Abstract
UV filters are the shield ingredients of sunscreens against the negative effects of solar radiation. Since the discovery of the first UV filter, nearly 30 filters have become commercially available. Over the years, innovation and regulatory updates have driven their use by the cosmetic industry. This work aimed to characterize commercial sunscreens and unveil the main trends by analyzing the labels of 444 sunscreen formulations that are currently being marketed. Avobenzone, octocrylene, and bis-ethylhexyloxyphenol methoxyphenyl triazine were the three UV filters with the highest usage frequencies (>40%). Emulsified preparations and sprays were the preferred forms, while the most frequent SPF was 50+. Differences were noted between adult and children’s sunscreens, namely the higher usage of inorganic filters for the latter. Over the past few years, the five most used UV filters remained the same, but octocrylene, ethylhexyl salicylate, and nano methylene bis-benzotriazolyl tetramethylbutylphenol had decreased usage. An increasing tendency towards the use of the inorganic UV filter titanium oxide was also observed. Overall, this study characterized the true market impact of approved UV filters and how the market has evolved over recent years. This insight can help pave the way for the design of new UV filters and is helpful for the assessment of environmental risks.
1. Introduction
The noxious impact of solar radiation was known to many ancient civilizations, and individuals consequently tried to avoid photo-induced damage. Egyptians used natural extracts such as almond oil, calcite powder and clay, rice, and jasmine due to their photoprotective and healing properties [1,2,3]. Greeks protected themselves with oils and sands containing minerals during exercise, and these were later used in India for medicinal purposes [1,4,5]. Experimental studies about the damaging effects of UV radiation (UVR) in skin started in the early 1800’s [3]. Twenty years later, Everard Home researched the ability of a group of substances to absorb UVR. Acidified quinine sulfate (AQS) was the first compound that was proven to reduce sunburns induced by UVR [5]. After that, Friedrich Hammer, in 1891, combined AQS in lotions and created the first sunscreen [4]. The development of UV filters progressed, and, in 1894, Paul Unna and Dubreuilh confirmed that some skin disorders, such as erythema and skin cancer, were associated with excessive sunlight exposure without protection [5]. With this evidence, Hausser and Vahler started their research in this field, culminating in 1928 with the creation of the first formulation offering UVB protection, an emulsion containing benzyl cinnamate and benzyl salicylate [2,6]. Despite all these advances, the first commercially available sunscreen was only marketed in 1935, a creation of Eugene Shueller, the founder of L’Oréal. This sunscreen was called “Ambre Solaire” and contained benzyl salicylate [3,7], the same UVB filter present in Hausser and Vahler’s formulation [2,6]. In the 1940’s, p-aminobenzoic acid (PABA) and its derivatives were studied, with PABA derivatives revealed to be more efficient, namely the mono and dialkyl PABA ester derivatives [8]. Over the years, other methodologies were developed to prove the effectiveness and safety of UV filters. PABA was confirmed to be photounstable and a promotor of allergic reactions [9]. Further research confirmed its low photostability, as well as toxic effects, culminating in its ban in the European Union in 2008 [10]. In 1969, the dangerous effects of UVA radiation for the skin became more apparent [11]. The first UVA filters available were benzophenone derivatives. In 1980, only 1% of European sunscreens contained this substance, though this had increased to 35% ten years later [12]. It is important to mention that after the creation of the first sunscreen formulation, “Ambre Solaire”, this research topic stimulated the scientific community and increased the desire to understand the mechanisms behind sunscreen activity.
Sunscreens are products used worldwide but with different definitions and classifications across countries. The United States of America (USA), New Zealand, Canada, and Australia apply the drug regulations to sunscreens [13]. In contrast, in China, Japan, South Africa, and in some countries in South America, sunscreens are regulated as cosmetic products, similarly to the European Union (EU) [13]. UV filters are defined in Cosmetic Regulation (EC) n° 1223/2009 as “substances which are exclusively or mainly intended to protect the skin against certain UV radiation by absorbing, reflecting, or scattering UV radiation” [14]. Annex VI of this regulation mentions the list of approved UV filters for cosmetic use, with a total of 28 compounds being listed, including two inorganics (zinc oxide (ZnO) and titanium oxide (TiO2)), which can be present in non-nano or nano forms, and 26 organic UV filters. Of all the 26 organic UV filters, bisoctrizole and trisbiphenyl triazine can also be present in nano forms. One of the controlled parameters is the maximum concentration allowed in the formulation. Over the years, some alterations to the list of European approved UV filters were noted, including both approvals and withdrawals. In 2015, the EU removed 3-benzylidene camphor from the list of approved UV filters because of its toxicity towards marine organisms [15] and endocrine-disruptor potential [16]; in contrast, ZnO was approved in the following year (2016) [13,14,17]. Additionally, the effectiveness of the sunscreen formulations should be tested by standardized testing methods, namely ISO guidelines which include in vivo methods for determination of UVB SPF [18,19], in vitro and in vivo evaluation of UVA protection [20,21,22], and also evaluation of the water resistance of the product [10,23]. Considering the importance of the market characterization of sunscreens and in order to understand the usage frequency of UV filters in these photoprotective formulations, this work aims to provide readers with a market study focused on products commercialized in 2021 in pharmacies and parapharmacies. The usage frequency of UV filters was also evaluated with regard to adults’ and children’s photoprotective formulations. The evolution of UV filters was assessed with a comparison of sunscreens launched in 2015, unveiling the main trends and their underlying causes.
