Volatiles in Berries: Biosynthesis, Composition, Bioavailability, and Health Benefits
Abstract
1. Introduction
2. Biosynthesis of Volatiles in Plants
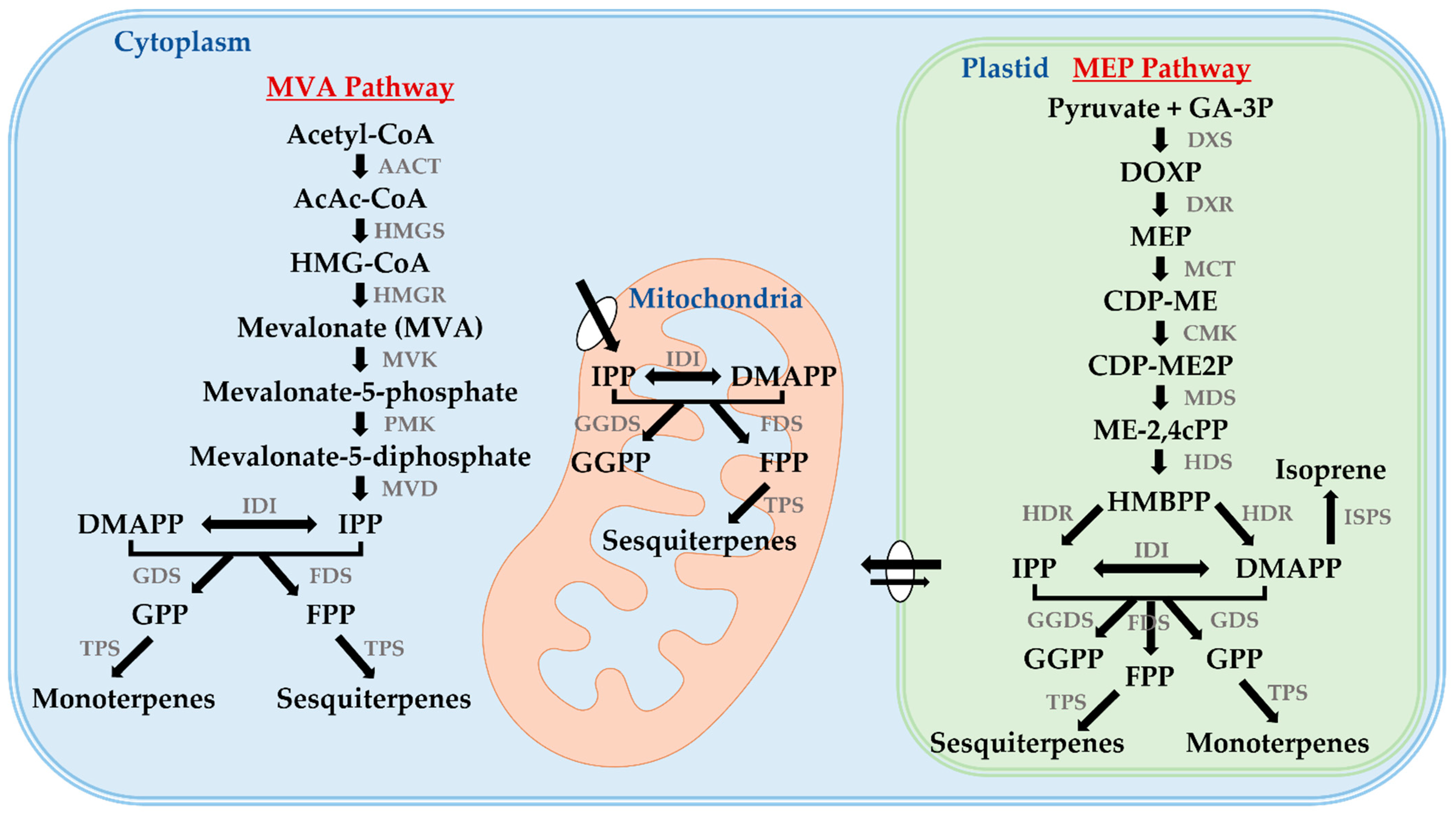
2.1. Biosynthesis of Terpenes
2.2. MVA and MEP Pathways
2.3. Biosynthesis of Other Plant Volatiles
2.3.1. Phenylpropanoids/Benzenoids
2.3.2. Volatile Fatty Acid Derivatives
2.4. Application of Plant Volatile Biosynthesis
3. The Chemical Composition of Volatile Compounds in Berries
3.1. Strawberry
3.2. Blueberry
3.3. Raspberry
3.4. Blackberry
3.5. Cranberry
4. Bioavailability of Berry Volatiles
5. Health Benefits of Berry Volatiles
5.1. Inflammation
5.1.1. Modulation of Pro-Inflammatory Mediators
5.1.2. Regulation of Inflammatory Transcription Factors and Signal Transduction
5.1.3. Attenuation of Oxidative Stress and Autophagy
5.2. Cancer
5.3. Obesity
5.4. Diabetes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kader, A.A. Flavor Quality of Fruits and Vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Du, X.; Qian, M. Flavor chemistry of small fruits: Blackberry, raspberry, and blueberry. In Flavor and Health Benefits of Small Fruits; Qian, M.C., Rimando, A.M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; Volume 1035, pp. 27–43. [Google Scholar]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.A.; Klee, H.J. Plant Volatile Compounds: Sensory Cues for Health and Nutritional Value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Forney, C.F.; Song, J. Flavors and Aromas: Chemistry and Biological Functions. In Fruit and Vegetable Phytochemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 515–540. ISBN 978-1-119-15804-2. [Google Scholar]
- Horvat, R.J.; Schlotzhauer, W.S.; Chortyk, O.T.; Nottingham, S.F.; Payne, J.A. Comparison of Volatile Compounds from Rabbiteye Blueberry (Vaccinium Ashei) and Deerberry (V. Stamineum) during Maturation. J. Essent. Oil Res. 1996, 8, 645–648. [Google Scholar] [CrossRef]
- Ibáñez, E.; López-Sebastián, S.; Ramos, E.; Tabera, J.; Reglero, G. Analysis of Volatile Fruit Components by Headspace Solid-Phase Microextraction. Food Chem. 1998, 63, 281–286. [Google Scholar] [CrossRef]
- de Ancos, B.; Ibañez, E.; Reglero, G.; Cano, M.P. Frozen storage effects on anthocyanins and volatile compounds of raspberry fruit. J. Agric. Food Chem. 2000, 48, 873–879. [Google Scholar] [CrossRef]
- Wang, C.Y. Maintaining Postharvest Quality of Raspberries with Natural Volatile Compounds. Int. J. Food Sci. Technol. 2003, 38, 869–875. [Google Scholar] [CrossRef]
- Duan, W.; Sun, P.; Chen, L.; Gao, S.; Shao, W.; Li, J. Comparative Analysis of Fruit Volatiles and Related Gene Expression between the Wild Strawberry Fragaria pentaphylla and cultivated Fragaria × ananassa. Eur. Food Res. Technol. 2018, 244, 57–72. [Google Scholar] [CrossRef]
- Yuan, F.; Cheng, K.; Gao, J.; Pan, S. Characterization of Cultivar Differences of Blueberry Wines Using GC-QTOF-MS and Metabolic Profiling Methods. Molecules 2018, 23, 2376. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Brouwer, B.; Oud, N.; Verdonk, J.C.; Tikunov, Y.; Woltering, E.; Schouten, R.; Pereira da Silva, F. Sensory, GC-MS and PTR-ToF-MS Profiling of Strawberries Varying in Maturity at Harvest with Subsequent Cold Storage. Postharvest Biol. Technol. 2021, 182, 111719. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Forney, C.F. Flavour Volatile Production and Regulation in Fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Bood, K.G.; Zabetakis, I. The Biosynthesis of Strawberry Flavor (II): Biosynthetic and Molecular Biology Studies. J. Food Sci. 2002, 67, 2–8. [Google Scholar] [CrossRef]
- Siegmund, B. Biogenesis of aroma compounds: Flavour formation in fruits and vegetables. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2015; pp. 127–149. [Google Scholar]
- Giuggioli, N.R.; Briano, R.; Baudino, C.; Peano, C. Effects of Packaging and Storage Conditions on Quality and Volatile Compounds of Raspberry Fruits. CyTA-J. Food 2015, 13, 512–521. [Google Scholar] [CrossRef]
- Saftner, R.; Polashock, J.; Ehlenfeldt, M.; Vinyard, B. Instrumental and Sensory Quality Characteristics of Blueberry Fruit from Twelve Cultivars. Postharvest Biol. Technol. 2008, 49, 19–26. [Google Scholar] [CrossRef]
- Dymerski, T.; Namieśnik, J.; Leontowicz, H.; Leontowicz, M.; Vearasilp, K.; Martinez-Ayala, A.L.; González-Aguilar, G.A.; Robles-Sánchez, M.; Gorinstein, S. Chemistry and biological properties of berry volatiles by two-dimensional chromatography, fluorescence and Fourier transform infrared spectroscopy techniques. Food Res. Int. 2016, 83, 74–86. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-Inflammatory Effect of Strawberry Extract against LPS-Induced Stress in RAW 264.7 Macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.S.; Neto, B.P.S.; Lopes, E.M.; Cunha, F.V.M.; Araújo, A.R.; Wanderley, C.W.S.; Wong, D.V.T.; Júnior, R.C.P.L.; Ribeiro, R.A.; Sousa, D.P.; et al. Anti-Inflammatory Effect of the Monoterpene Myrtenol Is Dependent on the Direct Modulation of Neutrophil Migration and Oxidative Stress. Chem.-Biol. Interact. 2017, 273, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Relationship between Volatile Composition and Bioactive Potential of Vegetables and Fruits of Regular Consumption—An Integrative Approach. Molecules 2021, 26, 3653. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The Formation and Function of Plant Volatiles: Perfumes for Pollinator Attraction and Defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral Volatiles: From Biosynthesis to Function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Dewick, P.M. The Biosynthesis of C5–C25 Terpenoid Compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Croteau, R. Terpenoid Metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar] [CrossRef]
- Zuzarte, M.; Salgueiro, L. Essential Oils Chemistry. In Bioactive Essential Oils and Cancer; de Sousa, D.P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–61. ISBN 978-3-319-19144-7. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). In Biochemistry and Molecular Biology of Plants; American Society of Plant Physiologists: Rockville, MD, USA, 2000. [Google Scholar]
- Liu, Y.; Luo, S.-H.; Schmidt, A.; Wang, G.-D.; Sun, G.-L.; Grant, M.; Kuang, C.; Yang, M.-J.; Jing, S.-X.; Li, C.-H.; et al. A Geranylfarnesyl Diphosphate Synthase Provides the Precursor for Sesterterpenoid (C25) Formation in the Glandular Trichomes of the Mint Species Leucosceptrum canum. Plant Cell 2016, 28, 804–822. [Google Scholar] [CrossRef]
