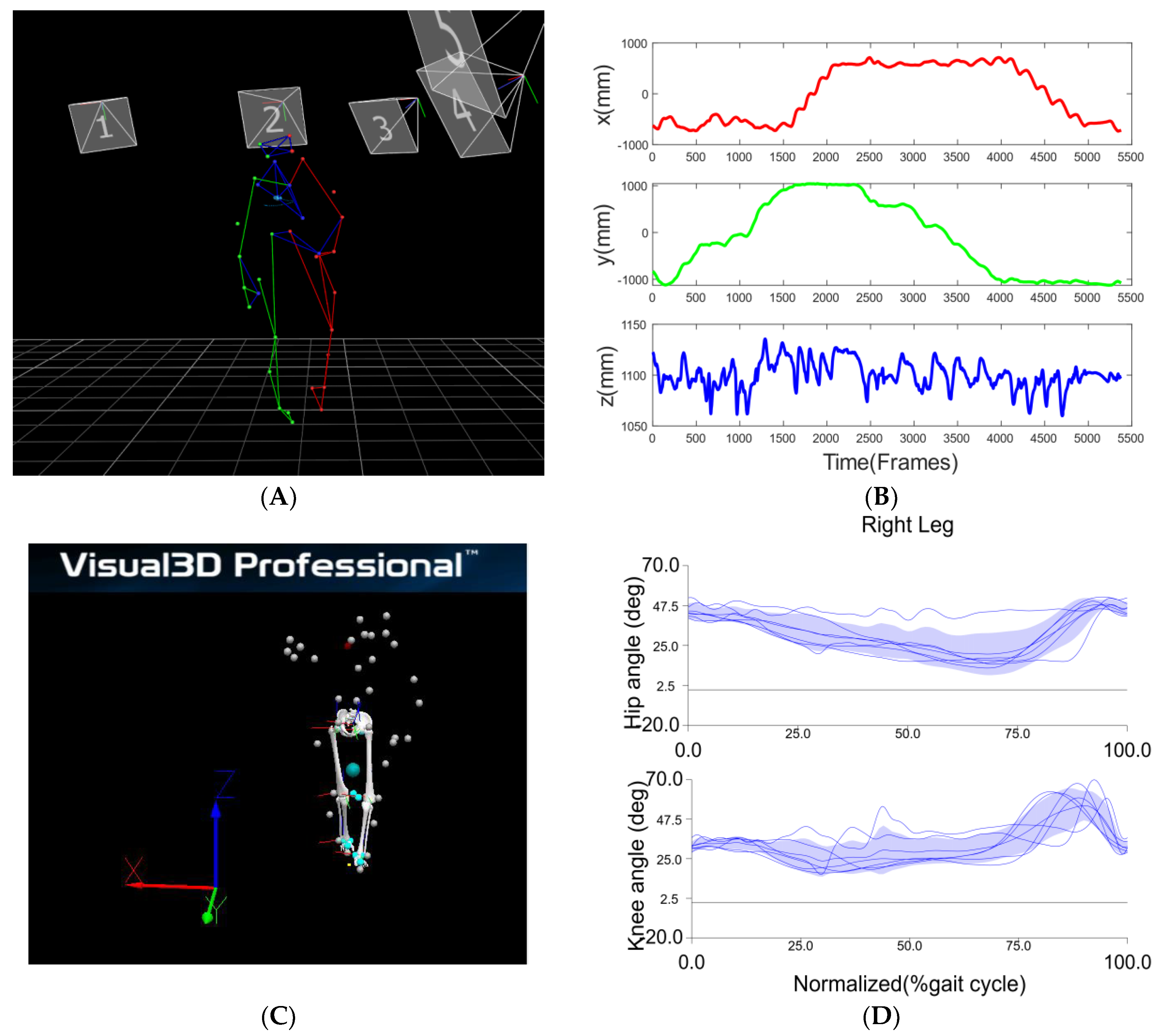
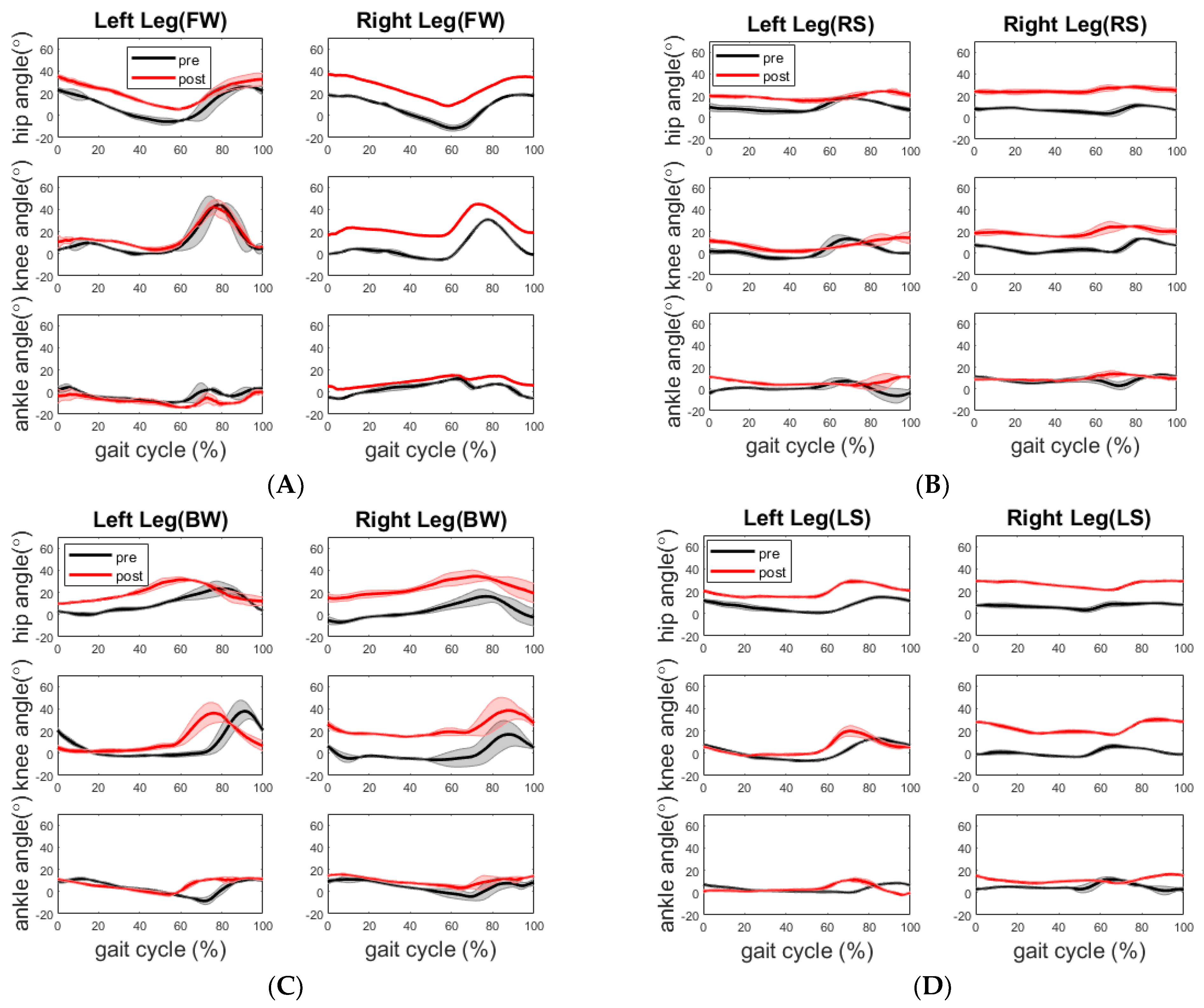
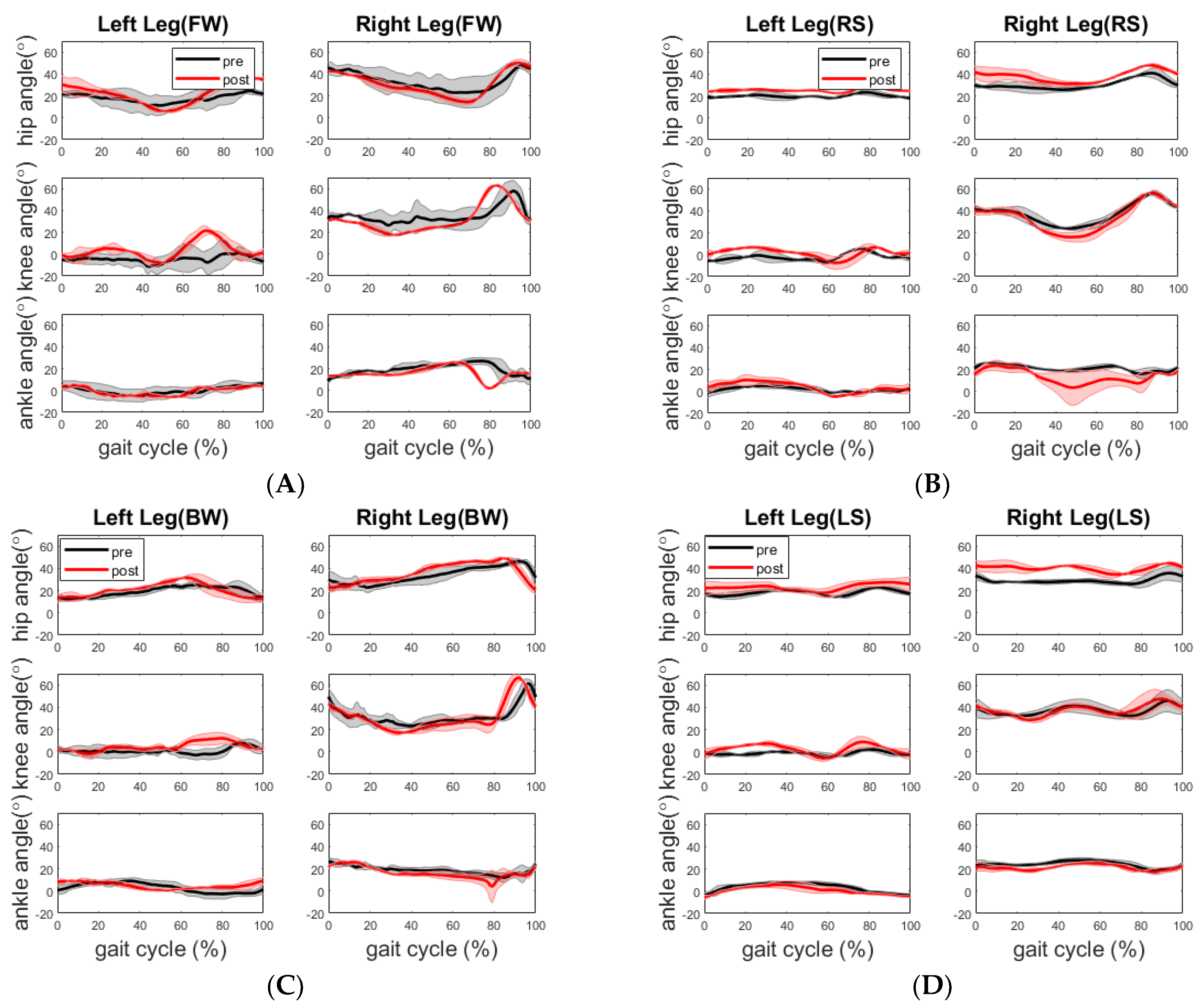
The healthy participant displayed a more flexed hip and knee on the right side across all walking sections post assessment, as shown in
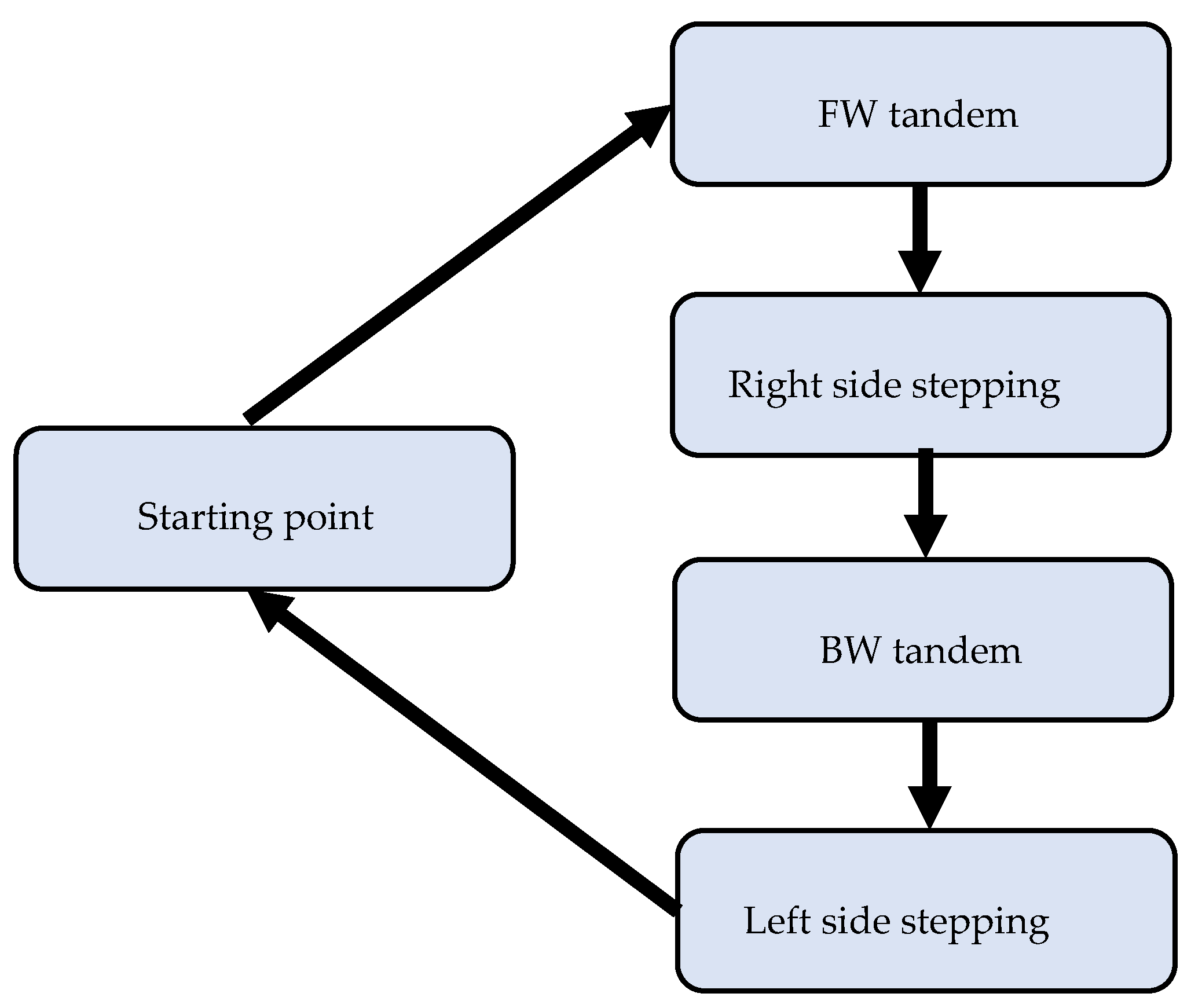
Figure 6. Although there is no significant improvement in walking time and step length during post assessment, the training may have led to the more balanced walking strategy in which the healthy participant walked along the path with reduced speed but better compliance of “heel-to-toe” tandem forward and backward walking. The more balanced walking strategy is also indicated by less difference between the cadences of the two legs. During the pre-assessment, the cadence of his right leg was higher than that of his left leg for forward and the right side stepping, whereas the cadence of the right leg was lower than