2. Materials and Methods
2.1. Data Collection
Label information was collected from a pool of 444 sunscreen formulations (379 for adults and 65 for children) from 43 international cosmetic brands marketed in Portuguese parapharmacies and pharmacies in 2021. Analysis of this composition was carried out by identifying the UV filters in the label. Information regarding galenic forms and SPF was also compiled. All the information available in the product labels was collected, along with information available on the manufacturers’ websites. Considering UV filter characterization, it is possible to group UV filters into organic or inorganic filters, and further group them according to their ability to absorb UVR or their classification as UVA and/or UVB filters. To analyse the evolution of the usage of UV filters, a comparison was performed among the sunscreens marketed in the same distribution channel in 2015 (205 sunscreens for adults and 31 for children). Each UV filter was searched for on the PubMed, Scopus, and Farmácias Portuguesas online databases using the keywords “INCI name”, “sunscreens”, OR “UV filters”.
2.2. Data Analysis
The UV filters contained in sunscreens were listed according to International Nomenclature of Cosmetic Ingredient (INCI) names. The collected data were analyzed regarding the UV filters and their association, galenic forms, and SPF value. The number of sunscreen products containing UV filters on their labels was evaluated and expressed as a percentage. The UV filters were identified according to INCI lists and ranked in descending order of occurrence to disclose the most used UV filters. The association of UV filters in terms of being only organic, only inorganic, and organic and inorganic was also studied, and presented as a percentage, for adult and children’s sunscreens separately. The galenic form of the sunscreen formulations was also unveiled for adult and children’s sunscreens. Sunscreens were also studied, grouped, and analyzed according to their SPF value.
3. Results
3.1. Usage Frequency of UV Filters in Sunscreens for Adults and Children
Among the 28 UV filters approved by Regulation (EC) n° 1223/2009, 24 were identified in the market study performed. Through the analysis of adult sunscreens, a higher usage of organic UV filters was observed (20 of the 24, Figure 1) when compared to inorganic filters, including both nano and non-nano forms (4 of the 24, Figure 1). Avobenzone (BMDBM), octocrylene (OC), and bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S®) were the three most frequently used UV filters with percentages that varied between 45% and 75%, all belonging to the organic UV filters category (Figure 1).
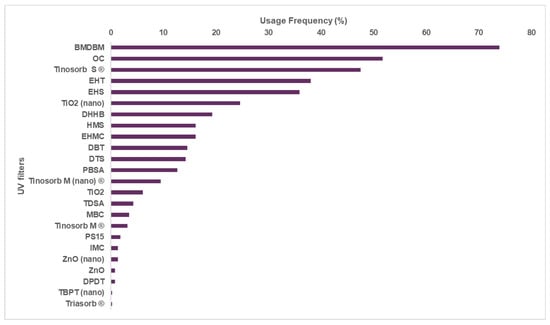
Figure 1.
Usage frequency of the UV filters in sunscreens for adults. (BMDBM: butyl methoxydibenzoylmethane, OC: octocrylene, Tinosorb S®: bis-ethylhexyloxyphenol methoxyphenyl triazine, EHT: ethylhexyl triazone, EHS: ethylhexyl salicylate, DHHB: diethylamino hydroxybenzoyl hexyl benzoate, EHMC: ethylhexyl methoxycinnamate, DBT: diethylhexyl butamido triazone, DTS: dometrizole trisiloxane, PBSA: phenylbenzimidazole sulfonic acid, Tinosorb M®: methylene bis-benzotriazolyl tetramethylbutylphenol, TDSA: terephthalydidene dicamphor sulfonic acid, MBC: 4-methylbenzylidene camphor, PS15: polysilicone-15, IMC: isoamyl p-methoxycinnamate, DPDT: disodium phenyl dibenzimidazole tetrasulfonate, TBPT: tris-biphenyl triazine, Triasorb®: phenylene bis-diphenyltriazine.)
Avobenzone, also known as BMDBM, is widely used in adult sunscreens and had a usage frequency of 73.9%. This UV filter absorbs UVA radiation, conferring protection against one of the most harmful radiations that is directly associated with skin photoaging and indirect damage to DNA through stimulation of the production of reactive species, inducing an oxidative stress process [24]. Octocrylene (51.7%) and bis-ethylhexyloxyphenol methoxyphenyl triazine (47.5%), which absorb UVB and UVA/UVB radiation, respectively, were also frequently used in adult sunscreen formulations. Ethylhexyl triazone (EHT) (38.0%) and ethylhexyl salicylate (EHS) (35.9%), ranked fourth and fifth, are also UV filters with the ability to absorb UVB radiation.