- Loza-Tavera, H. Monoterpenes in Essential Oils. In Chemicals via Higher Plant Bioengineering; Shahidi, F., Kolodziejczyk, P., Whitaker, J.R., Munguia, A.L., Fuller, G., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999; pp. 49–62. ISBN 978-1-4615-4729-7. [Google Scholar]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Pulido, P.; Perello, C.; Rodriguez-Concepcion, M. New Insights into Plant Isoprenoid Metabolism. Mol. Plant 2012, 5, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Chappell, J. Isoprenoid Biosynthesis in Plants: Carbon Partitioning Within the Cytoplasmic Pathway. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M.; Knani, M.; Simonin, P.; Sutter, B.; Sahm, H. Isoprenoid Biosynthesis in Bacteria: A Novel Pathway for the Early Steps Leading to Isopentenyl Diphosphate. Biochem. J. 1993, 295, 517–524. [Google Scholar] [CrossRef]
- Lange, B.M.; Wildung, M.R.; Stauber, E.J.; Sanchez, C.; Pouchnik, D.; Croteau, R. Probing Essential Oil Biosynthesis and Secretion by Functional Evaluation of Expressed Sequence Tags from Mint Glandular Trichomes. Proc. Natl. Acad. Sci. USA 2000, 97, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2015; pp. 63–106. ISBN 978-3-319-20107-8. [Google Scholar]
- Berthelot, K.; Estevez, Y.; Deffieux, A.; Peruch, F. Isopentenyl Diphosphate Isomerase: A Checkpoint to Isoprenoid Biosynthesis. Biochimie 2012, 94, 1621–1634. [Google Scholar] [CrossRef]
- Rohdich, F.; Hecht, S.; Gärtner, K.; Adam, P.; Krieger, C.; Amslinger, S.; Arigoni, D.; Bacher, A.; Eisenreich, W. Studies on the Nonmevalonate Terpene Biosynthetic Pathway: Metabolic Role of IspH (LytB) Protein. Proc. Natl. Acad. Sci. USA 2002, 99, 1158–1163. [Google Scholar] [CrossRef]
- Tritsch, D.; Hemmerlin, A.; Bach, T.J.; Rohmer, M. Plant Isoprenoid Biosynthesis via the MEP Pathway: In Vivo IPP/DMAPP Ratio Produced by (E)-4-Hydroxy-3-Methylbut-2-Enyl Diphosphate Reductase in Tobacco BY-2 Cell Cultures. FEBS Lett. 2010, 584, 129–134. [Google Scholar] [CrossRef]
- Vranová, E.; Coman Schmid, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile Terpenoids: Multiple Functions, Biosynthesis, Modulation and Manipulation by Genetic Engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Hoeffler, J.-F.; Meyer, O.; Tritsch, D.; Kagan, I.A.; Grosdemange-Billiard, C.; Rohmer, M.; Bach, T.J. Cross-Talk between the Cytosolic Mevalonate and the Plastidial Methylerythritol Phosphate Pathways in Tobacco Bright Yellow-2 Cells *. J. Biol. Chem. 2003, 278, 26666–26676. [Google Scholar] [CrossRef]
- Laule, O.; Fürholz, A.; Chang, H.-S.; Zhu, T.; Wang, X.; Heifetz, P.B.; Gruissem, W.; Lange, M. Crosstalk between Cytosolic and Plastidial Pathways of Isoprenoid Biosynthesis in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The Nonmevalonate Pathway Supports Both Monoterpene and Sesquiterpene Formation in Snapdragon Flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A. Plant Volatile Terpenoid Metabolism: Biosynthetic Genes, Transcriptional Regulation and Subcellular Compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.M.; Herrmann, K.M. Dynamics of the Shikimate Pathway in Plants. Trends Plant Sci. 1997, 2, 346–351. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of Essential Oil Production in Plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Shrivastava, G.; Rogers, M.; Wszelaki, A.; Panthee, D.R.; Chen, F. Plant Volatiles-Based Insect Pest Management in Organic Farming. Crit. Rev. Plant Sci. 2010, 29, 123–133. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant Lipoxygenases. Physiological and Molecular Features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef]
- Tholl, D. Terpene Synthases and the Regulation, Diversity and Biological Roles of Terpene Metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.-P. Practical Approaches to Plant Volatile Analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef]
- Gershenzon, J.; Croteau, R. Regulation of monoterpene biosynthesis in higher plants. In Biochemistry of the Mevalonic Acid pathway to Terpenoids; Springer: Boston, MA, USA, 1990; pp. 99–160. [Google Scholar]
- Schenkel, D.; Maciá-Vicente, J.G.; Bissell, A.; Splivallo, R. Fungi Indirectly Affect Plant Root Architecture by Modulating Soil Volatile Organic Compounds. Front. Microbiol. 2018, 9, 1847. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Effects of Exogenous Methyl Jasmonate on Quality and Preservation of Postharvest Fruits: A Review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef] [PubMed]
- Chanjirakul, K.; Wang, S.Y.; Wang, C.Y.; Siriphanich, J. Natural Volatile Treatments Increase Free-Radical Scavenging Capacity of Strawberries and Blackberries. J. Sci. Food Agric. 2007, 87, 1463–1472. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Spinelli, F.; Pastore, C.; Farneti, B.; Savioli, S.; Rodriguez-Estrada, M.T.; Donati, I. Contribution of Fruit Microbiome to Raspberry Volatile Organic Compounds Emission. Postharvest Biol. Technol. 2022, 183, 111742. [Google Scholar] [CrossRef]
- Egea, M.B.; Bertolo, M.R.V.; de Oliveira Filho, J.G.; Lemes, A.C. A Narrative Review of the Current Knowledge on Fruit Active Aroma Using Gas Chromatography-Olfactometry (GC-O) Analysis. Molecules 2021, 26, 5181. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, L.F.V.; Johnson, T.S.; Benevenuto, J.; Edger, P.P.; Colquhoun, T.A.; Munoz, P.R. Genome-Wide Association of Volatiles Reveals Candidate Loci for Blueberry Flavor. New Phytol. 2020, 226, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F.R. Metabolomic Selection for Enhanced Fruit Flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ban, Z.; Wang, K.; Li, D.; Li, D.; Poverenov, E.; Li, L.; Luo, Z. Aroma Volatiles, Sensory and Chemical Attributes of Strawberry (Fragaria × Ananassa Duch.) Achenes and Receptacle. Int. J. Food Sci. Technol. 2017, 52, 2614–2622. [Google Scholar] [CrossRef]
- Yan, J.; Ban, Z.; Lu, H.; Li, D.; Poverenov, E.; Luo, Z.; Li, L. The Aroma Volatile Repertoire in Strawberry Fruit: A Review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Forney, C.F. Horticultural and Other Factors Affecting Aroma Volatile Composition of Small Fruit. HortTechnology 2001, 11, 529–538. [Google Scholar] [CrossRef]
- Du, X.; Plotto, A.; Song, M.; Olmstead, J.; Rouseff, R. Volatile Composition of Four Southern Highbush Blueberry Cultivars and Effect of Growing Location and Harvest Date. J. Agric. Food Chem. 2011, 59, 8347–8357. [Google Scholar] [CrossRef]
