Size-Dependent Cytotoxic and Molecular Study of the Use of Gold Nanoparticles against Liver Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
2.2. Methods
2.2.1. Synthesis of Gold Nanoparticles (GNPs)
2.2.2. Materials’ Characterizations
2.3. Cell Culture
2.4. Exposure of Material
2.4.1. Nanoparticles’ Exposure
2.4.2. MTT Assay
2.4.3. Neutral Red Uptake Assay
2.5. Reactive Oxygen Species (ROS) Detection
2.6. Morphological Changes
2.7. RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR) Analysis of Apoptotic Marker Genes
2.8. Statistical Analysis
3. Results
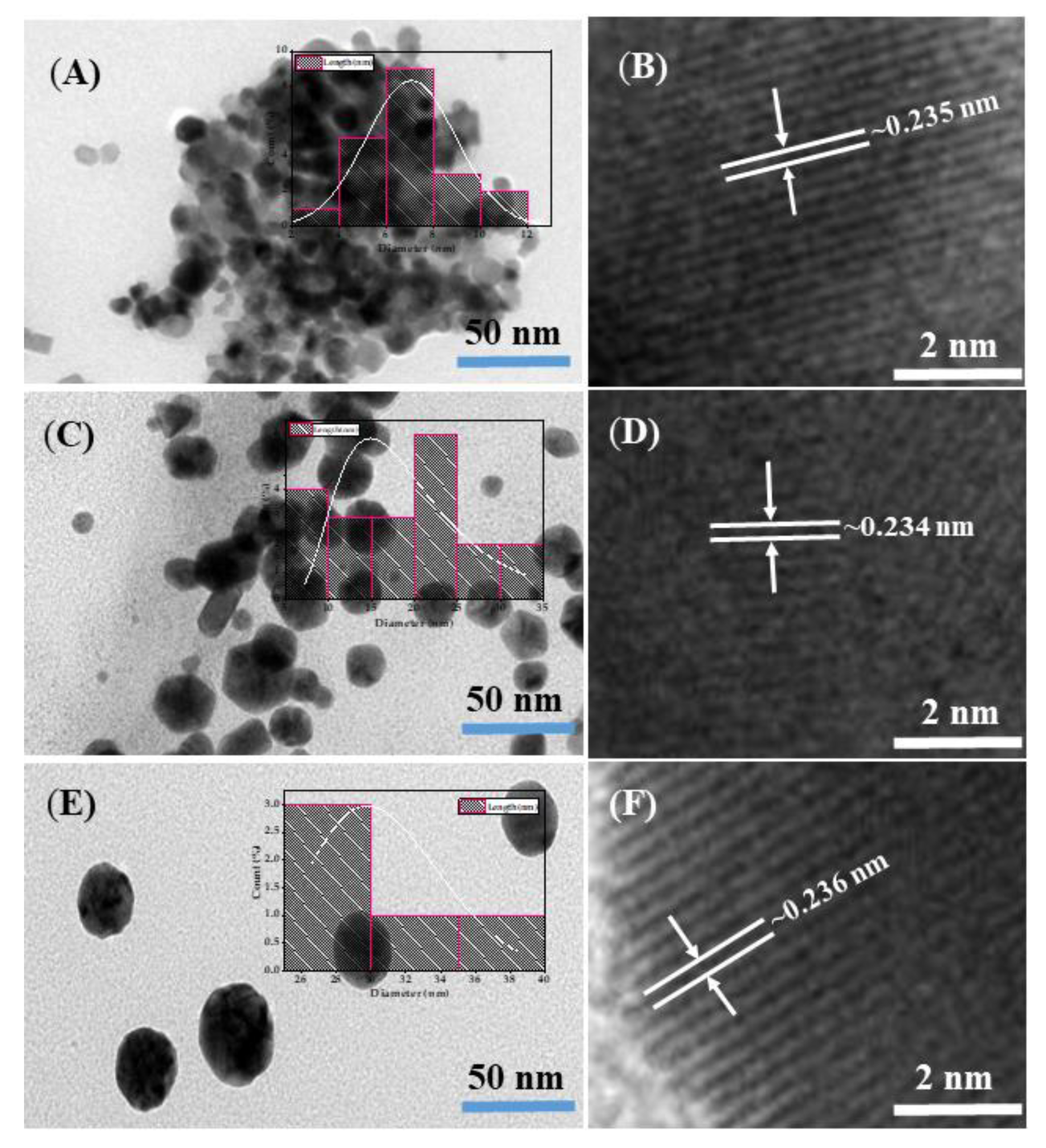
3.1. Morphological Characterization of GNPs
3.2. Hydrodynamic Size and Zeta Potential of GNPs
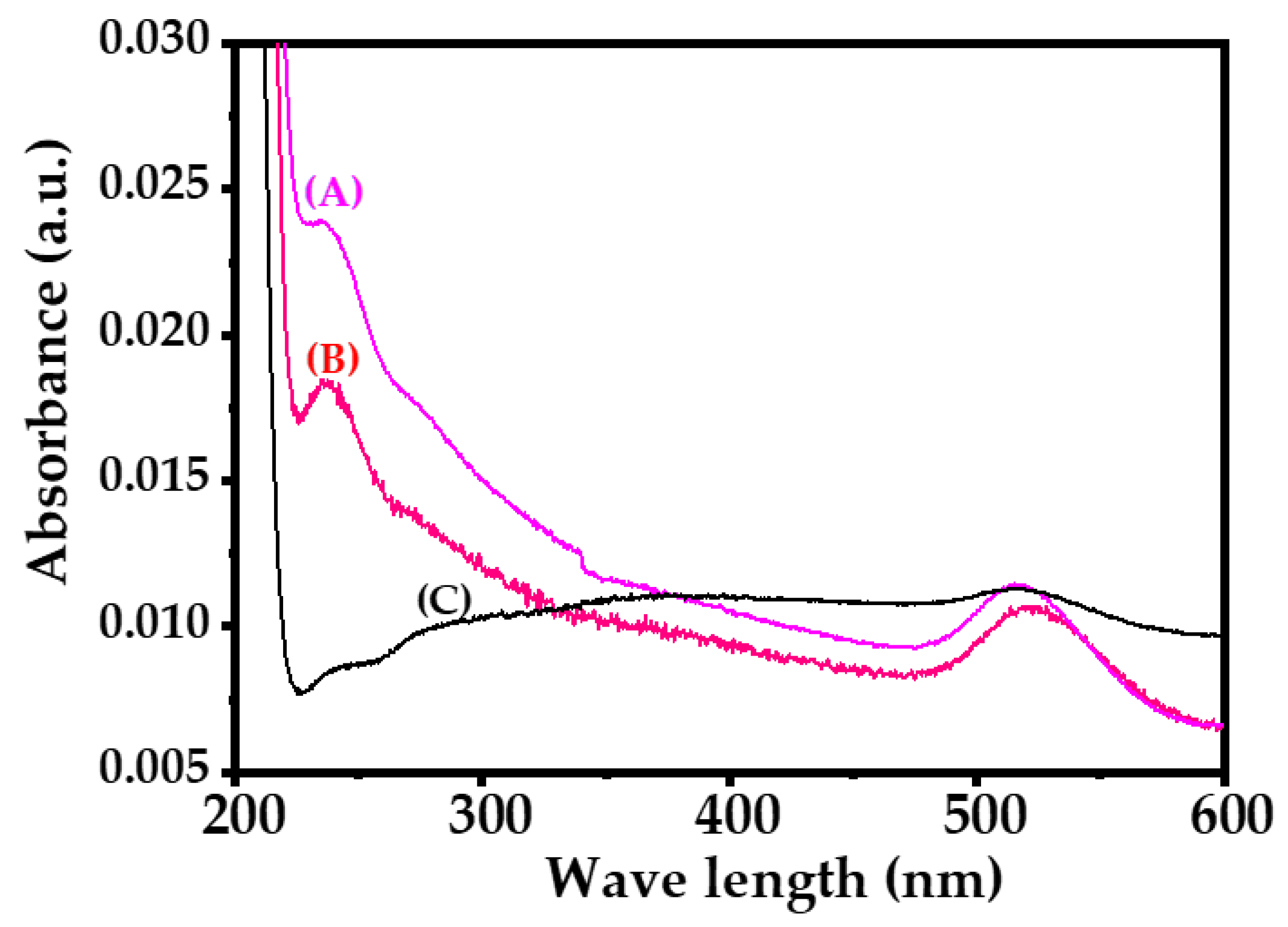
3.3. UV-Visible Spectroscopy
3.4. Morphological Changes of Cancer Cells (HepG2) Control and Treated with GNPs
3.5. Cytotoxicity Assessment with MTT Assay in HepG2 Cells with GNPs
3.6. Cytotoxicity Assessment via NRU Assay in HepG2 Cells with GNPs
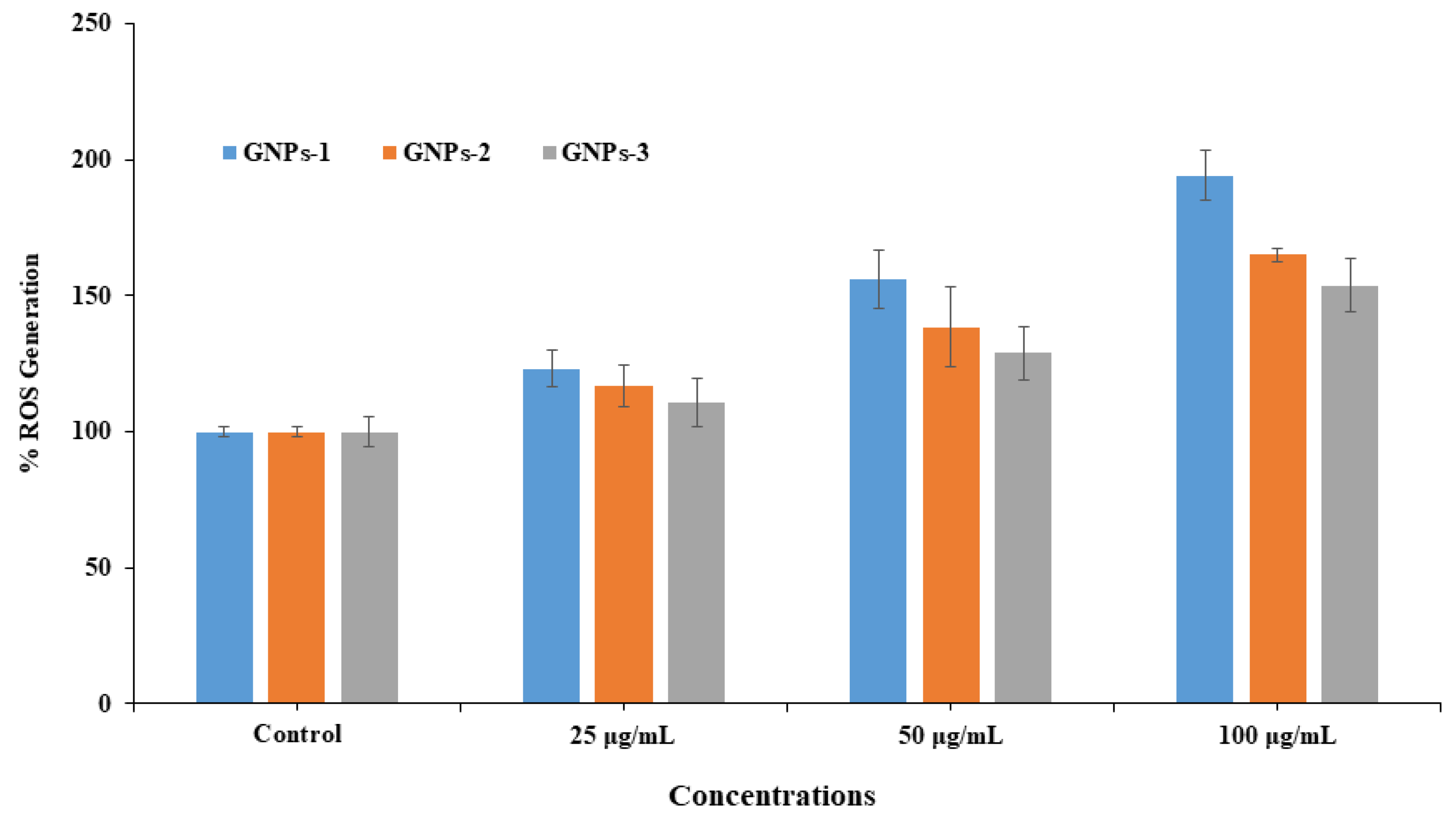
3.7. ROS Generation in HepG2 with and without Exposure GNPs
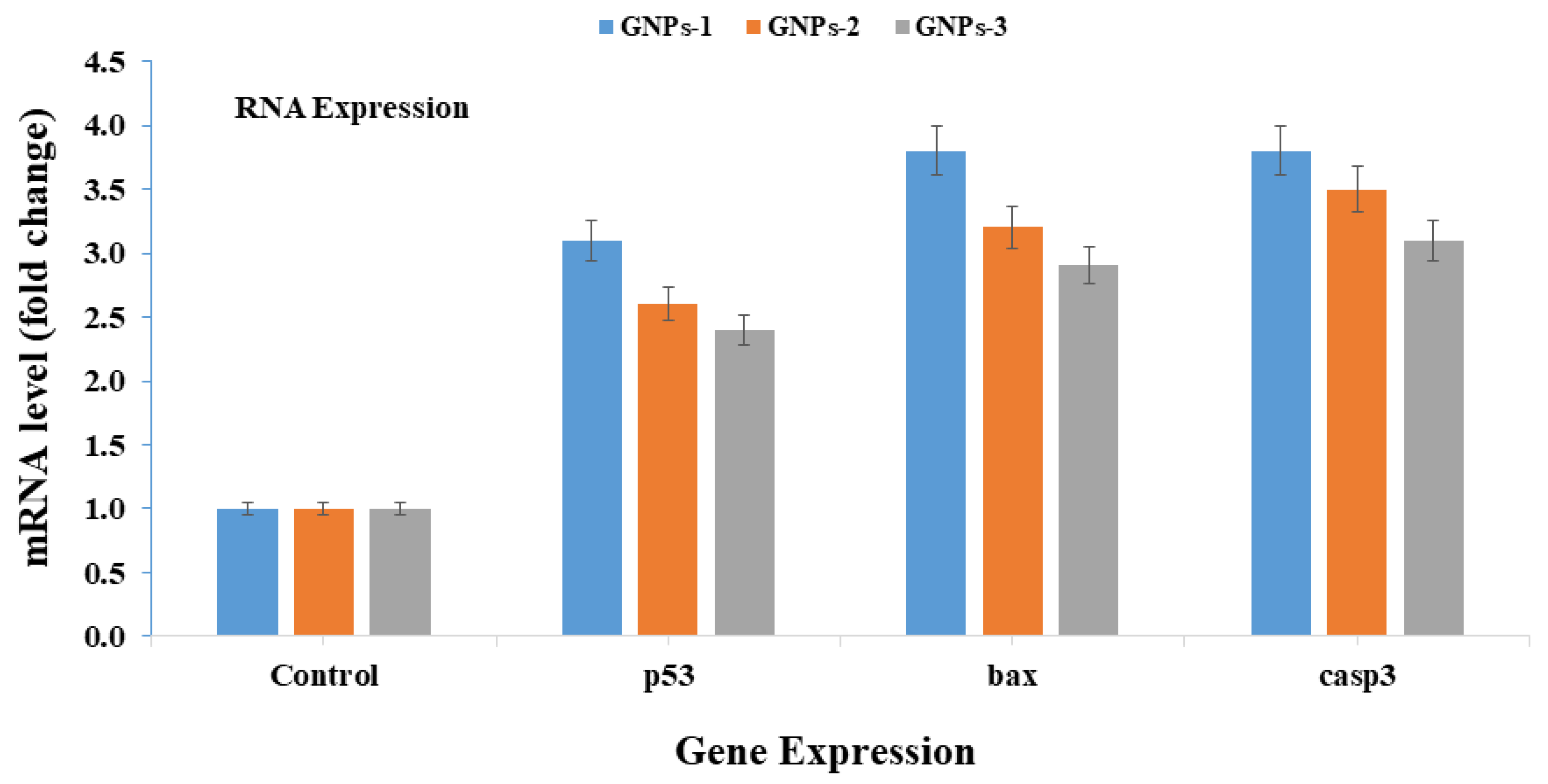
3.8. mRNA Expressions
3.9. Discussion
3.10. Techno-Economic Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| cDNA | Complementary DNA |
| DMSO | Dimethylsulfoxide |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco’s modified eagle medium |
| DCF-DA | 2, 7-dichlorofluorescin diacetate |
| FBS | Fetal bovine serum |
| GNPs | Gold nanoparticles |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HR-TEM | High-resolution transmission electron microscopy |
| HepG2 | Liver cancer cells |
| TEM | Transmission electron microscopy |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NRU | Neutral red uptake |
| qPCR | Quantitative polymerase chain reaction |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| SEM | Scanning electron microscope |
| NMs | Nanomaterials |
References