Photostability is critical for the effective photoprotective action of the UV filters. Nevertheless, some currently used UV filters have demonstrated low photostability [17]. Avobenzone is photounstable and suffers photoisomerization and/ or photodegradation when exposed to UVR [25]. Phenacyl and benzoyl radicals are the main photodegradation products formed; however, their combination could generate more reactive and toxic species for human beings and the environment [25,26], thus losing its photoprotective action [25]. Additionally, octocrylene was recently reported for its transformation through retro-aldol condensation into a phototoxic benzophenone derivative [27]. Interestingly, some UV filters could act as photostabilizers. Octocrylene and bis-ethylhexyloxyphenol methoxyphenyl triazine filters are examples of UV filters that possess dual activity, acting as photoprotective agents against UV radiation but also as photostabilizers of other filters, such as avobenzone [28]. Octocrylene has been reported to prevent around 50% of avobenzone’s degradation after 1 h of UVR exposure [29]. It is important to highlight that these three UV filters exhibit a high percentage of use, probably not only for their photoprotective activity, but also for the advantageous effects when combined together. Additionally, the combination of avobenzone with octocrylene could amplify photoprotective action towards both regions of the UV spectrum (UVA and UVB), ensuring broad-spectrum action. Bis-ethylhexyloxyphenol methoxyphenyl triazine (broad-spectrum UV filter) and ethylhexyl triazone (strong UVB absorber) appear in third and fourth, respectively. These filters were two of the last five UV filters approved by European regulations and have been observed exhibiting effective protective action against UV radiation and good photostability [30]. This has led to these filters gaining special attention from cosmetic industries, pushing the demand for expanded production capacity [31]. Regarding inorganic UV filters, nano-sized TiO2 is much more frequent in adult sunscreens than non-nano form TiO2, and the latter is the inorganic UV filter with the highest usage frequency (24.5%). The approved use of the nano forms of certain UV filters was only observed after 2010. In 2014, the first nano UV filter, tris-biphenyl triazine, was approved for use in the EU [14]. Zinc oxide (2016) and nano-sized methylene bis-benzotriazoyl tetramethyl butylphenol (MBBT) (2018) have also been approved in recent years [32,33]. More recently, in 2019, the European Commission amended the entry regarding the nano form of TiO2 as a UV filter in Annex VI [30,34,35]. Reducing particle size from micro to nano particles decreases the appearance of white residue after skin application, which consequently improves the attractiveness of the product [36].
Regarding the analyzed children’s sunscreens, the most used organic filters are essentially the same as those used in adult photoprotective formulations (Figure 2). The inorganic filter TiO2 (non-nano form) is much more used in children’s sunscreens, increasing from 6.07% to 35.4% when compared to the photoprotectors used for adult formations. A lower number of UV filters was used in sunscreens for children (21 UV filters) compared to the adults’ sunscreens (24 UV filters). Isoamyl p-methoxycinnamate, tris-biphenyl triazine, and phenylene bis-diphenyl triazine were the UV filters included in the adults’ UV-protective formulations but not in children’s sunscreens. The photosensibilization potential of isoamyl p-methoxycinnamate has been previously reported, which may account for the observed difference.
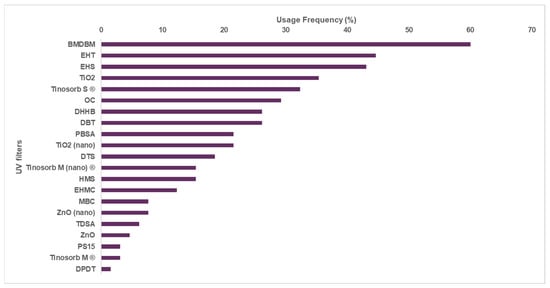
Figure 2.
Frequency of use for the different UV filters in sunscreens for children. (BMDBM: butyl methoxydibenzoylmethane, EHT: ethylhexyl triazone, EHS: ethylhexyl salicylate, Tinosorb S®: bis-ethylhexyloxyphenol methoxyphenyl triazine, OC: octocrylene, DHHB: diethylamino hydroxybenzoyl hexyl benzoate, DBT: diethylhexyl butamido triazone, PBSA: phenylbenzimidazole sulfonic acid, DTS: dometrizole trisiloxane, HMS: homosalate, EHMC: ethylhexyl methoxycinnamate, MBC: 4-methylbenzylidene camphor, TDSA: terephthalydidene dicamphor sulfonic acid, PS15: polysilicone-15, Tinosorb M®: methylene bis-benzotriazolyl tetramethylbutylphenol, and DPDT: disodium phenyl dibenzimidazole tetrasulfonate.)
3.2. Association of Organic/Inorganic UV Filters in Adults’ and Children’s Sunscreens
The existence of sunscreens with only organic UV filters, with only inorganic UV filters, or with both organic and inorganic filters was also explored (Figure 3). The association of diverse organic UV filters in the same product, such as octocrylene and avobenzone, has been confirmed as beneficial regarding stability, safety, and effectiveness [37]. Clinical studies performed to understand the effects of the combination of octocrylene with avobenzone demonstrated that it is non-phototoxic and non-photo-allergenic and provided evidence of broad photoprotective activity [38]. Regarding the combination of UV filters in adults’ sunscreens, the use of only organic UV filters (234 of 379) is more common, with a percentage of 61.7%, followed by photoprotectors containing both organic and inorganic filters (122 of 379) with 32.19%. Generally, organic and inorganic UV filters are used in association to boost photoprotective action, thus ensuring protection against UVA and UVB radiation and higher SPF. Regarding children’s sunscreens, among all the products marketed for paediatric use, photoprotectors containing only organic filters (30 of 65) represented 46.1%. A significant increase in the use of organic–inorganic filters (28 of 65) in children’s sunscreens (43.1%) was observed compared to their usage in adults’ sunscreens (32.2%). This variation could be justified by the ability of the inorganic filters to absorb, reflect, and disperse UVR, thus promoting higher SPF values [39]. The existence of products incorporating only inorganic UV filters is also higher among children’s sunscreens (10.8%) than in adults’ photoprotectors (6.07%). As mentioned before, this finding could be explained by the need to afford higher photoprotection to children, which requires the use of sunscreens with higher SPF values. Some studies have also reported that the use of organic UV filters in skin care products from an early age increases the probability of developing sensitization reactions [40].