- Gu, I.; Brownmiller, C.; Stebbins, N.B.; Mauromoustakos, A.; Howard, L.; Lee, S.-O. Berry Phenolic and Volatile Extracts Inhibit Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-ΚB Signaling Pathway. Antioxidants 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.; Howard, L.; Tipton, J.; Lavefve, L.; Brownmiller, C.; Lee, S.-O.; Gu, I.; Liyanage, R.; Lay, J.O. Blackberry Phenolic and Volatile Extracts Inhibit Cytokine Secretion in LPS-Inflamed RAW264.7 Cells. J. Food Bioact. 2021, 16, 34–47. [Google Scholar] [CrossRef]
- Wang, Y.; Finn, C.; Qian, M.C. Impact of Growing Environment on Chickasaw Blackberry (Rubus, L.) Aroma Evaluated by Gas Chromatography Olfactometry Dilution Analysis. J. Agric. Food Chem. 2005, 53, 3563–3571. [Google Scholar] [CrossRef] [PubMed]
- Oz, A.T.; Baktemur, G.; Kargi, S.P.; Kafkas, E. Volatile Compounds of Strawberry Varieties. Chem. Nat. Compd. 2016, 52, 507–509. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y. Seasonal Variation of Volatile Composition and Odor Activity Value of ’Marion’ (Rubus Spp. Hyb) and ’Thornless Evergreen’ (R. Laciniatus, L.) Blackberries. J. Food Sci. 2005, 70, C13–C20. [Google Scholar] [CrossRef]
- Du, X.; Rouseff, R. Aroma Active Volatiles in Four Southern Highbush Blueberry Cultivars Determined by Gas Chromatography–Olfactometry (GC-O) and Gas Chromatography–Mass Spectrometry (GC-MS). J. Agric. Food Chem. 2014, 62, 4537–4543. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the Key Aroma Volatile Compounds in Cranberry (Vaccinium Macrocarpon Ait.) Using Gas Chromatography–Olfactometry (GC-O) and Odor Activity Value (OAV). J. Agric. Food Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Ruse, K.; Sabovics, M.; Rakcejeva, T.; Dukalska, L.; Galoburda, R.; Berzina, L. The Effect of Drying Conditions on the Presence of Volatile Compounds in Cranberries. Int. J. Agric. Biosyst. Eng. 2012, 6, 163–169. [Google Scholar]
- Farneti, B.; Khomenko, I.; Grisenti, M.; Ajelli, M.; Betta, E.; Algarra, A.A.; Cappellin, L.; Aprea, E.; Gasperi, F.; Biasioli, F.; et al. Exploring Blueberry Aroma Complexity by Chromatographic and Direct-Injection Spectrometric Techniques. Front. Plant Sci. 2017, 8, 617. [Google Scholar] [CrossRef]
- Malowicki, S.M.M.; Martin, R.; Qian, M.C. Volatile Composition in Raspberry Cultivars Grown in the Pacific Northwest Determined by Stir Bar Sorptive Extraction−Gas Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 4128–4133. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.F.; Sanz, J.; Sanz, M.L.; Giuffrè, A.M.; Sicari, V.; Soria, A.C. Optimization of a Solid-Phase Microextraction Method for the Gas Chromatography–Mass Spectrometry Analysis of Blackberry (Rubus Ulmifolius Schott) Fruit Volatiles. Food Chem. 2015, 178, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.L.; Schwieterman, M.L.; Colquhoun, T.A.; Clark, D.G.; Olmstead, J.W. Potential for Increasing Southern Highbush Blueberry Flavor Acceptance by Breeding for Major Volatile Components. HortScience 2013, 48, 835–843. [Google Scholar] [CrossRef]
- Hirvi, T.; Honkanen, E.; Pyysalo, T. The Aroma of Cranberries. Z. Für Lebensm. -Unters. Und Forsch. 1981, 172, 365–367. [Google Scholar] [CrossRef]
- Moore, K.; Howard, L.; Brownmiller, C.; Gu, I.; Lee, S.-O.; Mauromoustakos, A. Inhibitory Effects of Cranberry Polyphenol and Volatile Extracts on Nitric Oxide Production in LPS Activated RAW 264.7 Macrophages. Food Funct. 2019, 10, 7091–7102. [Google Scholar] [CrossRef]
- Khomych, G.; Matsuk, Y.; Nakonechnaya, J.; Oliynyk, N.; Medved, L. Study of the Chemical Composition of Cranberry and the Use of Berries in Food Technology. EEJET 2017, 6, 59–65. [Google Scholar] [CrossRef][Green Version]
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J.; et al. Strawberry Flavor: Diverse Chemical Compositions, a Seasonal Influence, and Effects on Sensory Perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef]
- Aharoni, A.; Giri, A.P.; Verstappen, F.W.A.; Bertea, C.M.; Sevenier, R.; Sun, Z.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Gain and Loss of Fruit Flavor Compounds Produced by Wild and Cultivated Strawberry Species. Plant Cell 2004, 16, 3110–3131. [Google Scholar] [CrossRef]
- Giuggioli, N.R.; Girgenti, V.; Baudino, C.; Peano, C. Influence of Modified Atmosphere Packaging Storage on Postharvest Quality and Aroma Compounds of Strawberry Fruits in a Short Distribution Chain. J. Food Process. Preserv. 2015, 39, 3154–3164. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and Anticancer Properties of Berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Ménager, I.; Jost, M.; Aubert, C. Changes in Physicochemical Characteristics and Volatile Constituents of Strawberry (Cv. Cigaline) during Maturation. J. Agric. Food Chem. 2004, 52, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, E.; Türemiş, N.; Bilgili, B.; Zarifikhosroshahi, M.; Burgut, A.; Kafkas, S. Aroma Profiles of Organically Grown ‘Benicia’ and ‘Albion’ Strawberries. Acta Hortic. 2017, 703–708. [Google Scholar] [CrossRef]
- Song, C.; Hong, X.; Zhao, S.; Liu, J.; Schulenburg, K.; Huang, F.-C.; Franz-Oberdorf, K.; Schwab, W. Glucosylation of 4-Hydroxy-2,5-Dimethyl-3(2H)-Furanone, the Key Strawberry Flavor Compound in Strawberry Fruit. Plant Physiol. 2016, 171, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of Selected Aroma-Active Compounds in Strawberries by Headspace Solid-Phase Microextraction Gas Chromatography and Correlation with Sensory Descriptive Analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; Mosandl, A.; Wüst, M. Biosynthesis of Mono- and Sesquiterpenes in Strawberry Fruits and Foliage: 2H Labeling Studies. J. Agric. Food Chem. 2006, 54, 1473–1478. [Google Scholar] [CrossRef]
- Urrutia, M.; Rambla, J.L.; Alexiou, K.G.; Granell, A.; Monfort, A. Genetic Analysis of the Wild Strawberry (Fragaria Vesca) Volatile Composition. Plant Physiol. Biochem. 2017, 121, 99–117. [Google Scholar] [CrossRef]
- Hook, G.L.; Kimm, G.L.; Hall, T.; Smith, P.A. Solid-Phase Microextraction (SPME) for Rapid Field Sampling and Analysis by Gas Chromatography-Mass Spectrometry (GC-MS). TrAC Trends Anal. Chem. 2002, 21, 534–543. [Google Scholar] [CrossRef]
- Curran, A.M.; Rabin, S.I.; Prada, P.A.; Furton, K.G. Comparison of the Volatile Organic Compounds Present in Human Odor Using Spme-GC/MS. J. Chem. Ecol. 2005, 31, 1607–1619. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Stein-Chisholm, R.E.; Lloyd, S.W.; Bett-Garber, K.L.; Grimm, C.C.; Watson, M.A.; Lea, J.M. Volatile, Anthocyanidin, Quality and Sensory Changes in Rabbiteye Blueberry from Whole Fruit through Pilot Plant Juice Processing. J. Sci. Food Agric. 2017, 97, 469–478. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Yu, N.; Wei, Y.; Zhang, J.; Hou, Y.; Sun, A. Evaluation of Microbial, Physicochemical Parameters and Flavor of Blueberry Juice after Microchip-Pulsed Electric Field. Food Chem. 2019, 274, 146–155. [Google Scholar] [CrossRef]