- Nguyen, H.P.T.; Arafin, S.; Piao, J.; Cuong, T.V. Nanostructured Optoelectronics: Materials and Devices. J. Nanomater. 2016, 2016, 2051908. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Samykano, M. Progress in one-dimensional nanostructures. Mater. Charact. 2021, 179, 111373. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. One-dimensional and two-dimensional synergized nanostructures for high-performing energy storage and conversion. InfoMat 2019, 2, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Zeng, M.; Chen, M.; Huang, D.; Lei, S.; Zhang, X.; Wang, L.; Cheng, Z. Engineered two-dimensional nanomaterials: An emerging paradigm for water purification and monitoring. Mater. Horiz. 2021, 8, 758–802. [Google Scholar] [CrossRef]
- Song, G.; Gong, W.; Cong, S.; Zhao, Z.-G. Ultrathin Two-Dimensional Nanostructures: Surface Defects for Morphology-Driven Enhanced Semiconductor SERS. Angew. Chem. Int. Ed. 2020, 60, 5505–5511. [Google Scholar] [CrossRef]
- Wahab, R.; Khan, F.; Yang, Y.B.; Hwang, I.H.; Shin, H.-S.; Ahmad, J.; Dwivedi, S.; Khan, S.T.; Siddiqui, M.A.; Saquib, Q.; et al. Zinc oxide quantum dots: Multifunctional candidates for arresting C2C12 cancer cells and their role towards caspase 3 and 7 genes. RSC Adv. 2016, 6, 26111–26120. [Google Scholar] [CrossRef]
- Wahab, R.; Dwivedi, S.; Khan, F.; Mishra, Y.K.; Hwang, I.; Shin, H.-S.; Musarrat, J.; Al-Khedhairy, A.A. Statistical analysis of gold nanoparticle-induced oxidative stress and apoptosis in myoblast (C2C12) cells. Colloids Surf. B Biointerfaces 2014, 123, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.U.; Lin, J.; Younas, M.R.; Liu, X.; Shen, L. Synthesis of gold nanorods and their performance in the field of cancer cell imaging and photothermal therapy. Cancer Nanotechnol. 2021, 12, 20. [Google Scholar] [CrossRef]
- Feng, C.-J.; Guan, A.-Q.; Xie, Z. First-principle study of Ag-Au double wall nanotube and nanowire. Phys. Lett. A 2021, 407, 127468. [Google Scholar] [CrossRef]
- Ansari, S.G.; Ansari, Z.A.; Wahab, R.; Kim, Y.S.; Khang, G.; Shin, H.S. Glucose sensor based on nano-baskets of tin oxide templated in porous alumina by plasma enhanced CVD. Biosens. Bioelectron. 2008, 23, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Nanostructured metal oxides and its hybrids for photocatalytic and biomedical applications. Adv. Colloid Interface Sci. 2020, 281, 102178. [Google Scholar] [CrossRef] [PubMed]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Broadhead, E.J.; Tibbetts, K.M. Fabrication of Gold–Silicon Nanostructured Surfaces with Reactive Laser Ablation in Liquid. Langmuir 2020, 36, 10120–10129. [Google Scholar] [CrossRef] [PubMed]