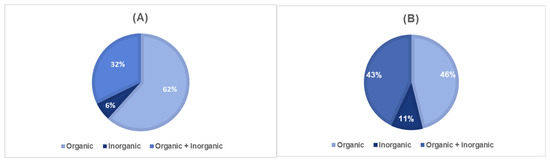
Figure 3.
Usage frequency of sunscreens containing only organic, only inorganic, or a combination of organic and inorganic UV filters in (A) adults’ and in (B) children’s sunscreens.
3.3. Galenic Forms in Adults’ and Children’s Sunscreens
The correct choice of vehicle is also very important for the acceptance of the formulation by the consumer. For people to maintain a regular photoprotective routine, and therefore enjoy its beneficial effects, the application of the product should be easy and lead to a pleasant sensation on the skin. The application of an appropriate amount of the cosmetic formulation and the renewal of the application are key factors for maximizing protective effects. Formulations come in various physical forms. When analyzing the different sunscreen products, the galenic form designations that were used were those attributed by the brands and mentioned on the packaging labels.
Various forms were found, namely cream, fluid, lotion, spray, emulsion, mist, gel, mousse, gel cream, oil gel, oil, and stick forms. An overlap in the names used by the different commercial brands was also noticed. More specifically, names such as milk, lotion, cream, cream-gel, and emulsion are all emulsified preparations, except for lotion, which can be an emulsion or another type of liquid preparation, such as a suspension or solution. Given this categorization, emulsified preparations appear as the main galenic form of sunscreens for adults, while spray is the most frequent for children (Figure 4). The galenic forms most commonly found in adult and children’s sunscreens were cream (24.5% and 15.4%), fluid (18.7% and 29.2%), spray (16.6% and 32.3%), lotion (11.4% and 6.15%), and gel (6.33% and 615%). Oil, stick, and oil gel were only present in adults’ sunscreens, with a usage frequency under 6%.
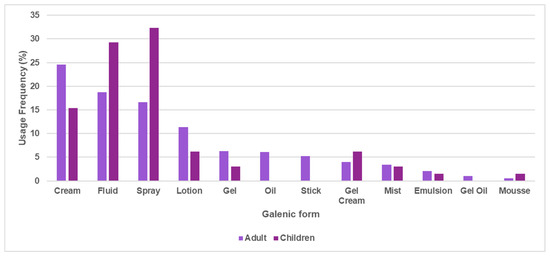
Figure 4.
Usage frequency of different galenic forms in adults’ and children’ sunscreens.
Sprays are easy to apply on children, hence they were the forms most common in children’s photoprotectors, while creams are the best for dry skin conditions [41]. The main disadvantage of using a spray form are inadequate application, which could diminish the photoprotective action towards UVR, and toxic effects after inhalation [42]. For this reason, in 2019, the European Union cosmetics regulations amended a restriction to the use of nano form TiO2 in spray products in the list of European approved UV filters [13,30].
3.4. SPF Values in Adults’ and Children’s Sunscreens
The SPF values of the analyzed sunscreens varied between 15 (minimum) and 50+ (maximum) (Figure 5). The maximum value (SPF50+) was the most frequently found both for adults’ (59.4%) and children’s sunscreens (81.5%). It is important to highlight the absence of sunscreens with an SPF below 30 in paediatric photoprotectors, as well as residual use in adult sunscreens. The percentage of products containing SPF 30 in adults’ sunscreens (24.5%) is higher than the next protection available, SPF 50 (11.1%), but much lower than that of SPF50+ products (59.4%).
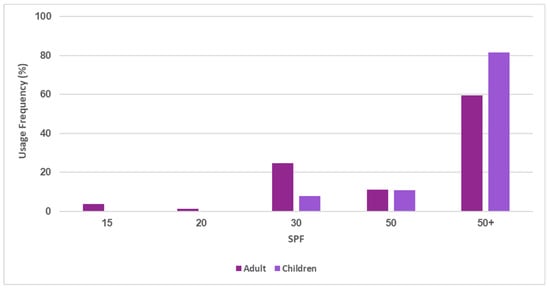
Figure 5.
Frequency of SPF values in adults’ and children’s sunscreens.