- Bett-Garber, K.L.; Lea, J.M.; Watson, M.A.; Grimm, C.C.; Lloyd, S.W.; Beaulieu, J.C.; Stein-Chisholm, R.E.; Andrzejewski, B.P.; Marshall, D.A. Flavor of Fresh Blueberry Juice and the Comparison to Amount of Sugars, Acids, Anthocyanidins, and Physicochemical Measurements. J. Food Sci. 2015, 80, S818–S827. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Jamali, B.; Rowshan, V. Headspace Analysis of Aroma Composition and Quality Changes of Selva Strawberry (Fragaria x Ananassa Duch.), Fruits as Influenced by Salinity Stress and Application Timing of Nitric Oxide. Anal. Chem. Lett. 2014, 4, 178–189. [Google Scholar] [CrossRef]
- Dymerski, T.; Namieśnik, J.; Vearasilp, K.; Arancibia-Avila, P.; Toledo, F.; Weisz, M.; Katrich, E.; Gorinstein, S. Comprehensive Two-Dimensional Gas Chromatography and Three-Dimensional Fluorometry for Detection of Volatile and Bioactive Substances in Some Berries. Talanta 2015, 134, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Klesk, K.; Qian, M.; Martin, R.R. Aroma Extract Dilution Analysis of Cv. Meeker (Rubus Idaeus, L.) Red Raspberries from Oregon and Washington. J. Agric. Food Chem. 2004, 52, 5155–5161. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.-M.S.; Frandsen, H.L.; Fromberg, A. Authenticity of Raspberry Flavor in Food Products Using SPME-Chiral-GC-MS. Food Sci. Nutr. 2016, 4, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ghaste, M.S. Comprehensive Mapping of Volatile Organic Compounds in Fruits. Ph.D. Thesis, University of Trento, Trento, Italy, 2015. [Google Scholar]
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile Compounds of Raspberry Fruit: From Analytical Methods to Biological Role and Sensory Impact. Molecules 2015, 20, 2445–2474. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Rosales, P.U.; Ragazzo-Sánchez, J.A.; Ruiz-Montañez, G.; Ortiz-Basurto, R.I.; Luna-Solano, G.; Calderón-Santoyo, M. Saccharomyces Cerevisiae Mixed Culture of Blackberry (Rubus Ulmifolius, L.) Juice: Synergism in the Aroma Compounds Production. Sci. World J. 2014, 2014, e163174. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive Compounds, Antioxidant Activity, and Biological Effects of European Cranberry (Vaccinium Oxycoccos). Molecules 2019, 24, 24. [Google Scholar] [CrossRef]
- Research, C. for D.E. and Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs—General Considerations. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioavailability-and-bioequivalence-studies-submitted-ndas-or-inds-general-considerations (accessed on 10 August 2022).
- Porrini, M.; Riso, P. Factors Influencing the Bioavailability of Antioxidants in Foods: A Critical Appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef]
- Winstanley, P.; Orme, M. The Effects of Food on Drug Bioavailability. Br. J. Clin. Pharmacol. 1989, 28, 621–628. [Google Scholar] [CrossRef]
- Caldwell, J.; Gardner, I.; Swales, N. An Introduction to Drug Disposition: The Basic Principles of Absorption, Distribution, Metabolism, and Excretion. Toxicol. Pathol. 1995, 23, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of Nutraceuticals: Role of the Food Matrix, Processing Conditions, the Gastrointestinal Tract, and Nanodelivery Systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of Hydrophobic and Low-Soluble Food Bioactive Compounds within Different Nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Kohlert, C.; van Rensen, I.; März, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and Pharmacokinetics of Natural Volatile Terpenes in Animals and Humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef]
- Verma, P.; Thakur, A.S.; Deshmukh, K.; Jha, A.K.; Verma, S. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. [Google Scholar]
- Mun, H.; Townley, H.E. Nanoencapsulation of Plant Volatile Organic Compounds to Improve Their Biological Activities. Planta Med. 2021, 87, 236–251. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Nutrition, Well-Being and Health; BoD—Books on Demand: Norderstedt, Germany, 2012; ISBN 978-953-51-0125-3. [Google Scholar]
- Igimi, H.; Nishimura, M.; Kodama, R.; Ide, H. Studies on the Metabolism of D-Limonene (p-Mentha-1,8-Diene): I. The Absorption, Distribution and Excretion of d-Limonene in Rats. Xenobiotica 1974, 4, 77–84. [Google Scholar] [CrossRef]
- Miyazawa, M.; Shindo, M.; Shimada, T. Metabolism of (+)- and (−)-Limonenes to Respective Carveols and Perillyl Alcohols by CYP2C9 and CYP2C19 in Human Liver Microsomes. Drug Metab. Dispos. 2002, 30, 602–607. [Google Scholar] [CrossRef]
- Eriksson, K.; Levin, J.-O. Gas Chromatographic-Mass Spectrometric Identification of Metabolites from α-Pinene in Human Urine after Occupational Exposure to Sawing Fumes. J. Chromatogr. B Biomed. Sci. Appl. 1996, 677, 85–98. [Google Scholar] [CrossRef]
- Papada, E.; Gioxari, A.; Amerikanou, C.; Galanis, N.; Kaliora, A.C. An Absorption and Plasma Kinetics Study of Monoterpenes Present in Mastiha Oil in Humans. Foods 2020, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Chadha, A.; Madyastha, K.M. Metabolism of Geraniol and Linalool in the Rat and Effects on Liver and Lung Microsomal Enzymes. Xenobiotica 1984, 14, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Madyastha, K.M.; Srivatsan, V. Biotransformations of α-Terpineol in the Rat: Its Effects on the Liver Microsomal Cytochrome P-450 System. Bull. Environ. Contam. Toxicol. 1988, 41, 17–25. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H. Potential of Essential Oils; BoD—Books on Demand: Norderstedt, Germany, 2018; ISBN 978-1-78923-779-5. [Google Scholar]
- Erasto, P.; Viljoen, A.M. Limonene-a review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1934578X0800300728. [Google Scholar] [CrossRef]
- de Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current Knowledge on Food Source, Metabolism, and Health Effects. Crit. Rev. Food Sci. Nutr. 2021, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and Cardiovascular Disease Mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef]
- Ahmed, A.U. An Overview of Inflammation: Mechanism and Consequences. Front. Biol. 2011, 6, 274. [Google Scholar] [CrossRef]
- Chi, H.; Barry, S.P.; Roth, R.J.; Wu, J.J.; Jones, E.A.; Bennett, A.M.; Flavell, R.A. Dynamic Regulation of Pro- and Anti-Inflammatory Cytokines by MAPK Phosphatase 1 (MKP-1) in Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2274–2279. [Google Scholar] [CrossRef]