- Danielson, E.; Sontakke, V.A.; Porkovich, A.J.; Wang, Z.; Kumar, P.; Ziadi, Z.; Yokobayashi, Y.; Sowwan, M. Graphene basedfield-effect transistor biosensors functionalized using gas-phase synthesized gold nanoparticles. Sens. Actuators B Chem. 2020, 320, 128432. [Google Scholar] [CrossRef]
- Teker, K.; AlKhaldi, A. Impact of gold nanoparticles on low-voltage operating GaN ultraviolet photodetector. Opt. Eng. 2020, 59, 127110. [Google Scholar] [CrossRef]
- Liu, X.; He, F.; Zhang, F.; Zhang, Z.; Huang, Z.; Liu, J. Dopamine and Melamine Binding to Gold Nanoparticles Dominates Their Aptamer-Based Label-Free Colorimetric Sensing. Anal. Chem. 2020, 92, 9370–9378. [Google Scholar] [CrossRef]
- Ding, J.; Lu, Z.; Wang, R.; Shen, G.; Xiao, L. Piezoelectric immunosensor with gold nanoparticles enhanced competitive immunoreaction technique for 2,4-dichlorophenoxyacetic acid quantification. Sens. Actuators B Chem. 2014, 193, 568–573. [Google Scholar] [CrossRef]
- Zhao, D.-W.; Yu, M.-Y.; Zheng, L.-L.; Li, M.; Dai, S.-J.; Chen, D.-C.; Lee, T.-C.; Yun, D.-Q. Enhanced Efficiency and Stability of Planar Perovskite Solar Cells Using a Dual Electron Transport Layer of Gold Nanoparticles Embedded in Anatase TiO2 Films. ACS Appl. Energy Mater. 2020, 3, 9568–9575. [Google Scholar] [CrossRef]
- Selopal, G.S.; Mohammadnezhad, M.; Besteiro, L.V.; Cavuslar, O.; Liu, J.; Zhang, H.; Pardo, F.N.; Liu, G.; Wang, M.; Durmusoglu, E.G.; et al. Synergistic Effect of Plasmonic Gold Nanoparticles Decorated Carbon Nanotubes in Quantum Dots/TiO2 for Optoelectronic Devices. Adv. Sci. 2020, 7, 2001864. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, D.; Jeong, S.; Do, H.T.; Kim, J.H. Photocatalytic Degradation of Rhodamine B Dye by TiO2 and Gold Nanoparticles Supported on a Floating Porous Polydimethylsiloxane Sponge under Ultraviolet and Visible Light Irradiation. ACS Omega 2020, 5, 4233–4241. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- He, M.Q.; Yu, Y.L.; Wang, J.H. Biomolecule-tailored assembly and morphology of gold nanoparticlesfor LSPR applications. Nano Today 2020, 35, 101005. [Google Scholar] [CrossRef]