that of the left leg for backward and left side stepping. In comparison, during the post-assessment, the cadence of his left leg increased during the forward walking section and decreased during the other three sections, whereas the cadence of his right leg increased during the backward walking section and decreased during the other three sections. Analysis of his EMG activity provided evidence for this shift. This finding might be due to the fact that the healthy elderly participant used a different side of leg to lead the forward (left leg lead) and backward (right leg lead) walking during post evaluation. We noticed that the healthy participant started from point A in the pre-assessment trial but unintentionally started from point D in the post-assessment trial. However, both healthy and stroke participants followed the same walking order.
In terms of joint angle, for the stroke participant, her joint ROMs during forward walking did not improve to the level of the reported minimally clinically important difference (MCID) from the straight-line walking study by Guzik et al. [
22,
23]. Her impaired left leg had a significant increase in hip joint ROM (5.8 ± 2.4 deg,
p = 0.019) during backward walking and a marginally significant increase in knee joint ROM (9.0 ± 6.7 deg,
p = 0.062) during the left side stepping. Her right hip joint ROM increased significantly (4.1 ± 3.7 deg,
p = 0.046) and the right knee joint ROM increased significantly (8.1 ± 2.9 deg,
p = 0.007) during backward walking. For the healthy participant, the significant increases were only found in his right knee joint ROM (4.2 ± 3.0 deg,
p = 0.031) and left ankle joint ROM (5.5 ± 0.4 deg,
p = 0.006) during the left side stepping. Currently, we did not find any studies that explicitly report MCIDs of backward walking and side stepping. However, the