- Xie, C.; Kang, J.; Ferguson, M.E.; Nagarajan, S.; Badger, T.M.; Wu, X. Blueberries Reduce Pro-Inflammatory Cytokine TNF-α and IL-6 Production in Mouse Macrophages by Inhibiting NF-ΚB Activation and the MAPK Pathway. Mol. Nutr. Food Res. 2011, 55, 1587–1591. [Google Scholar] [CrossRef]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, Costs, and Natural Variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK Signal Transduction Pathways Activated by Stress and Inflammation: A 10-Year Update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed]
- Roger, T.; David, J.; Glauser, M.P.; Calandra, T. MIF Regulates Innate Immune Responses through Modulation of Toll-like Receptor 4. Nature 2001, 414, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Shin, J.-S.; Jang, D.S.; Lee, K.-T. Cnidilide, an Alkylphthalide Isolated from the Roots of Cnidium Officinale, Suppresses LPS-Induced NO, PGE2, IL-1β, IL-6 and TNF-α Production by AP-1 and NF-ΚB Inactivation in RAW 264.7 Macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Scheidereit, C. The IκB Kinase Complex in NF-ΚB Regulation and Beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Bragado, P.; Armesilla, A.; Silva, A.; Porras, A. Apoptosis by Cisplatin Requires P53 Mediated P38α MAPK Activation through ROS Generation. Apoptosis 2007, 12, 1733–1742. [Google Scholar] [CrossRef]
- Pellegrini, M.; Baldari, C.T. Apoptosis and Oxidative Stress-Related Diseases: The P66Shc Connection. Curr. Mol. Med. 2009, 9, 392–398. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.-S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Amorim, J.L.; Simas, D.L.R.; Pinheiro, M.M.G.; Moreno, D.S.A.; Alviano, C.S.; da Silva, A.J.R.; Fernandes, P.D. Anti-Inflammatory Properties and Chemical Characterization of the Essential Oils of Four Citrus Species. PLoS ONE 2016, 11, e0153643. [Google Scholar] [CrossRef]
- Chi, G.; Wei, M.; Xie, X.; Soromou, L.W.; Liu, F.; Zhao, S. Suppression of MAPK and NF-ΚB Pathways by Limonene Contributes to Attenuation of Lipopolysaccharide-Induced Inflammatory Responses in Acute Lung Injury. Inflammation 2013, 36, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the Anti-Inflammatory, Anti-Catabolic and pro-Anabolic Effects of E-Caryophyllene, Myrcene and Limonene in a Cell Model of Osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yan, J.; Sun, Z. D-Limonene Exhibits Anti-Inflammatory and Antioxidant Properties in an Ulcerative Colitis Rat Model via Regulation of INOS, COX-2, PGE2 and ERK Signaling Pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Ramalho, T.R.; de Oliveira, M.T.P.; de Lima, A.L.A.; Bezerra-Santos, C.R.; Piuvezam, M.R. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015, 81, 1248–1254. [Google Scholar] [CrossRef]
- Jeong, J.B.; Jeong, H.J. Rheosmin, a Naturally Occurring Phenolic Compound Inhibits LPS-Induced INOS and COX-2 Expression in RAW264.7 Cells by Blocking NF-ΚB Activation Pathway. Food Chem. Toxicol. 2010, 48, 2148–2153. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, P.; Lu, X.; Li, Y.; Liu, J.; Liu, B.; Fu, Y.; Cao, Y.; Zhang, N. In Vivo and In Vitro Study on the Efficacy of Terpinen-4-Ol in Dextran Sulfate Sodium-Induced Mice Experimental Colitis. Front. Immunol. 2017, 8, 558. [Google Scholar] [CrossRef]
- Ning, J.; Xu, L.; Zhao, Q.; Zhang, Y.; Shen, C. The Protective Effects of Terpinen-4-Ol on LPS-Induced Acute Lung Injury via Activating PPAR-γ. Inflammation 2018, 41, 2012–2017. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool Reverses Neuropathological and Behavioral Impairments in Old Triple Transgenic Alzheimer’s Mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef]
- Lee, S.-C.; Wang, S.-Y.; Li, C.-C.; Liu, C.-T. Anti-Inflammatory Effect of Cinnamaldehyde and Linalool from the Leaf Essential Oil of Cinnamomum Osmophloeum Kanehira in Endotoxin-Induced Mice. J. Food Drug Anal. 2018, 26, 211–220. [Google Scholar] [CrossRef]
- Kim, M.-G.; Kim, S.-M.; Min, J.-H.; Kwon, O.-K.; Park, M.-H.; Park, J.-W.; Ahn, H.I.; Hwang, J.-Y.; Oh, S.-R.; Lee, J.-W.; et al. Anti-Inflammatory Effects of Linalool on Ovalbumin-Induced Pulmonary Inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef]
- Ma, J.; Xu, H.; Wu, J.; Qu, C.; Sun, F.; Xu, S. Linalool Inhibits Cigarette Smoke-Induced Lung Inflammation by Inhibiting NF-ΚB Activation. Int. Immunopharmacol. 2015, 29, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-Inflammatory Effects of Linalool in RAW 264.7 Macrophages and Lipopolysaccharide-Induced Lung Injury Model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yu, L.; Qiu, J.; Shen, B.; Wang, D.; Soromou, L.W.; Feng, H. Linalool Attenuates Lung Inflammation Induced by Pasteurella Multocida via Activating Nrf-2 Signaling Pathway. Int. Immunopharmacol. 2014, 21, 456–463. [Google Scholar] [CrossRef]
- Li, Y.; Lv, O.; Zhou, F.; Li, Q.; Wu, Z.; Zheng, Y. Linalool Inhibits LPS-Induced Inflammation in BV2 Microglia Cells by Activating Nrf2. Neurochem Res 2015, 40, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-ΚB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Major Selected Monoterpenes α-Pinene and 1,8-Cineole Found in Salvia Lavandulifolia (Spanish Sage) Essential Oil as Regulators of Cellular Redox Balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef]
- Lemes, R.S.; Alves, C.C.F.; Estevam, E.B.B.; Santiago, M.B.; Martins, C.H.G.; Santos, T.C.L.D.; Crotti, A.E.M.; Miranda, M.L.D. Chemical Composition and Antibacterial Activity of Essential Oils from Citrus Aurantifolia Leaves and Fruit Peel against Oral Pathogenic Bacteria. An. Acad. Bras. Ciênc. 2018, 90, 1285–1292. [Google Scholar] [CrossRef]
- Rehman, M.U.; Tahir, M.; Khan, A.Q.; Khan, R.; Oday-O-Hamiza; Lateef, A.; Hassan, S.K.; Rashid, S.; Ali, N.; Zeeshan, M.; et al. D-Limonene Suppresses Doxorubicin-Induced Oxidative Stress and Inflammation via Repression of COX-2, INOS, and NFκB in Kidneys of Wistar Rats. Exp. Biol. Med. 2014, 239, 465–476. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-ΚB: A Key Role in Inflammatory Diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative Stress and Apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thymus Mastichina, L. Essential Oils from Murcia (Spain): Composition and Antioxidant, Antienzymatic and Antimicrobial Bioactivities. PLoS ONE 2018, 13, e0190790. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Bae, H.C.; Park, E.-J.; Lee, C.R.; Kim, B.-J.; Lee, S.; Park, H.H.; Kim, S.-J.; So, I.; Kim, T.W.; et al. Geraniol Inhibits Prostate Cancer Growth by Targeting Cell Cycle and Apoptosis Pathways. Biochem. Biophys. Res. Commun. 2011, 407, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, Y.R.; Kim, S.-H.; Park, E.-J.; Kang, M.J.; So, I.; Chun, J.N.; Jeon, J.-H. Geraniol Suppresses Prostate Cancer Growth through Down-Regulation of E2F8. Cancer Med. 2016, 5, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, G.; Zhao, H.; Dong, X.; Yang, Z. Inhibiting the JNK/ERK Signaling Pathway with Geraniol for Attenuating the Proliferation of Human Gastric Adenocarcinoma AGS Cells. J. Biochem. Mol. Toxicol. 2021, 35, e22818. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Qing, C.; Wang, W.; Yang, Y. In Vitro and In Vivo Efficacy Studies of Lavender Angustifolia Essential Oil and Its Active Constituents on the Proliferation of Human Prostate Cancer. Integr. Cancer Ther. 2017, 16, 215–226. [Google Scholar] [CrossRef]