According to European recommendations regarding sunscreens, the minimum labelled SPF should be 6 and the maximum 50+. The number of SPF levels should be restricted to eight (SPF 6, 10, 15, 20, 25, 30, 50, 50+) to facilitate comparison between different products without reducing consumer choice [30]. Their effectiveness must be indicated on the label with reference to the following categories: “low” (SPF 6 and 10), “medium” (SPF 15, 20, and 25), “high” (SPF 30 and 50), and “very high” (SPF 50+) [2]. With growing consumer awareness of the damaging effects of UVR, there is an increased demand for high SPF photoprotectors (30, 50 and 50+) [2]. In response to this recent and growing concern, manufacturers are marketing sunscreen products with very high SPF values, thus justifying the absence of sunscreens categorized with low SPF protection in this study. Currently, the maximum numerical indication, expressed as 50+, has been agreed upon, as it is understood that SPFs above this value could mislead consumers.
3.5. Evolution of the Use of UV Filters
When compared to 2015, the top five UV filters in sunscreens marketed in 2021 remained the same (Figure 6). It is relevant to highlight that avobenzone was the most used organic UV filter in both periods of analysis, with usage frequencies higher than 65%. On the contrary, a change in the usage frequency of bis-ethylhexyloxyphenol methoxyphenyl triazine, ethylhexyl salicylate, and octocrylene was observed. The usage frequency of bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S®) (2015: 24.6% and 2021: 45.3%) almost doubled in a timespan of six years, probably due to its high photoprotective effectiveness. This triazine UV filter is a broad-spectrum photoprotective agent, and additionally possesses interesting photostable characteristics that make it a good photoprotective and photostabilizing agent of unstable UV filters, which might explain the increased usage. In contrast to what happened to triazine UV filters, the usage of ethylhexyl salicylate (2015: 39.4% and 2021: 36.9%) and octocrylene (2015: 51.7% and 2021: 48.4%) decreased over the analyzed period by around 10%. Ethylhexyl salicylate is a good UVB absorber; however, it tends to suffer from photoisomerization and potentially induces some environmental toxicity [26]. On the other side, octocrylene could be transformed into a benzophenone derivative through a retro-aldol condensation reaction, which increases its phototoxic potential and leads to negative environmental effects [27]. Additionally, this toxic benzophenone derivative possesses chemical similarities to a hydroxylated derivative of benzophenone-type compounds that were already reported as possessing endocrine disrupting activity [27,43], evidencing possible cytotoxic effects and its potential to act as an endocrine disrupter agent.
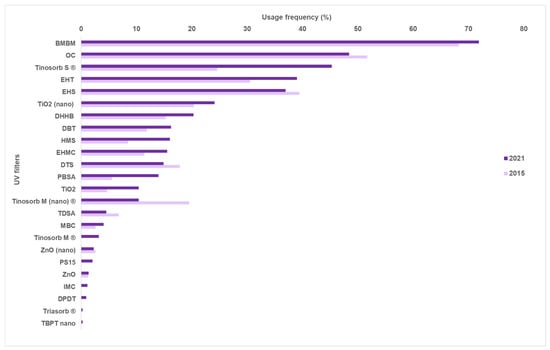
Figure 6.
Frequency of use for UV filters found in sunscreens in 2015 and 2021. (BMDBM: butyl methoxydibenzoylmethane, OC: octocrylene, Tinosorb S®: bis-ethylhexyloxyphenol methoxyphenyl triazine, EHT: ethylhexyl triazone, EHS: ethylhexyl salicylate, DHHB: diethylamino hydroxybenzoyl hexyl benzoate, DBT: diethylhexyl butamido triazone, HMS: homosalate, EHMC: ethylhexyl methoxycinnamate, DTS: dometrizole trisiloxane, PBSA: phenylbenzimidazole sulfonic acid, Tinosorb M®: methylene bis-benzotriazolyl tetramethylbutylphenol, TDSA: terephthalydidene dicamphor sulfonic acid, MBC: 4-methylbenzylidene camphor, PS15: polysilicone-15, IMC: isoamyl p-methoxycinnamate, DPDT: disodium phenyl dibenzimidazole tetrasulfonate, Triasorb®: phenylene bis-diphenyltriazine, TBPT: tris-biphenyl triazine.)
Among the available forms of inorganic UV filters, the collected data between 2015 and 2021 showed an increasing tendency towards the use of the inorganic UV filter TiO2, reaching usage frequencies of 24.1% and 10.4% in 2021 for its nano and non-nano forms, respectively. This is particularly evident for non-nano TiO2, whose usage more than doubled in six years (from 4.7% to 10.4%). With respect to ZnO, decreased usage was found for the non-nano form, while nano ZnO was similarly used in both periods of analysis. The reasons for the increased use of TiO2 are not clear but might be explained by its ability to offer broad-spectrum action and higher SPF values, as well as by the toxicity issues that affect other organic filters.
Methylene bis-benzotriazolyl tetramethylbutylphenol (Tinosorb M®) is considered a hybrid UV filter due to its physical–chemical behavior and ability to reflect and absorb UV radiation [44]. Additionally, it can also act as a good photostabilizer of other UV filters [44]. However, in the period of analysis, a decrease of almost 50% in the usage frequency of its nano form in sunscreens was observed (2015: 19.5% and 2021: 10.4%). The revised document related to this nano filter, published by the Scientific Committee on Consumer Safety (SCCS), reported that studies about acute oral and dermal toxicity, skin irritation, and skin sensitization for the nano-sized material were not provided [45]. Additionally, some allergic contact cases were reported concerning this UV filter in its non-nano form (11 cases in European countries) [46]. Consequently, it was recommended that Tinosorb M® be included into the European photopatch test baseline series [47], which could have influenced the decrease in use of this filter in sunscreens available on the market in 2021.