- Ma, F.H.; Li, C.; Liu, Y.; Shi, L. Mimicking Molecular Chaperones to Regulate Protein Folding. Adv. Mater. 2020, 32, e1805945. [Google Scholar] [CrossRef]
- He, Z.; Yin, H.; Chang, C.-C.; Wang, G.; Liang, X. Interfacing DNA with Gold Nanoparticles for Heavy Metal Detection. Biosensors 2020, 10, 167. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Huang, H.; Chen, X.; Leng, Y.; Lai, W.; Huang, X.; Xiong, Y. Engineered gold nanoparticles as multicolor labels for simultaneous multi-mycotoxin detection on the immunochromatographic test strip nanosensor. Sens. Actuators B Chem. 2020, 316, 128107. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef]
- Lvarez, R.G.; Chen, L.; Nedilko, A.; Iglesias, A.S.; Rix, A.; Lederle, W.; Pathak, V.; Lammers, T.; Plessen, G.V.; Kostarelos, K.; et al. Optimizing the Geometry of Photoacoustically Active Gold Nanoparticles for Biomedical Imaging. ACS Photonics 2020, 7, 646–652. [Google Scholar]
- Li, H.; Pan, S.; Xia, P.; Chang, Y.; Fu, C.; Kong, W.; Yu, Z.; Wang, K.; Yang, X.; Qi, Z. Advances in the application of gold nanoparticles in bone tissue engineering. J. Biol. Eng. 2020, 14, 14–15. [Google Scholar] [CrossRef]
- Munawer, U.; Raghavendra, V.B.; Ningaraju, S.; Krishna, K.L.; Ghosh, A.R.; Melappa, G.; Pugazhendhi, A. Biofabrication of gold nanoparticles mediated by the endophytic Cladosporium species: Photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int. J. Pharm. 2020, 588, 119729. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Yang, Y.B.; Umar, A.; Singh, S.; Hwang, I.H.; Shin, H.-S.; Kim, Y.-S. Platinum quantum dots and their cytotoxic effect towards myoblast cancer cells (C2C12). J. Biomed. Nanotechnol. 2012, 8, 424–431. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jia, J.; Jiang, C.; Zhai, S. Gold Nanoparticle-Induced Cell Death and Potential Applications in Nanomedicine. Int. J. Mol. Sci. 2018, 19, 754. [Google Scholar] [CrossRef] [Green Version]