- Mirza, M.B.; Elkady, A.I.; Al-Attar, A.M.; Syed, F.Q.; Mohammed, F.A.; Hakeem, K.R. Induction of Apoptosis and Cell Cycle Arrest by Ethyl Acetate Fraction of Phoenix Dactylifera, L. (Ajwa Dates) in Prostate Cancer Cells. J. Ethnopharmacol. 2018, 218, 35–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, X.; Wang, G.; Liao, Y.; Qing, C. Linalool Inhibits 22Rv1 Prostate Cancer Cell Proliferation and Induces Apoptosis. Oncol. Lett. 2020, 20, 289. [Google Scholar] [CrossRef]
- Ou-Yang, F.; Tsai, I.-H.; Tang, J.-Y.; Yen, C.-Y.; Cheng, Y.-B.; Farooqi, A.A.; Chen, S.-R.; Yu, S.-Y.; Kao, J.-K.; Chang, H.-W. Antiproliferation for Breast Cancer Cells by Ethyl Acetate Extract of Nepenthes Thorellii × (Ventricosa × Maxima). Int. J. Mol. Sci. 2019, 20, 3238. [Google Scholar] [CrossRef]
- Abou Baker, D.H. Achillea Millefolium, L. Ethyl Acetate Fraction Induces Apoptosis and Cell Cycle Arrest in Human Cervical Cancer (HeLa) Cells. Ann. Agric. Sci. 2020, 65, 42–48. [Google Scholar] [CrossRef]
- Noor, R.; Astuti, I. Cytotoxicity of α-terpineol in HeLa cell line and its effects to apoptosis and cell cycle. J. Med. Sci. (Berk. Ilmu Kedokt.) 2014, 46, 1–9. [Google Scholar]
- Chang, M.-Y.; Shieh, D.-E.; Chen, C.-C.; Yeh, C.-S.; Dong, H.-P. Linalool Induces Cell Cycle Arrest and Apoptosis in Leukemia Cells and Cervical Cancer Cells through CDKIs. Int. J. Mol. Sci. 2015, 16, 28169–28179. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, G. Linalool monoterpene exerts potent antitumor effects in OECM 1 human oral cancer cells by inducing sub-G1 cell cycle arrest, loss of mitochondrial membrane potential and inhibition of PI3K/AKT biochemical pathway. J BUON 2019, 24, 323–328. [Google Scholar] [PubMed]
- Pushpalatha, R.; Sindhu, G.; Sharmila, R.; Tamizharasi, G.; Vennila, L.; Vijayalakshmi, A. Linalool Induces Reactive Oxygen Species Mediated Apoptosis in Human Oral Squamous Carcinoma Cells. Indian J. Pharm. Sci. 2021, 83, 906–917. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, M.A.; Crespo, R.; Galle, M.; de Bravo, M.G. Anti-Cancer Mechanisms of Linalool and 1,8-Cineole in Non-Small Cell Lung Cancer A549 Cells. Heliyon 2020, 6, e05639. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-Limonene Exhibits Antitumor Activity by Inducing Autophagy and Apoptosis in Lung Cancer. OTT 2018, 11, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. D-Limonene Rich Volatile Oil from Blood Oranges Inhibits Angiogenesis, Metastasis and Cell Death in Human Colon Cancer Cells. Life Sci. 2012, 91, 429–439. [Google Scholar] [CrossRef]
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J BUON/Off. J. Balk. Union. Oncol. 2020, 25, 280–285. [Google Scholar]
- Viana, A.F.S.C.; Lopes, M.T.P.; Oliveira, F.T.B.; Nunes, P.I.G.; Santos, V.G.; Braga, A.D.; Silva, A.C.A.; Sousa, D.P.; Viana, D.A.; Rao, V.S.; et al. (−)-Myrtenol Accelerates Healing of Acetic Acid-Induced Gastric Ulcers in Rats and in Human Gastric Adenocarcinoma Cells. Eur. J. Pharmacol. 2019, 854, 139–148. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.; Liang, Y. Myrcene Exerts Anti-Tumor Effects on Oral Cancer Cells in Vitro via Induction of Apoptosis. Trop. J. Pharm. Res. 2022, 21, 933–938. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Su, M.; Sun, L.; Zhang, S.; Wang, D.; Liu, Z.; Yuan, Y.; Liu, Y.; Li, Y. Geraniol Improves Endothelial Function by Inhibiting NOX-2 Derived Oxidative Stress in High Fat Diet Fed Mice. Biochem. Biophys. Res. Commun. 2016, 474, 182–187. [Google Scholar] [CrossRef]
- de Sousa, G.M.; Cazarin, C.B.B.; Junior, M.R.M.; de Lamas, C.A.; Quitete, V.H.A.C.; Pastore, G.M.; Bicas, J.L. The Effect of α-Terpineol Enantiomers on Biomarkers of Rats Fed a High-Fat Diet. Heliyon 2020, 6, e03752. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, H.; Dou, H.; Guo, L.; Huang, W. Microcapsule of Sweet Orange Essential Oil Changes Gut Microbiota in Diet-Induced Obese Rats. Biochem. Biophys. Res. Commun. 2018, 505, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Yun, J.W. Monoterpene Limonene Induces Brown Fat-like Phenotype in 3T3-L1 White Adipocytes. Life Sci. 2016, 153, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ruiz, L.A.; Ortega-Pérez, L.G.; Piñón-Simental, J.S.; Magaña-Rodriguez, O.R.; Meléndez-Herrera, E.; Rios-Chavez, P. Role of the Major Terpenes of Callistemon Citrinus against the Oxidative Stress during a Hypercaloric Diet in Rats. Biomed. Pharmacother. 2022, 153, 113505. [Google Scholar] [CrossRef]
- Bacanlı, M.; Anlar, H.G.; Aydın, S.; Çal, T.; Arı, N.; Ündeğer Bucurgat, Ü.; Başaran, A.A.; Başaran, N. D-Limonene Ameliorates Diabetes and Its Complications in Streptozotocin-Induced Diabetic Rats. Food Chem. Toxicol. 2017, 110, 434–442. [Google Scholar] [CrossRef]
- Murali, R.; Saravanan, R. Antidiabetic Effect of D-Limonene, a Monoterpene in Streptozotocin-Induced Diabetic Rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- More, T.A.; Kulkarni, B.R.; Nalawade, M.L.; Arvindekar, A.U. Antidiabetic activity of linalool and limonene in streptozotocin-induced diabetic rat: A combinatorial therapy approach. Int. J. Pharm. Pharm. Sci. 2014, 6, 159–163. [Google Scholar]
- Murali, R.; Karthikeyan, A.; Saravanan, R. Protective Effects of D-Limonene on Lipid Peroxidation and Antioxidant Enzymes in Streptozotocin-Induced Diabetic Rats. Basic Clin. Pharmacol. Toxicol. 2013, 112, 175–181. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Ghaleb, H.; Elberry, A.A.; Balamash, K.S.; Ghareib, S.A.; Azhar, A.; Banjar, Z. Geraniol Alleviates Diabetic Cardiac Complications: Effect on Cardiac Ischemia and Oxidative Stress. Biomed. Pharmacother. 2017, 88, 1025–1030. [Google Scholar] [CrossRef]
- Kamble, S.P.; Ghadyale, V.A.; Patil, R.S.; Haldavnekar, V.S.; Arvindekar, A.U. Inhibition of GLUT2 Transporter by Geraniol from Cymbopogon Martinii: A Novel Treatment for Diabetes Mellitus in Streptozotocin-Induced Diabetic Rats. J. Pharm. Pharmacol. 2020, 72, 294–304. [Google Scholar] [CrossRef]
