Functionalized Mesoporous Silica Nanoparticles as Delivery Systems for Doxorubicin: Drug Loading and Release
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods and Materials
2.2. Synthesis of Native MSN, MSN-PLH and MSN-PLH-TAM
2.3. Loading of DOX onto MSN-Based Systems
2.4. In-Vitro Release Kinetics
3. Results and Discussion
3.1. Drug Loading
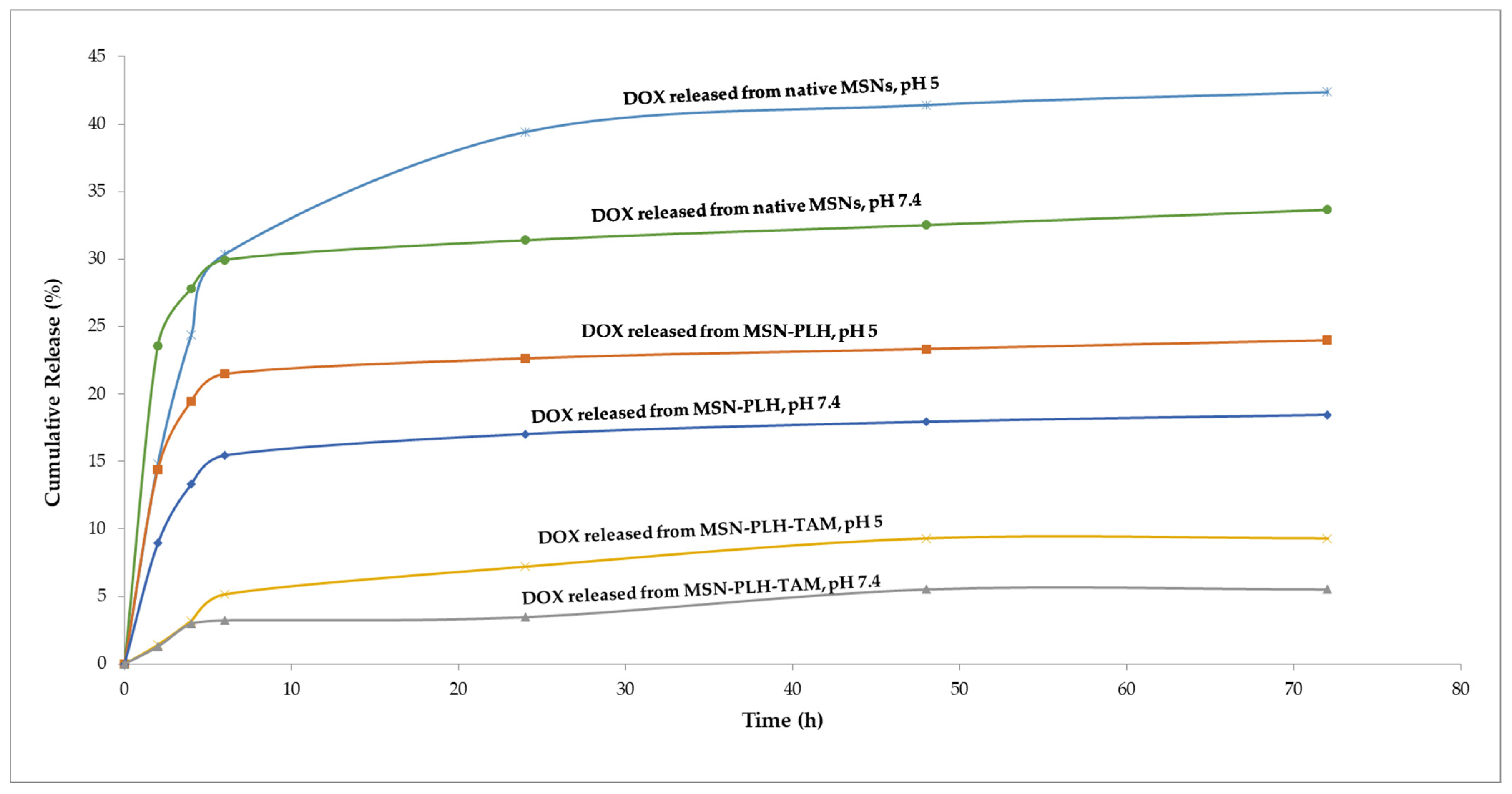
3.2. Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bleeker, E.A.; de Jong, W.H.; Geertsma, R.E.; Groenewold, M.; Heugens, E.H.; Koers-Jacquemijns, M.; van de Meent, D.; Popma, J.R.; Rietveld, A.G.; Wijnhoven, S.W.; et al. Considerations on the EU definition of a nanomaterial: Science to support policy making. Regul. Toxicol. Pharmacol. 2013, 65, 119–125. [Google Scholar] [CrossRef]
- Day, C.M.; Hickey, S.M.; Song, Y.; Plush, S.E.; Garg, S. Novel Tamoxifen Nanoformulations for Improving Breast Cancer Treatment: Old Wine in New Bottles. Molecules 2020, 25, 1182. [Google Scholar] [CrossRef]
- Day, C.M.; Barclay, T.G.; Song, Y.; Garg, S. Swelling-controlled Drug Delivery Systems. In Biomaterials Science Series; The Royal Society of Chemistry: London, UK, 2018; Volume 2018, pp. 232–264. [Google Scholar]
- Jena, S.K.; Sangamwar, A.T. Polymeric micelles: A promising tool for tamoxifen delivery in cancer? Ther. Deliv. 2017, 8, 109–111. [Google Scholar] [CrossRef][Green Version]
- Xuan, Q.J.; Wang, J.X.; Nanding, A.; Wang, Z.P.; Liu, H.; Lian, X.; Zhang, Q.-Y. Tumor-Associated Macrophages are Correlated with Tamoxifen Resistance in the Postmenopausal Breast Cancer Patients. Pathol. Oncol. Res. 2014, 20, 619–624. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Day, C.M.; Sweetman, M.J.; Garg, S. Mesoporous silica nanoparticles for drug combination delivery in cancer therapy: Background, current insights, and future perspectives. In Nanocarriers for the Delivery of Combination Drugs; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2021, pp. 119–162. [Google Scholar]
- Keshavarz, M.; Ahmad, N. Characterization and Modification of Mesoporous Silica Nanoparticles Prepared by Sol-Gel. J. Nanoparticles 2013, 2013, 102823. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Cell Selective Apoptosis Induced by Polymorphic Alteration of Self-Assembled Silica Nanowebs. ACS Appl. Mater. Interfaces 2017, 9, 6292–6305. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Interaction of Mesoporous Silica Nanoparticles with Human Red Blood Cell Membranes: Size and Surface Effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef]