studies on walking strategies reported that backward walking involves conscious extension of the hip joint extension which is different from forward walking. The conscious extension of the hip joint during backward walking is difficult not only for hemiplegia but also for healthy elderly individuals due to the sensory conflicts. It has been reported that peak hip joint extension and peak knee joint flexion, ankle joint plantar flexion movement range, walking speed and cadence are significantly lower in backward walking than in forward walking for hemiplegia and healthy individuals. As for the side stepping, Sparto et al. reported two different strategies of lateral step behaviors to initiate stepping in older adults based on the profile of vertical ground reaction force (VGRF): one strategy involving a single postural adjustment (PA) made to directly unload the stepping leg which is adopted by younger adults; another one involving two PAs prior to liftoff, with the stepping leg initially loading to propel the center of mass toward the stance leg, followed by the VGRF decreasing to unload the stepping leg so that the step could be taken [
24]. Two PAs to initiate stepping may be caused by aging related declines in hip abductor muscle composition and torque generation [
25]. It was found that participants with a preferred strategy of generating two PAs had greater frequency of fall than those with a preferred strategy of generating one PA [
24]. Borrelli et al. reported the similar findings that older adults are prone to multistep responses when exposed to lateral pull at waist level, which are associated with an increased fall risk [
26]. We argue that the survivor of stroke with hemiplegia will tend to use the preferred strategy of generating two PAs, especially when the impaired leg initiates the stepping.