Four organic UV filters, namely disodium phenyl dibenzimidazole tetrasulfonate (DPDT), phenylene bis-diphenyl triazine (Triasorb®), nano tris-biphenyl triazine (TBTP), and isoamyl p-methoxycinnamate (IMC), only appeared in the analysis performed in 2021, with usage frequencies lower than 5%.
4. Conclusions
Sunscreens are available to consumers in different forms, with different compositions, and with different characteristics. This work aimed to describe the composition and general features of UV filters by focusing on the photoprotective formulations available in 2021 in Portuguese pharmacies and parapharmacies, which is representative of the European market. The incorporation of UVA filters in the composition of sunscreens was demonstrated by the fact that the most used filter by far (>75%), avobenzone, belongs to this category. This finding can be interpreted as a result of growing knowledge regarding the harmful effects of UVA radiation, as well as the goal of cosmetic manufacturers to offer sunscreens affording broad-spectrum protection.
The design of sunscreens was influenced by the target consumers, both regarding the UV filters used and the sunscreen form chosen. The combination of both organic and inorganic UV filters in the same product was substantially higher in children’s photoprotectors (43.1%) than in adult sunscreens (32.2%), as was the use of inorganic UV filters (>50%). The formulation of photoprotectors for children with higher amounts of inorganic filters might have a dual purpose: to increase SPF and minimize sensitization reactions due to organic filters. Regarding galenic forms, 12 different galenic forms were identified in adult sunscreen formulations, which was slightly higher than the ten available for children’s sunscreens. Emulsions were the preferred form for adults’ sunscreens, while for children sprays were preferred, probably due to convenience of use. As to information about the SPF values, there were no sunscreens with an SPF value below 30 for children. The trendiest SPF value, both in adults (59.4%) and children (81.5%), was SPF 50+, which shows the intention of industries to place sunscreens with highly effective photoprotection in the market (in particular in the pharmacy sector), thus targeting health concerns related to sun overexposure, particularly skin cancer.
Analyzing the evolution of the usage frequency of UV filters from 2015 to 2021, some conclusions can be drawn. In a timespan of six years, avobenzone maintained its position as the most used UV filter (usage frequency >65%). The incorporation of bis-ethylhexyloxyphenol methoxyphenyl triazine in sunscreens doubled in 2021. In contrast, a decrease in the usage of octocrylene and ethylhexyl salicylate (up to 10%), and particularly of nano methylene bis-benzotriazolyl tetramethylbutylphenol (Tinosorb M®) (nearly 50%), was also observed, probably related to human and environmental safety issues. On the other hand, the inorganic UV filter TiO2 is being increasingly used. Recent data on the safety and photostability of UV filters and consumer concerns seem to be, at least partially, being considered in the reformulation of commercial sunscreens, which substantiates the focus of the cosmetic industry on designing safer products.
The use of UV filters in cosmetic formulations is a must-have in order to afford protection against UV radiation. However, the choice of which UV filters should be used is a challenge. There are numerous studies that report some of the drawbacks of the actual UV filters, such as photostability issues, skin sensitization reactions, and human and environmental toxicity, especially towards marine species [17]. Thus, it is important to continue to monitor the use of UV filters in marketed sunscreens, with the aim of staying up to date regarding the main trends in this area. The opportunity for innovation and scientific support are two important benefits that this type of study could bring to the development of new sun protection products in terms of informing the choice UV filters. This insight is also important as it helps to improve sunscreen recommendations by increasing awareness of the wide range of products available.
Ultimately, this work allows for characterization of the true market impact of approved UV filters and how the market has evolved in the last six years. This insight is also relevant for the assessment of environmental risks, such as those associated with the presence of UV filters in aquatic ecosystems.
Author Contributions
Conceptualization, I.F.A.; Data collection, I.A. and J.D.; Data analysis, A.J. and I.A.; Supervision, J.M.S.L. and I.F.A.; Writing—original draft preparation and final manuscript, A.J.; Writing—review and editing, E.S., H.C., M.T.C., J.M.S.L. and I.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by national funds from FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of projects UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences (UCIBIO) and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy (i4HB). This research was also supported by national funds through FCT (Foundation for Science and Technology) within the scope of UIDB/04423/2020, UIDP/04423/2020 (Group of Natural Products and Medicinal Chemistry—CIIMAR), as well as a structured program of R&D&I ATLANTIDA (NORTE-01-0145-FEDER-000040), supported by NORTE2020 through ERDF. This work was also financed by the European Regional Development Fund (ERDF) through the Centro 2020 Regional Operational Programme under project CENTRO-01-0145-FEDER-000012 (HealthyAging2020), and through the Northern Regional Operational Program (NORTE2020) under the project 47239—Cork2Cosmetic (NORTE-01-0247-FEDER-047239). Ana Jesus acknowledges the Ph.D. research grant UI/BD/151319/2021, fully supported by national funding from FCT, the Foundation for Science and Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shaath, N. Sunscreens: Regulations and Commercial Development, 3rd ed.; Taylor & Francis: Abingdon, UK, 2005; Volume 28. [Google Scholar]
- Geoffrey, K.; Mwangi, A.N.; Maru, S.M. Sunscreen products: Rationale for use, formulation development and regulatory considerations. Saudi Pharm. J. 2019, 27, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Abiola, T.T.; Whittock, A.L.; Stavros, V.G. Unravelling the Photoprotective Mechanisms of Nature-Inspired Ultraviolet Filters Using Ultrafast Spectroscopy. Molecules 2020, 25, 3945. [Google Scholar] [CrossRef] [PubMed]
- Urbach, F. The historical aspects of sunscreens. J. Photochem. Photobiol. B Biol. 2001, 64, 99–104. [Google Scholar] [CrossRef]
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef]
- Hausser, K.W.; Vahle, W. Sonnenbrand und Sonnenbraunung. Wiss. Veraffentlichungen Siemens-Konzerns 1927, 6, 101–120. [Google Scholar] [CrossRef]
- L’Oreal. Garnier Ambre Solaire. 2020. Available online: https://www.loreal.com/it-it/italy/press-release/group/garnier-ambre-solaire/ (accessed on 6 January 2022).