- Slowing, I.I.; Wu, C.W.; Vivero-Escoto, J.L.; Lin, V.S. Mesoporous silica nanoparticles for reducing hemolytic activity towards mammalian red blood cells. Small 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Day, C.M.; Sweetman, M.J.; Hickey, S.M.; Song, Y.; Liu, Y.; Zhang, N.; Plush, S.E.; Garg, S. Concept Design, Development and Preliminary Physical and Chemical Characterization of Tamoxifen-Guided-Mesoporous Silica Nanoparticles. Molecules 2021, 26, 219. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Madani, J.; Puértolas, T.; Alvarez, M.; Hernández, A.; Pazo-Cid, R.; Artal, A.; Antón Torres, A. Liposomal Doxorubicin in the treatment of breast cancer patients: A review. J. Drug. Deliv. 2013, 2013, 456409. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Zhao, M.; Ding, X.F.; Shen, J.Y.; Zhang, X.P.; Ding, X.W.; Xu, B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. J. Zhejiang Univ. Sci. B 2017, 18, 15–26. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.K.; Mishra, D.K.; Rosling, A.; Rosenholm, J.M. Therapeutic Potential of Polymer-Coated Mesoporous Silica Nanoparticles. Appl. Sci. 2020, 10, 289. [Google Scholar] [CrossRef]
- Amolegbe, S.A.; Hirano, Y.; Adebayo, J.O.; Ademowo, O.G.; Balogun, E.A.; Obaleye, J.A.; Krettli, A.U.; Yu, C.; Hayami, S. Mesoporous silica nanocarriers encapsulated antimalarials with high therapeutic performance. Sci. Rep. 2018, 8, 3078. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Ma, Z.; Wang, D.; Wang, L.; Zhan, J.; She, L.; Yang, F. Hydrophilic mesoporous carbon nanospheres with high drug-loading efficiency for doxorubicin delivery and cancer therapy. Int. J. Nanomed. 2016, 11, 1793–1806. [Google Scholar]
- Ji, F.; Sun, H.; Qin, Z.; Zhang, E.; Cui, J.; Wang, J.; Li, S.; Yao, F. Engineering Polyzwitterion and Polydopamine Decorated Doxorubicin-Loaded Mesoporous Silica Nanoparticles as a pH-Sensitive Drug Delivery. Polymers 2018, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, K.; Liu, J.; Sun, G.; Yin, Q.; Lu, L. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 2015, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Gadag, S.; Cheruku, S.P.; Raichur, A.M.; Day, C.M.; Garg, S.; Manandhar, S.; Sreedhar, K.; Pai, R.; Suresh, A.; et al. Chitosan-glucuronic acid conjugate coated mesoporous silica nanoparticles: A smart pH-responsive and receptor-targeted system for colorectal cancer therapy. Carbohydr. Polym. 2021, 261, 117893. [Google Scholar] [CrossRef]
- Varache, M.; Bezverkhyy, I.; Weber, G.; Saviot, L.; Chassagnon, R.; Baras, F.; Bouyer, F. Loading of Cisplatin into Mesoporous Silica Nanoparticles: Effect of Surface Functionalization. Langmuir 2019, 35, 8984–8995. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, D.; Yin, X.; Jin, X.; Zhang, X.; Zheng, S.; Wang, C.; Liu, Y. Intracellular pH-responsive and rituxi-mab-conjugated mesoporous silica nanoparticles for targeted drug delivery to lymphoma B cells. J. Exp. Clin. Cancer. Res. 2017, 36, 24. [Google Scholar] [CrossRef] [PubMed]
- Subedi, R.K.; Kang, K.W.; Choi, H.K. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur. J. Pharm. Sci. 2009, 37, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.J.H.; Crommelin, D.J.A.; Storm, G.; Hulshoff, A. Doxorubicin decomposition on storage. Effect of pH, type of buffer and liposome encapsulation. Int. J. Pharm. 1985, 23, 1–11. [Google Scholar] [CrossRef]