- Hoenerhoff, M.J.; Pandiri, A.R.; Lahousse, S.A.; Hong, H.H.; Ton, T.V.; Masinde, T.; Auerbach, S.S.; Gerrish, K.; Bushel, P.R.; Shockley, K.R.; et al. Global gene profiling of spontaneous hepatocellular carcinoma in B6C3F1 mice: Similarities in the molecular landscape with human liver cancer. Toxicol. Pathol. 2011, 39, 678–699. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.X. Systemic treatment of hepatocellular carcinoma: Dawn of a new era? Ann. Surg. Oncol. 2010, 17, 1247–1256. [Google Scholar] [CrossRef]
- Nault, J.C.; Rossi, J.Z. Genetics of hepatobiliary carcinogenesis. Semin. Liver Dis. 2011, 31, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.B.; Zhu, A.X. Metabolic syndrome and hepatocellular carcinoma: Two growing epidemics with a potential link. Cancer 2009, 115, 5651–5661. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, A.; Khan, Z.; Alloghbi, A.; Ahmed, T.S.S.; Ashraf, M.; Hammouda, D.M. Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina 2019, 55, 526. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Das, A.; Raha, S.; Barui, A. Size-dependent apoptotic activity of gold nanoparticles on osteosarcoma cells correlated with SERS signal. J. Photochem. Photobiol. B Biol. 2020, 203, 111778. [Google Scholar] [CrossRef] [PubMed]
- Yohan, D.; Cruje, C.; Lu, X.; Chithrani, D.B. Size-Dependent Gold Nanoparticle Interaction at Nano–Micro Interface Using Both Monolayer and Multilayer (Tissue-Like) Cell Models. Nano-Micro Lett. 2015, 8, 44–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomić, S.; Đokić, J.; Vasilijić, S.; Ogrinc, N.; Rudolf, R.; Pelicon, P.; Vučević, D.; Milosavljević, P.; Janković, S.; Anžel, I.; et al. Size-Dependent Effects of Gold Nanoparticles Uptake on Maturation and Antitumor Functions of Human Dendritic Cells In Vitro. PLoS ONE 2014, 9, e96584. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Huang, J.; Feng, Q.; Chen, X.; Liu, X.; Li, X.; Zhang, T.; Xiao, S.; Li, H.; Zhong, Z.; et al. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int. J. Nanomed. 2019, 14, 6957–6970. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumara, S.; Ganesan, S. Size-dependent in vitro cytotoxicity assay of gold nanoparticles. Toxicol. Environ. Chem. 2013, 95, 277–287. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Rizk, M.G.H.; Kezimana, P.; Kirichuk, A.A.; Stanishevskiy, Y.M. Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics. ChemEngineering 2021, 5, 69. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-Dependent Localization and Penetration of Ultrasmall Gold Nanoparticles in Cancer Cells, Multicellular Spheroids, and Tumors In Vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef] [Green Version]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Alhussan, A.; Bozdoğan, E.; Chithrani, D. Combining Gold Nanoparticles with Other Radiosensitizing Agents for Unlocking the Full Potential of Cancer Radiotherapy. Pharmaceutics 2021, 13, 442. [Google Scholar] [CrossRef]
- Chauhan, A.; Khan, T.; Omri, A. Design and Encapsulation of Immunomodulators onto Gold Nanoparticles in Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 8037. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth proce -sses in the synthesis of colloidal gold, Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Singh, G.; Kashyap, M.P.; Khanna, V.K.; Yadav, S.; Chandra, D.; Pant, A.B. Influence of cytotoxic doses of 4-hydroxynonenal on selected neurotransmitter receptors in PC-12 cells. Toxicol. In Vitro 2008, 22, 1681–1688. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Kashyap, M.P.; Kumar, V.; Al-Khedhairy, A.A.; Musarrat, J.; Pant, A.B. Protective potential of trans-resveratrol against 4-hydroxynonenal induced damage in PC12 cells. Toxicol. In Vitro 2010, 24, 1592–1598. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Liang, W.S.; Geng, C.Y. Coalescence Behavior of Gold Nano particles. Nanoscale Res. Lett. 2009, 4, 684–688. [Google Scholar] [CrossRef] [Green Version]
- Arunachalam, K.D.; Annamalai, S.K.; Hari, S. One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int. J. Nanomed. 2013, 8, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Balmes, O.; Malm, J.-O.; Pettersson, N.; Karlsson, G.; Bovin, J.-O. Imaging Atomic Structure in Metal Nanoparticles Using High-Resolution Cryo-TEM. Microsc. Microanal. 2005, 12, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Zeta Potential—An Introduction in 30 Minutes. 2005. Available online: https://www.malvernpanalytical.com/ (accessed on 4 December 2021).