- Kumler, W.D.; Daniels, T.C. Sunscreen Compounds. J. Am. Pharm. Assoc. 1948, 37, 474–476. [Google Scholar] [CrossRef]
- Macki, B.S.; Mackie, L.E. Historical perspective: The PABA story. Australas. J. Dermatol. 1999, 40, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive 2008/123/EC of 18 December 2008 Amending Council Directive 76/768/EEC, Concerning Cosmetic Products, for the Purpose of Adapting Annexes II and VII Thereto to Technical Progress. 2008. Available online: https://www.legislation.gov.uk/eudr/2008/123/contents (accessed on 20 April 2022).
- Kligman, A.M. Early Destructive Effect of Sunlight on Human Skin. J. Am. Med. Assoc. 1969, 210, 2377–2380. [Google Scholar] [CrossRef]
- Roelandts, R.; Vanhee, J.; Bonamie, A.; Kerkhofs, L.; Degreef, H. A Survey of Ultraviolet Absorbers in Commercially Available Sun Products. Int. J. Dermatol. 1983, 22, 247–255. [Google Scholar] [CrossRef]
- Chisvert, A.; Salvador, A. Ultraviolet Filters in Cosmetics: Regulatory Aspects and Analytical Methods. In Analysis of Cosmetic Products, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–106. [Google Scholar]
- Regulation (EC) n°1223/2009 of the European Parliament and of the Council: Current Consolidated Version (01/03/2022). 2022, Official Journal of the European Union. 02009R1223—EN—03.12.2020—025.001—(1–389). Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwimro215MX7AhUUslYBHficAmwQFnoECA0QAQ&url=https%3A%2F%2Feur-lex.europa.eu%2Flegal-content%2FEN%2FTXT%2FPDF%2F%3Furi%3DCELEX%3A02009R1223-20201203%26rid%3D3&usg=AOvVaw0EQqca4O9KBjZxFMiFsLy0 (accessed on 6 January 2022).
- Scheil, V.; Triebskorn, R.; Kohler, H.R. Cellular and stress protein responses to the UV filter 3-benzylidene camphor in the amphipod crustacean Gammarus fossarum (Koch 1835). Arch. Environ. Contam. Toxicol. 2008, 54, 684–689. [Google Scholar] [CrossRef]
- Holbech, H.N.; Korsgaard, B.; Bjerregaard, P. The Chemical UV-Filter 3-Benzylidene Camphor Causes an Oestrogenic Effect in an in vivo Fish Assay. Pharmacol. Toxicol. 2002, 91, 204–208. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- ISO 24444:2010; Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF). ISO: Geneva, Switzerland, 2010.
- ISO 24444:2019; Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF). ISO: Geneva, Switzerland, 2019.
- ISO 24443:2012; Determination of Sunscreen UVA Photoprotection In Vitro. ISO: Geneva, Switzerland, 2012.
- ISO 24443:2021; Cosmetics—Determination of Sunscreen UVA Photoprotection In Vitro. ISO: Geneva, Switzerland, 2021.
- ISO 24442:2011; Cosmetics—Sun Protection Test Methods—In Vivo Determination of Sunscreen UVA Protection. ISO: Geneva, Switzerland, 2011.
- Moyal, D.; Passeron, T.; Josso, M.; Douezan, S.; Delvigne, V.; Seité, S. Formulation of sunscreens for optimal efficacy. J. Cosmet. Sci. 2020, 71, 199–206. [Google Scholar]
- Dahmane, R.; Pandel, R.; Trebse, P.; Poljsak, B. The role of sun exposure in skin aging. In Sun Exposure: Risk Factors, Protection Practices and Health Effects; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 1–40. [Google Scholar]
- Bonda, C.A.; Lott, D. Sunscreen photostability. In Principles and Practice of Photoprotection; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 247–273. [Google Scholar]
- Tarras-Wahlberg, N.; Stenhagen, G.; Larko, O.; Rosen, A.; Wennberg, A.M.; Wennerstrom, O. Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation. J. Investig. Dermatol. 1999, 113, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; DiNardo, J.C.; Stien, D.; Rodrigues, A.M.S.; Lebaron, P. Benzophenone Accumulates over Time from the Degradation of Octocrylene in Commercial Sunscreen Products. Chem. Res. Toxicol. 2021, 34, 1046–1054. [Google Scholar] [CrossRef]
- Herzog, B.; Giesinger, J.; Settels, V. Insights into the stabilization of photolabile UV-absorbers in sunscreens. Photochem. Photobiol. Sci. 2020, 19, 1636–1649. [Google Scholar] [CrossRef]
- Wolverton, S.E.; Wu, J.J. Comprehensive Dermatologic Drug Therapy, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Regulation (EC) n° 1223/2009 of the European Parliament and of the Council: Current Consolidated Version (01/10/2021), Official Journal of the European Union L.342/59-209. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223 (accessed on 6 January 2022).