- Hakeem, A.; Zahid, F.; Zhan, G.; Yi, P.; Yang, H.; Gan, L.; Yang, X. Polyaspartic acid-anchored mesoporous silica nanoparticles for pH-responsive doxorubicin release. Int. J. Nanomed. 2018, 13, 1029–1040. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.; Parikh, A.; Joyce, P.; Chung, R.; Liu, L.; Afinjuomo, F.; Hayball, J.D.; Petrovsky, N.; Barclay, T.G.; et al. Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation. Pharmaceutics 2019, 11, 581. [Google Scholar] [CrossRef]
- Sun, J.G.; Jiang, Q.; Zhang, X.P.; Shan, K.; Liu, B.; Zhao, C.; Yan, B. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int. J. Nanomed. 2019, 14, 1489–1501. [Google Scholar] [CrossRef]
- Shen, J.; He, Q.; Gao, Y.; Shi, J.; Li, Y. Mesoporous silica nanoparticles loading doxorubicin reverse multidrug resistance: Performance and mechanism. Nanoscale 2011, 3, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, P.; Tziveleka, L.A.; Varlasa, S.; Iatrou, H. pH-Sensitive nanogates based on poly(l-histidine) for controlled drug release from mesoporous silica nanoparticles. Polym. Chem. 2016, 7, 1475–1485. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Zhu, D.; Zong, S.; Yang, L.; Zhong, Y.; Cui, Y. pH and Thermo Dual-Stimuli-Responsive Drug Carrier Based on Mesoporous Silica Nanoparticles Encapsulated in a Copolymer–Lipid Bilayer. ACS Appl. Mater. Interfaces 2013, 5, 10895–10903. [Google Scholar] [CrossRef] [PubMed]
- Duo, Y.; Li, Y.; Chen, C.; Liu, B.; Wang, X.; Zeng, X.; Chen, H. DOX-loaded pH-sensitive mesoporous silica nanoparticles coated with PDA and PEG induce pro-death autophagy in breast cancer. RSC Adv. 2017, 7, 39641–39650. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Day, C.M.; Sweetman, M.J.; Song, Y.; Plush, S.E.; Garg, S. Functionalized Mesoporous Silica Nanoparticles as Delivery Systems for Doxorubicin: Drug Loading and Release. Appl. Sci. 2021, 11, 6121. https://doi.org/10.3390/app11136121
Day CM, Sweetman MJ, Song Y, Plush SE, Garg S. Functionalized Mesoporous Silica Nanoparticles as Delivery Systems for Doxorubicin: Drug Loading and Release. Applied Sciences. 2021; 11(13):6121. https://doi.org/10.3390/app11136121
Chicago/Turabian StyleDay, Candace M., Martin J. Sweetman, Yunmei Song, Sally E. Plush, and Sanjay Garg. 2021. "Functionalized Mesoporous Silica Nanoparticles as Delivery Systems for Doxorubicin: Drug Loading and Release" Applied Sciences 11, no. 13: 6121. https://doi.org/10.3390/app11136121
APA StyleDay, C. M., Sweetman, M. J., Song, Y., Plush, S. E., & Garg, S. (2021). Functionalized Mesoporous Silica Nanoparticles as Delivery Systems for Doxorubicin: Drug Loading and Release. Applied Sciences, 11(13), 6121. https://doi.org/10.3390/app11136121