- Babukumar, S.; Vinothkumar, V.; Sankaranarayanan, C.; Srinivasan, S. Geraniol, a Natural Monoterpene, Ameliorates Hyperglycemia by Attenuating the Key Enzymes of Carbohydrate Metabolism in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 2017, 55, 1442–1449. [Google Scholar] [CrossRef]
- El-Said, Y.A.M.; Sallam, N.A.A.; Ain-Shoka, A.A.-M.; Abdel-Latif, H.A.-T. Geraniol Ameliorates Diabetic Nephropathy via Interference with MiRNA-21/PTEN/Akt/MTORC1 Pathway in Rats. Naunyn-Schmiedeberg’s Arch Pharm. 2020, 393, 2325–2337. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; Elberry, A.A.; Ghareib, S.A. Geraniol Improves the Impaired Vascular Reactivity in Diabetes and Metabolic Syndrome through Calcium Channel Blocking Effect. J. Diabetes Complicat. 2016, 30, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Mahdavifard, S.; Nakhjavani, M. Effect of linalool on the activity of glyoxalase-I and diverse glycation products in rats with type 2 diabetes. J. Maz. Univ. Med. Sci. 2020, 30, 24–33. [Google Scholar]
- Deepa, B.; Venkatraman Anuradha, C. Effects of Linalool on Inflammation, Matrix Accumulation and Podocyte Loss in Kidney of Streptozotocin-Induced Diabetic Rats. Toxicol. Mech. Methods 2013, 23, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Xuemei, L.; Qiu, S.; Chen, G.; Liu, M. Myrtenol Alleviates Oxidative Stress and Inflammation in Diabetic Pregnant Rats via TLR4/MyD88/NF-ΚB Signaling Pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22904. [Google Scholar] [CrossRef] [PubMed]

| Compound | Strawberry | Blueberry | Raspberry | Blackberry | Cranberry |
|---|---|---|---|---|---|
| Esters | |||||
| Methyl butanoate | [65,66] | ||||
| Methyl hexanoate | [65,66,67] | ||||
| Ethyl acetate | [65] | [68] | [69] | [70] | |
| Hexyl acetate | [65] | ||||
| Ethyl butanoate | [66,69] | [71] | |||
| Ethyl hexanoate | [66,72] | [73] | |||
| Ethyl 2-methylbutanoate | [67] | [74] | [71,73] | [75] | |
| Ethyl 2-methylpropanoate | [67] | [76] | |||
| 3-methylbutylacetate | [67] | ||||
| Hexyl butanoate | [69] | ||||
| Ethyl propanoate | [74] | ||||
| Methyl 2-methylbutanoate | [74] | ||||
| Methyl 3-methylbutanoate | [74] | ||||
| Ethyl 3-methylbutanoate | [74] | ||||
| (Z)-3-hexyl acetate | [74] | ||||
| (E)-2-hexyl acetate | [74] | ||||
| Geranyl acetate | [74] | ||||
| 3-cis-hexenyl formate | [77] | ||||
| Ethyl benzoate | [69] | ||||
| Ketones | |||||
| 2-heptanone | [67] | [74,78] | [79] | [73] | |
| 2,3-butanedione | [67] | [80] | |||
| 1-octen-3-one | [74] | ||||
| 2-nonanone | [74] | ||||
| 6-methyl-5-hepten-2-one | [74] | ||||
| Raspberry ketone | [76] | ||||
| β-damascenone | [76] | [71] | |||
| 3-hydroxy-2-butanone | [80] | ||||
| 2-undecanone | [73] | ||||
| Isophorone | [69] | ||||
| Terpenes | |||||
| Limonone | [65] | [74] | [69,73] | ||
| α-terpinene | [65] | ||||
| Linalool | [65,66] | [69,74,78] | [67,76] | [71,73] | [69] |
| Nerolidol | [66] | ||||
| Myrtenol | [69] | [69] | [69,73] | ||
| Geraniol | [68,74] | [76,79] | [71] | ||
| Citronellol | [74,81] | [69] | |||
| α-terpineol | [69,74,81] | [69,76] | [70] | [69,77,82,83] | |
| Nerol | [74] | [76] | |||
| Eucalyptol (1,8-cineolo) | [74,78] | [69,83] | |||
| Dihydrolinalool oxide | [74] | ||||
| δ-elemene | [78] | ||||
| (E)-caryophyllene | [78] | ||||
| Caryophyllene oxide | [78] | ||||
| Linalool oxide | [69] | [83] | |||
| β-ionone | [69] | [76,79] | [73] | [75] | |
| α-ionone | [69,76,79] | [73] | |||
| β-pinene | [76] | ||||
| Terpinen-4-ol | [79] | [69] | |||
| α-pinene | [73] | ||||
| p-cymene | [73] | ||||
| Sabinene | [73] | ||||
| Acids | |||||
| Butanoic acid | [69] | [69] | [77] | ||
| Hexanoic acid | [78] | [69] | [69,73] | ||
| Octanoic acid | [78] | ||||
| Nonanoic acid | [78] | ||||
| Decanoic acid | [78] | [73] | |||
| 3-methylbutanoic acid | [69] | [73] | |||
| 2-methylbutanoic acid | [73,80] | [69,75] | |||
| Acetic acid | [73] | ||||
| Alcohols | |||||
| Cis-3-hexen-1-ol | [65] | ||||
| (E)-2-hexenol | [68] | ||||
| (Z)-3-hexenol | [68] | [76] | |||
| 2-phenylethanol | [81] | ||||
| (Z)-3-hexenol | [74] | ||||
| 2-heptanol | [74] | [73] | |||
| Phenylethyl alcohol | [69] | ||||
| 2-ethylhexanol | [69] | [69] | [69] | ||
| 1-octanol | [76] | [73,80] | |||
| (Z)-hexenol | [79] | ||||
| Ethanol | [73,80] | ||||
| 1-hexanol | [73,80] | ||||
| p-cymen-8-ol | [73] | ||||
| Nopol | [73] | ||||
| 4-methyl-1-pentanol | [69] | ||||
| 4-penten-2-ol | [77] | ||||
| Benzyl alcohol | [77,82,84] | ||||
| Aldehydes | |||||
| Hexanal | [65,85] | [68,74,78] | [76,79] | [73] | [75] |
| Trans-2-hexenal | [65] | [73,80] | |||
| Cis-3-hexenal | [67] | ||||
| (E)-2-hexenal | [68,74,78] | [76,79] | [75] | ||
| Vanilllin | [81] | [69] | |||
| (Z)-3-hexenal | [74,78] | ||||
| (E,Z)-2,6-nonadienal | [74] | ||||
| (E,E)-2,4-nonedienal | [74] | ||||
| Pentanal | [74] | [75] | |||
| Octanal | [74] | [75] | |||
| (E,E)-2,4-hexadienal | [74] | ||||
| Decanal | [74] | ||||
| Hexadienal | [78] | ||||
| Heptanal | [78] | [76] | |||
| Benzaldehyde | [76] | [77] | |||
| cuminaldehyde | [69] | ||||
| Methylbutanal | [80] | ||||
| Methional | [71] | ||||
| Trans,cis-2,6-nonadienal | [71] | ||||
| 3-methylbutanal | [73] | ||||
| 2-methylbutanal | [73] | ||||
| (E)-2-heptenal | [75] | ||||
| (E)-2-octenal | [75] | ||||
| (E)-2-nonenal | [75] | ||||
| Trans-2-decanal | [83] | ||||
| 2-octanal | [83] | ||||
| Surfurs | |||||
| methanethiol | [66] | ||||
| Norisoprenoids | |||||
| β-damascenone | [78] | ||||
| Cis-1,5-octadien-3-one | [71] | ||||
| Furanones | |||||
| Furaneol | [65,66] | [74] | [76] | [71] | |
| Mesifurane | [65,66,67,69] | ||||
| Hydrocarbons | |||||
| Octane | [78] | ||||
| Ethyl benzene | [78] | ||||
| p-xylene | [78] | ||||
| Mxylene | [78] | ||||
| Β-ocimene | [76] | ||||
| Lactones | |||||
| γ-decalactone | [65] | ||||
| Butyrolactone | [78] | ||||
| δ-octalactone | [79] | ||||
| δ-decalactone | [79] |
| Volatile Compound | Inflammation Model | Effect | References |
|---|---|---|---|
| Limonene | Carrageenan-induced mice subcutaneous air pouch mice model | IFN-γ, IL-1β, NO, and TNF-α production↓ | [142] |
| Limonene | LPS-induced acute lung injury mice model | NF-κB and MAPK activation↓ (IκBα, NF-κB p65, ERK, JNK, and p38 MAPK phosphorylation↓) | [143] |
| D-limonene | Doxorubicin-induced rat model | COX-2, iNOS, NO, PGE2, and TNF-α production↓ NF-κB activation↓ | [144] |
| D-limonene | Ulcerative colitis rat model | COX-2, iNOS, PGE2↓ | [145] |
| Limonene, myrcene | IL-1β-induced human chondrocyte model | JNK and p38 phosphorylation↓ NF-κB activation↓ | [146] |