- Fabian, F. BASF Pushes UV Filter Production Capacities. Available online: https://www.basf.com/global/en/media/news-releases/2019/12/p-19-422.html (accessed on 6 January 2022).
- Whitehouse, L. Nano-Sized UV Filter Passes EU Cosmetic Regulation 2018. Available online: https://www.cosmeticsdesign-europe.com/Article/2018/08/03/Nano-sized-UV-filter-passes-EU-Cosmetic-Regulation (accessed on 6 January 2022).
- BASF Gains EU Approval of Nano-Sized UV Filter in Cosmetics 2018. Available online: https://www.basf.com/global/en/media/news-releases/2018/07/p-18-276.html (accessed on 6 January 2022).
- EU Updates the Use of Nano Titanium Dioxide in Cosmetics with a Warning. Available online: https://www.sgs.com/en/news/2019/12/safeguards-17919-eu-updates-the-use-of-nano-titanium-dioxide-in-cosmetics-with-warning (accessed on 6 January 2022).
- Scientific Committee on Consumer Safety (SCCS). Opinion on Additional Coatings for Titanium Dioxide (Nano Form) as UV-Filter in Dermally Applied Cosmetic Products; Preliminary Version SCCS/1580/16; Unit C2, European Commission Health and Food Safety: Luxembourg, 2016. [Google Scholar]
- Palm, M.D.; O’Donoghue, M.N. Update on photoprotection. Dermatol. Ther. 2007, 20, 360–376. [Google Scholar] [CrossRef]
- Mendrok-Edinger, C. The Quest for Avobenzone Stabilizers and Sunscreen Photostability. Available online: https://www.cosmeticsandtoiletries.com/formulas-products/sun-care/article/21836968/the-quest-for-avobenzone-stabilizers-and-sunscreen-photostability (accessed on 15 November 2021).
- Benevenuto, C.G.; Guerra, L.O.; Gaspar, L.R. Combination of retinyl palmitate and UV-filters: Phototoxic risk assessment based on photostability and in vitro and in vivo phototoxicity assays. Eur. J. Pharm. Sci. 2015, 68, 127–136. [Google Scholar] [CrossRef]
- Nery, E.M.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. A short review of alternative ingredients and technologies of inorganic UV filters. J. Cosmet. Dermatol. 2021, 20, 1061–1065. [Google Scholar] [CrossRef]
- Kerr, A.; Ferguson, J. Photoallergic contact dermatitis. Photodermatol. Photoimmunol. Photomed. 2010, 26, 56–65. [Google Scholar] [CrossRef]
- Novick, R.; Anderson, G.; Miller, E.; Allgeier, D.; Unice, K. Factors that influence sunscreen application thickness and potential preservative exposure. Photodermatol. Photoimmunol. Photomed. 2015, 31, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Chaiyabutr, C.; Sukakul, T.; Kumpangsin, T.; Bunyavaree, M.; Charoenpipatsin, N.; Wongdama, S.; Boonchai, W. Ultraviolet filters in sunscreens and cosmetic products—A market survey. Contact Dermat. 2021, 85, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Mutsuga, M.; Kato, T.; Iida, M.; Tanamoto, K. Estrogenic and Anti-Androgenic Activities of Benzophenones in Human Estrogen and Androgen Receptor Mediated Mammalian Reporter Gene Assays. J. Health Sci. 2005, 51, 48–54. [Google Scholar] [CrossRef]
- Methylene Bis-Benzotriazolyl Tetramethylbutylphenol. Available online: https://incidecoder.com/ingredients/methylene-bis-benzotriazolyl-tetramethylbutylphenol (accessed on 20 May 2022).
- Scientific Committee on Consumer Safety. Opinion on 2,2′-Methylene-bis-(6-(2H-benzotriazol-2-yl)-4-(1,1,3,3tetramethylbutyl)phenol) (Nano Form): Submission III, COLIPA S79, European Commission 2018, SCCS/1546/15. Available online: https://data.europa.eu/doi/10.2875/101475 (accessed on 20 April 2022).
- de Groot, A.C.; van Zuuren, E.J.; Hissink, D. Contact allergy to Tinosorb(R) M: Recommendations for diagnostic improvement. Contact Dermat. 2014, 70, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Goncalo, M.; Ferguson, J.; Bonevalle, A.; Bruynzeel, D.P.; Gimenez-Arnau, A.; Goossens, A.; Kerr, A.; Lecha, M.; Neumann, N.; Niklasson, B.; et al. Photopatch testing: Recommendations for a European photopatch test baseline series. Contact Dermat. 2013, 68, 239–243. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).