- Cui, W.; Li, J.; Zhang, Y.; Rong, H.; Lu, W. Long Jiang, Nanomedicine: Nanotechnology. Biol. Med. 2012, 8, 46–53. [Google Scholar]
- Wahab, R.; Kaushik, N.; Khan, F.; Kaushik, N.K.; Lee, S.-J.; Choi, E.H.; A Al-Khedhairy, A. Gold quantum dots impair the tumorigenic potential of glioma stem-like cells via β-catenin downregulation in vitro. Int. J. Nanomed. 2019, 14, 1131–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrom, A.M.; Faase, R.A.; Marquart, G.W.; Baio, J.E.; Mackiewicz, M.R.; Harper, S.L. Size-Dependent Interactions of Lipid-Coated Gold Nanoparticles: Developing a Better Mechanistic Understanding through Model Cell Membranes and In Vivo Toxicity. Int. J. Nanomed. 2020, 15, 4091–4104. [Google Scholar] [CrossRef]
- Misawa, M.; Takahashi, J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV Irradiations. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 604–614. [Google Scholar] [CrossRef]
- Contini, C.; Hindley, J.W.; Macdonald, T.J.; Barritt, J.D.; Ces, O.; Quirke, N. Size depend ency of gold nanoparticles interacting with model membranes. Commun. Chem. 2020, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.A.M.; Tovar, G.N.; Castanon, G.M.; Gonzalez, C. Effect of gold nanoparticles (AuNPs) on isolated rat tracheal segments. Toxicol. Rep. 2021, 8, 1412–1418. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Dusetzina, S.B. Weighing Costs and Benefits in the Economics of Cancer Care. J. Clin. Oncol. 2020, 38, 289–291. [Google Scholar] [CrossRef]
- Meropol, N.J.; Schulman, K.A. Cost of Cancer Care: Issues and Implications. J. Clin. Oncol. 2007, 25, 180–186. [Google Scholar] [CrossRef]
- Taylor, D.G. The political economics of cancer drug discovery and pricing. Drug Discov. Today 2020, 25, 2149–2160. [Google Scholar] [CrossRef] [PubMed]



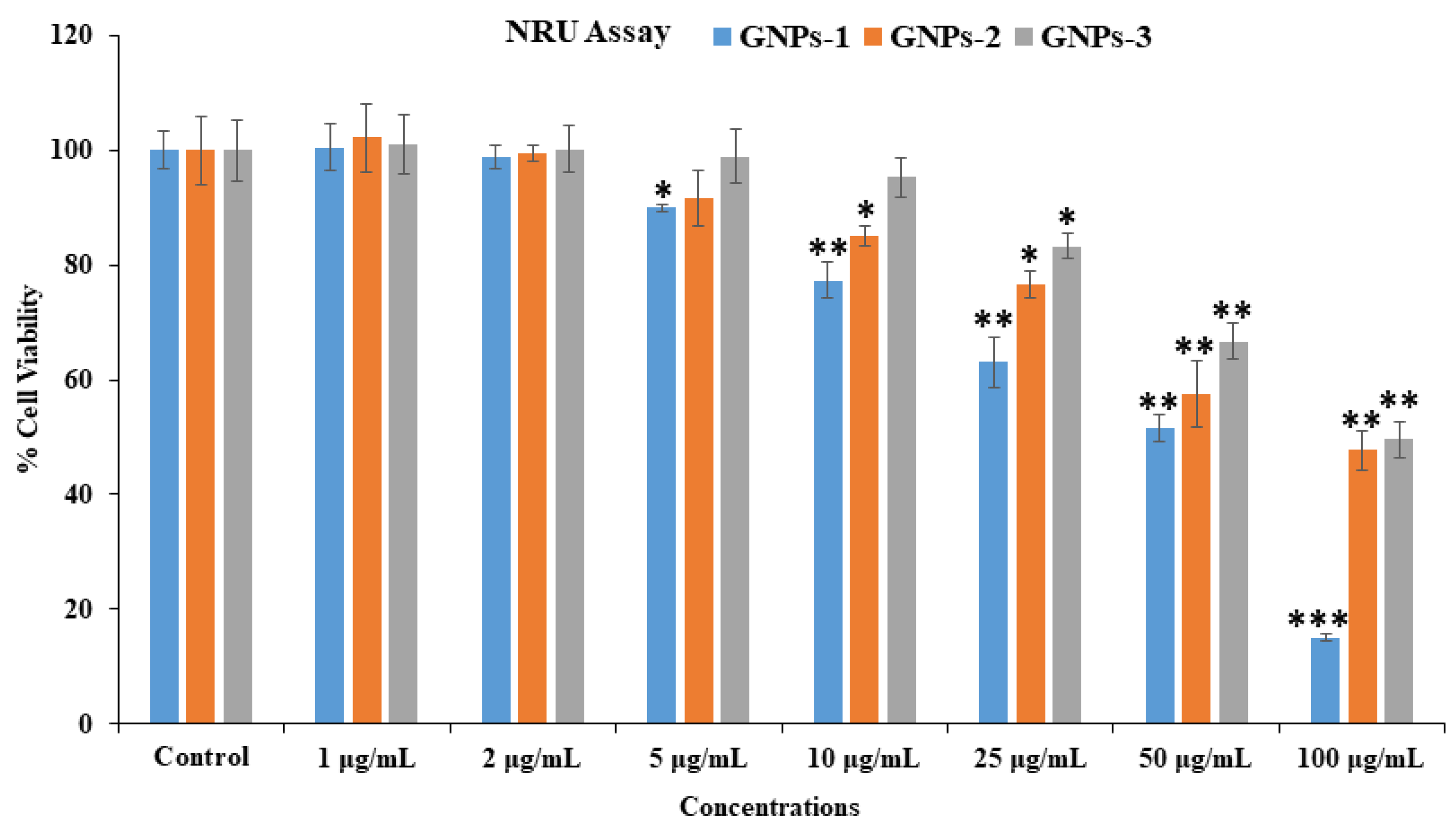
| Concentrations | GNPs-1 (% Cell Viability) | GNPs-2 (% Cell Viability) | GNPs-3 (% Cell Viability) |
|---|---|---|---|