Overall, the results show that these dependent variables, walking speed, step length and cadence in spatiotemporal measures, and knee and hip joint angles are more sensitive to gait improvement or learning effect, among all the dependent variables. The walking path chosen for this gait evaluation involved the participants in four types of walking exercises (forward, backward, and left- and right-side stepping). This path provided us evidence on how well the participant performed a set of walking exercises in a row and the transition in between in terms of joint kinematics, muscle activities and postural stability. Compared to an only-straight-line walking path, this multidirectional path would better indicate participants’ walking ability in their daily activities. Because the results shown here were limited to two participants (one healthy elderly and one stroke with hemiplegia), it is fairly early to say if the change of joint angle profile, spatiotemporal measures, as well as EMG activities is a common trend for each population.
Although this study focuses on the biomechanical analysis of walking, we had used two clinical metrics such as the balance error scoring system (BESS) and activities specific balance confidence (ABC) [
26] to evaluate the balance control. In the BESS assessment, the participants perform double-leg, single-leg and tandem stances as they stand on either hand or foam support surfaces with eyes closed [
26]. The BESS assesses the trunk deviation from its upright posture and the number of deviations are counted as errors for six 20-s trials per condition [
26]. The ABC assesses the participant’s balance confidence using a list of survey questions relating to the gait and balance [
26]. A score of 100 represents high balance level and a score between 50 and 80 represents a moderate balance level. The low balance level has an ABC score of less than 50. Some of the clinical metrics currently used are Fugl-Meyer Assessment (FMA) for Lower Extremity, National Institutes of Health Stroke Scale (NIHSS), Timed Up and Go Test (TUG) [
27] and Tinetti Gait and Balance Assessment Tool [
28]. The FMA Lower Extremity [
29] is used to assess the level of lower limb impairment by quantifying the joint movement in supine, sitting or standing positions. The assessment of hip, knee and ankle flexion extension under these static positions may not reflect well the capability of these joints during the actual walking. The NIHSS is a systematic assessment tool that provides a quantitative measure of stroke-related neurologic deficit [
30]. Only item 6 of NIHSS assesses the motor function related to the leg in a supine position. TUG is a Falls risk screening tools for falls among older adults. However, it has been reported that the TUG should no longer be used as a falls risk assessment in community-dwelling elderly people due to its limited predictive ability [
27]. Tinetti assessment covers both gait and balance. However, the scoring of performance in each task is based on the examiner’s subjective observation and also, its binary scoring metrics is rather rigid to indicate the progress.
Sensorimotor exercises were developed and are of interest because, if successful in improving balance, they can be eventually translated to home-based practices and training. With the aging population in the United States and worldwide rapidly increasing, our society is in great need of safe and effective solutions that can be easily accessed on a daily basis by people regardless of their socio-economic status [
31,
32]. Due to the pandemic and the evolution of technology, lifestyles are shifting to make daily activities gravitate more towards the home and local community. To meet this need and shift, exercise programs that can be implemented outside of the laboratory or hospital settings have been developed and studied for fall prevention practices [
33,
34,
35]. These programs reportedly reduced the rate of falls by improving balance, leg strength and function, and physical activity and balance confidence in older adults living in the community [
36,
37]. The benefits of these exercises programs and sensorimotor therapies have led us to develop a multi-sensorimotor training program that can be delivered to meet both needs of gait rehabilitation and fall prevention in home environment.