| Rheosmin | LPS-induced RAW264.7 cells | COX-2, iNOS, NO, and PGE2 production↓ | [147] |
| α-terpineol | LPS-induced RAW264.7 cells | NO production↓ | [83] |
| γ-terpinene | Carrageenan-induced peritonitis mice model | IL-1β and TNF-α production↓ | [146] |
| Terpinen-4-ol | DSS-induced colitis mice model | NF-κB activation↓ | [148] |
| Terpinen-4-ol | LPS-induced acute lung injury mice model | IL-1β and TNF-α production↓ IκBα and NF-κB p65 phosphorylation↓ | [149] |
| Linalool | Aged triple transgenic Alzheimer’s mice model | COX-2, IL-1β, and iNOS production↓ P38 MAPK production↓ | [150] |
| Linalool | Endotoxin-induced mice model | IFN-γ, IL-1β, IL-18, NO, and TNF-α production↓ TLR4 expression↓ NF-κB activation↓ | [151] |
| Linalool | Ovalbumin-induced pulmonary inflammation mice model | iNOS and MCP-1 production↓ MAPK and NF-κB activation↓ | [152] |
| Linalool | Cigarette smoke-induced acute lung inflammation mice model | IL-1β, IL-6, IL-8, MCP-1, and TNF-α production↓ NF-κB activation↓ | [153] |
| Linalool | LPS-induced RAW264.7 cells | IL-6 and TNF-α production↓ | [154] |
| Linalool | Pasteurella multocida-induced lung inflammation mice model | IL-6 and TNF-α production↓ Nrf2 nuclear translocation↑ | [155] |
| Linalool | LPS-induced BV2 microglia cells | IL-1β, NO, PGE2, and TNF-α production↓ NF-κB activation↓ Nrf2 nuclear translocation↑ HO-1 expression↑ | [156] |
| α-pinene | LPS-induced mouse peritoneal macrophages | COX-2, IL-6, iNOS, NO, and TNF-α production↓ MAPK and NF-κB activation↓ | [157] |
| α-pinene, 1,8-cineole | H2O2-stimulated U373-MG cells (human astrocytoma cell line) | ROS formation↓ | [158] |
| Berry volatile extracts | LPS-induced RAW264.7 cells | COX-2↓, IL-6↓, NO↓, PGE2↓, TNF-α↓ IκBα and NF-κB p65 phosphorylation↓ | [69] |
| Volatile Compound | Cancer Model | Effect | References |
|---|---|---|---|
| Geraniol | Human prostate cancer PC-3 cells, in vitro and in vivo xenograft mice model | Cell proliferation↓ Cell cycle arrest and apoptosis↑ Tumor volume and weight↓ Docetaxel sensitization↑ | [164] |
| Geraniol | Human prostate cancer PC-3 cells | E2F8 transcription factor↓ G2/M phase cell cycle arrest↑ | [165] |
| Geraniol | Gastric adenocarcinoma AGS cells | ERK, JNK, p38 MAPK activation↓ Apoptosis↑ | [166] |
| Linalool, linalyl acetate | Human prostate cancer PC-3 and DU145 cells, PC-3 cell-transplanted xenograft mice model | Apoptosis and G2/M phase cell cycle arrest↑ Tumor growth↓ | [167] |
| Linalool | Human prostate cancer 22Rv1 cells | Cell proliferation↓ Apoptosis↑ | [169] |
| Linalool | Human leukemia U937 cells and human cervical adenocarcinoma HeLa cells | Apoptosis and cell cycle arrest↑ | [173] |
| Linalool | Human oral cancer OECM1 and KB cells | Cell proliferation↓ Apoptosis and sub-G1 phase cell cycle arrest↑ | [174] [175] |
| Linalool, 1,8-cineole | Human lung adenocarcinoma A549 cells | Cell proliferation↓ Cell cycle arrest↑ No apoptosis | [176] |
| Ethyl acetate | Human prostate cancer PC-3 cells | Apoptosis and S phase cell cycle arrest↑ Oxidative stress↑ Mitochondrial membrane potential (MMP)↓ | [168] |
| Ethyl acetate | Human breast cancer MCF7 and SKBR3 cells | Sub G1 phase cell cycle arrest↑ ROS production↑ MMP↓ | [170] |
| Ethyl acetate | Human cervical cancer HeLa cells | Apoptosis and G2/M phase cell cycle arrest↑ | [171] |
| α-terpineol | Human cervical cancer HeLa cells | Apoptosis and G1 phase cell cycle arrest↑ | [172] |
| D-limonene | Lung cancer A549 and H1299 cells | Tumor growth↓ Apoptosis and autophagy-related gene expression↑ | [177] |
| Limonene | Human bladder cancer T24 cells | Cell proliferation↓ Apoptosis and G2/M cell cycle arrest↑ Bax, cleaved caspase-3, 8, and 9 expression↑ Bcl-2 expression↓ | [179] |
| Myrcene | Oral cancer SCC9 cells | Apoptosis↑ Cell migration↓ | [181] |
| Volatile Compound | Obesity Model | Effect | References |
|---|---|---|---|
| Geraniol | High-fat diet (HFD)-fed mice | Aortic NADPH oxidases, ROS production↓ | [182] |
| α-terpineol | HFD-induced obese rats | IL-1β and TNF-α↓ Serum TBARS↓ Insulin sensibility↑ | [183] |
| D-limonene-rich sweet orange essential oil | HFD-induced obese rats | Body weight↓ Relative abundance of Bifidobacterium↑ | [184] |
| Limonene | Mouse preadipocytes 3T3-L1 | Adipocyte browning↑ | [185] |
| Limonene, α-terpineol, 1,8-cineole | High-fat-sucrose diet (HFSD)-fed rats | Body weight↓ Fat deposition↓ Serum glucose level↓ Triacylglycerol level↓ | [186] |
| Volatile Compound | Diabetes Model | Effect | References |
|---|---|---|---|
| D-limonene | Streptozotocin-induced diabetic rat model | DNA damage↓ Antioxidant enzyme activities↑ | [187] |
| D-limonene | Streptozotocin-induced diabetic rat model | Antihyperglycemic activities↑ | [188] |
| Limonene, linalool | Streptozotocin-induced diabetic rat model | Blood glucose level↓ Antioxidant enzyme activities↑ | [189] |
| D-limonene | Streptozotocin-induced diabetic rat model | Lipid peroxidation↓ Antioxidant activity↑ | [190] |
| Geraniol | Streptozotocin-induced diabetic rat model | Oxidative stress↓ | [191] |
| Geraniol | Streptozotocin-induced diabetic rat model | GLUT2 expression↓ Kidney glucose release↓ | [192] |
| Geraniol | Streptozotocin-induced diabetic rat model | Insulin resistance↓ Plasma glucose level↓ | [193] |
| Geraniol | Streptozotocin-induced diabetic rat model | Redox balance↑ Lipid peroxidation↓ | [194] |
| Geraniol | Streptozotocin-induced diabetic rat model | Vasoconstriction↓ | [195] |
| Linalool | Streptozotocin-induced diabetic rat model | NF-κB and TGF-β1 expression↓ | [197] |
| Myrtenol | Streptozotocin-induced gestational diabetic pregnant rat model | Blood glucose level↓ Pro-inflammatory markers↓ HDL and antioxidant activity↑ | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, I.; Howard, L.; Lee, S.-O. Volatiles in Berries: Biosynthesis, Composition, Bioavailability, and Health Benefits. Appl. Sci. 2022, 12, 10238. https://doi.org/10.3390/app122010238
Gu I, Howard L, Lee S-O. Volatiles in Berries: Biosynthesis, Composition, Bioavailability, and Health Benefits. Applied Sciences. 2022; 12(20):10238. https://doi.org/10.3390/app122010238
Chicago/Turabian StyleGu, Inah, Luke Howard, and Sun-Ok Lee. 2022. "Volatiles in Berries: Biosynthesis, Composition, Bioavailability, and Health Benefits" Applied Sciences 12, no. 20: 10238. https://doi.org/10.3390/app122010238
APA StyleGu, I., Howard, L., & Lee, S.-O. (2022). Volatiles in Berries: Biosynthesis, Composition, Bioavailability, and Health Benefits. Applied Sciences, 12(20), 10238. https://doi.org/10.3390/app122010238