| Control | 100.00 ± 2.23 | 100.00 ± 2.93 | 100.00 ± 6.13 |
| 1 μg/mL | 101.91 ± 1.82 | 102.00 ± 2.89 | 100.86 ± 6.44 |
| 2 μg/mL | 96.39 ± 5.02 | 98.70 ± 2.49 | 99.02 ± 5.49 |
| 5 μg/mL | 86.86 ± 6.96 | 90.56 ± 3.64 | 94.77 ± 1.60 |
| 10 μg/mL | 69.08 ± 1.00 | 83.50 ± 3.92 | 87.35 ± 2.88 |
| 25 μg/mL | 61.87 ± 2.62 | 77.35 ± 1.86 | 79.58 ± 4.65 |
| 50 μg/mL | 48.23 ± 2.06 | 53.79 ± 2.23 | 67.89 ± 2.21 |
| 100 μg/mL | 17.05 ± 0.37 | 46.49 ± 1.52 | 55.94 ± 2.17 |
| Concentrations | GNPs-1 (% Cell Viability) | GNPs-2 (% Cell Viability) | GNPs-3 (% Cell Viability) |
|---|---|---|---|
| Control | 100.00 ± 3.24 | 100.00 ± 5.97 | 100.00 ± 5.31 |
| 1 μg/mL | 100.48 ± 3.95 | 102.16 ± 5.97 | 100.97 ± 5.12 |
| 2 μg/mL | 98.84 ± 1.93 | 99.35 ± 1.46 | 100.19 ± 4.07 |
| 5 μg/mL | 89.95 ± 0.69 | 91.57 ± 4.77 | 98.95 ± 4.80 |
| 10 μg/mL | 77.34 ± 3.20 | 85.02 ± 1.80 | 95.31 ± 3.45 |
| 25 μg/mL | 63.03 ± 4.46 | 76.49 ± 2.32 | 83.26 ± 2.19 |
| 50 μg/mL | 51.46 ± 2.35 | 57.54 ± 5.74 | 66.60 ± 3.14 |
| 100 μg/mL | 15.10 ± 0.52 | 47.66 ± 3.46 | 49.54 ± 3.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khedhairy, A.A.; Wahab, R. Size-Dependent Cytotoxic and Molecular Study of the Use of Gold Nanoparticles against Liver Cancer Cells. Appl. Sci. 2022, 12, 901. https://doi.org/10.3390/app12020901
Al-Khedhairy AA, Wahab R. Size-Dependent Cytotoxic and Molecular Study of the Use of Gold Nanoparticles against Liver Cancer Cells. Applied Sciences. 2022; 12(2):901. https://doi.org/10.3390/app12020901
Chicago/Turabian StyleAl-Khedhairy, Abdulaziz A., and Rizwan Wahab. 2022. "Size-Dependent Cytotoxic and Molecular Study of the Use of Gold Nanoparticles against Liver Cancer Cells" Applied Sciences 12, no. 2: 901. https://doi.org/10.3390/app12020901
APA StyleAl-Khedhairy, A. A., & Wahab, R. (2022). Size-Dependent Cytotoxic and Molecular Study of the Use of Gold Nanoparticles against Liver Cancer Cells. Applied Sciences, 12(2), 901. https://doi.org/10.3390/app12020901