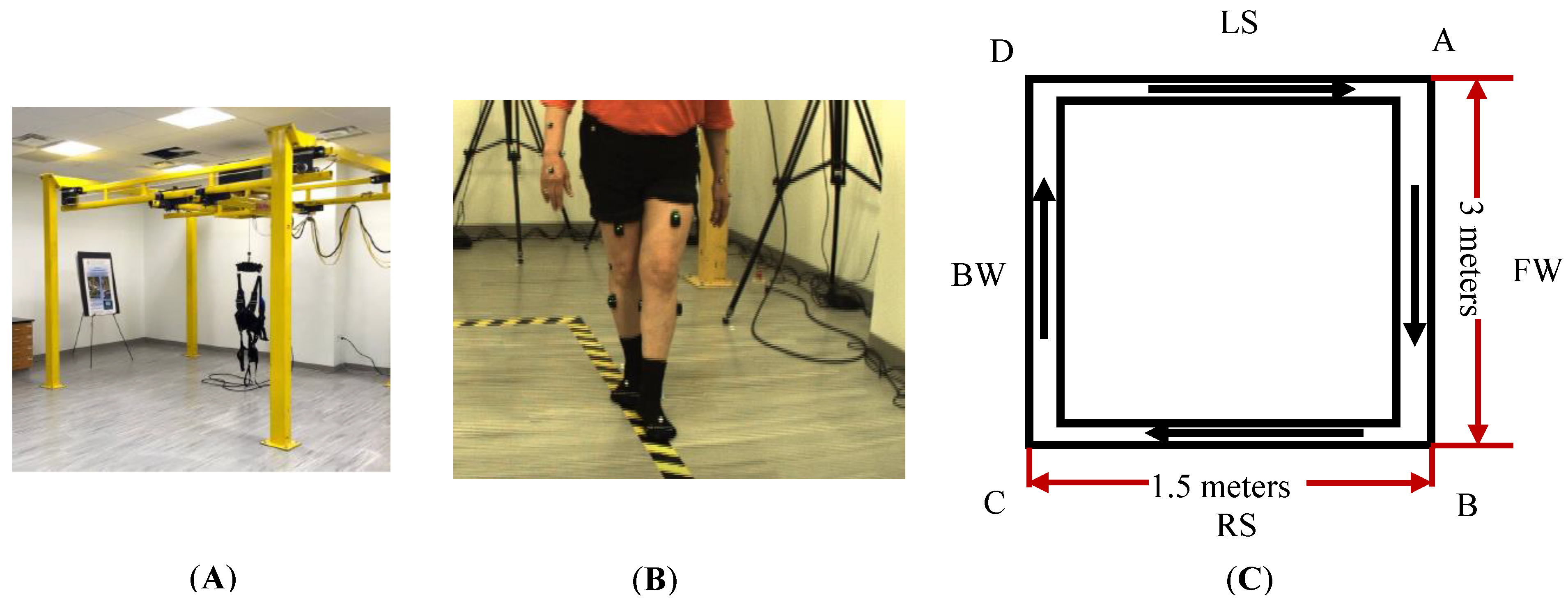
The present assessment protocol has two novelties. First, a rectangle walking path was used to assess the movement and balance. This path requires participants to follow the path and perform various walking patterns (tandem forward, side-stepping led by right leg, tandem backward, side-stepping led by left leg) and transitions in between. Performing these tasks in sequence and in short distance can assess the change of dynamic balancing and multisensory integration in different walking situations, which is critical for fall preventions. Second, we developed a data-processing pipeline that can produce spatiotemporal measures, the joint angle profile muscle activity profile with respect to the gait cycle, and can perform the statistical analysis.
Regarding the limitations of this study, first, the current training intensity of our 6-week SMT may not be sufficient for both participants to have clinically significant improvement in joint range of motion at each walking section. We plan to increase the training intensity from 30 min to 45 min and from 2 sessions a week to 3 sessions a week. The walking distance is relatively short on each walking segment as shown in
Figure 1C. Both participants may be not able to reach the steady-state walking pace, which may compromise the assessment of their movement and balance. Secondly, our motion capture system did not have the force plate to collect the ground reaction force (GRF) data which can provide more accurate time information on the gait events such as heel strike and toe off. The GRF data can also verify the surface EMG data we collected from four leg muscles, because the external moment calculated by GRF data should be balanced by internal muscle forces (internal moment). We therefore plan to install the force plate to collect GRF data. In addition, the current assessment protocol did not include the standardized gait assessment tasks. As we continue the gait analysis based on motion capture in more participants, we plan to include clinical assessments (e.g., ten-meter walk test (10MWT) to assess walking speed, six-minute walk test (6MWT)) to assess aerobic capacity and endurance [
38].