The Impact of Segmentation Method and Target Lesion Selection on Radiomic Analysis of 18F-FDG PET Images in Diffuse Large B-Cell Lymphoma
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Identification of the Optimal Lesion Segmentation Method
2.3. Radiomic Features Extraction
2.4. Radiomic and Clinical–Radiomic Predictive Models
2.5. Main Analysis and Sensitivity Analysis
3. Results
3.1. Identification of the Optimal Lesion Segmentation Method
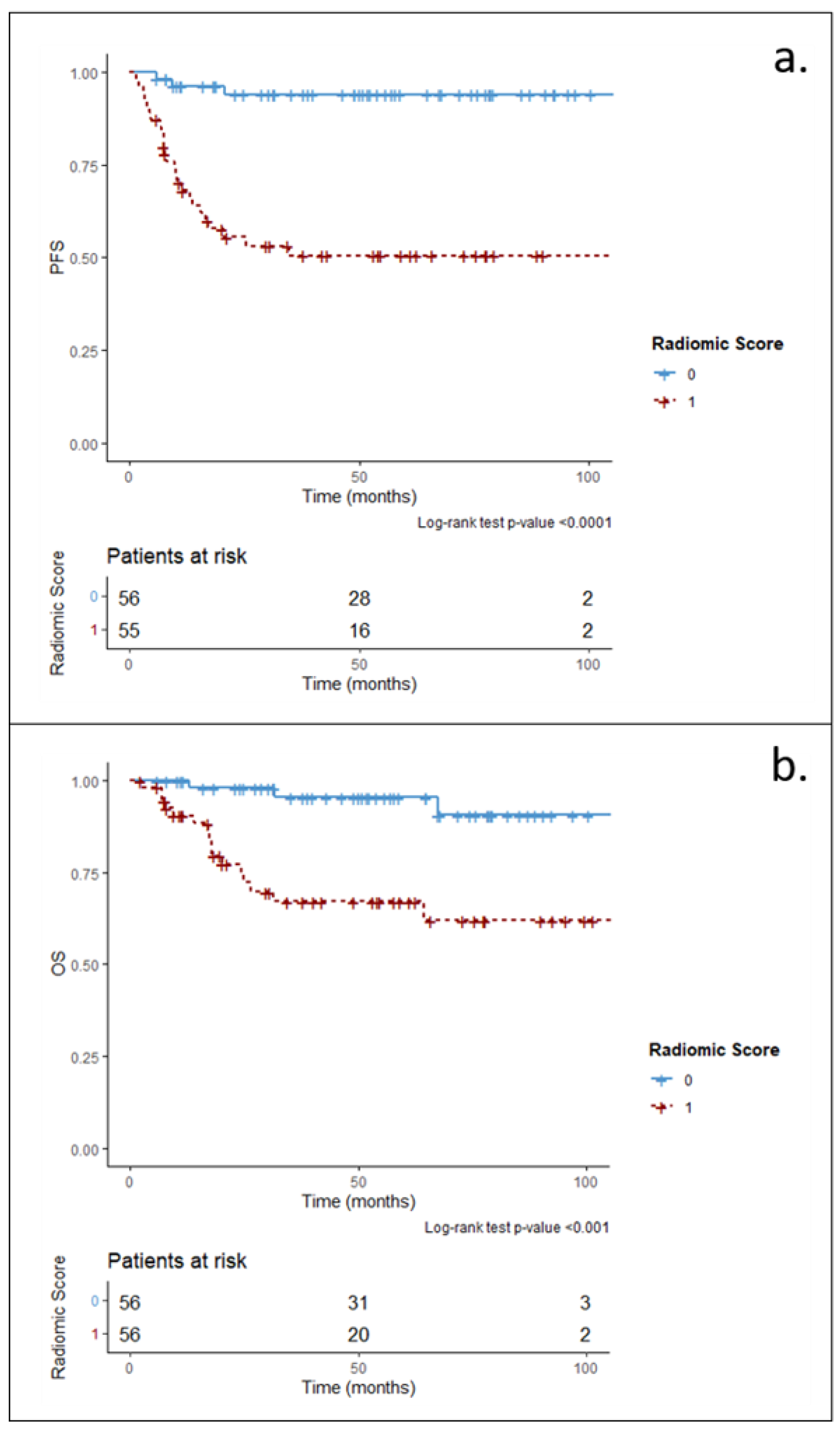
3.2. Main Analysis: Radiomic and Clinical–Radiomic Predictive Models
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sant, M.; Allemani, C.; Tereanu, C.; De Angelis, R.; Capocaccia, R.; Visser, O.; Marcos-Gragera, R.; Maynadié, M.; Simonetti, A.; Lutz, J.-M.; et al. Incidence of hematologic malignancies in Europe by morphologic subtype: Results of the HAEMACARE project. Blood 2010, 116, 3724–3734. [Google Scholar] [CrossRef]
- International Non-Hodgkin's Lymphoma Prognostic Factors Project A Predictive Model for Aggressive Non-Hodgkin's Lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [CrossRef] [PubMed]
- Harrysson, S.; Eloranta, S.; Ekberg, S.; Enblad, G.; Jerkeman, M.; Wahlin, B.E.; Andersson, P.-O.; Smedby, K.E. Incidence of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) including CNS relapse in a population-based cohort of 4243 patients in Sweden. Blood Cancer J. 2021, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Harrington, F.; Greenslade, M.; Talaulikar, D.; Corboy, G. Genomic characterisation of diffuse large B-cell lymphoma. Pathology 2021, 53, 367–376. [Google Scholar] [CrossRef]
- Khan, S.; Naim, S.; Bilwani, R.; Salem, A.; Gorlin, D.; Muhammad, A.; Gul, M.; Imam, M.H.; Chaudhry, A. Radiogenomics and Its Role in Lymphoma. Curr. Hematol. Malign. Rep. 2020, 15, 211–224. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Kim, J.; Hong, J.; Kim, S.G.; Hwang, K.H.; Kim, M.; Ahn, H.K.; Sym, S.J.; Park, J.; Cho, E.K.; Shin, D.B.; et al. Prognostic Value of Metabolic Tumor Volume Estimated by 18 F-FDG Positron Emission Tomography/Computed Tomography in Patients with Diffuse Large B-Cell Lymphoma of Stage II or III Disease. Nucl. Med. Mol. Imaging 2014, 48, 187–195. [Google Scholar] [CrossRef]
- Xie, M.; Zhai, W.; Cheng, S.; Zhang, H.; Xie, Y.; He, W. Predictive value of F-18 FDG PET/CT quantization parameters for progression-free survival in patients with diffuse large B-cell lymphoma. Hematology 2015, 21, 99–105. [Google Scholar] [CrossRef]
- Cottereau, A.-S.; Lanic, H.; Mareschal, S.; Meignan, M.; Vera, P.; Tilly, H.; Jardin, F.; Becker, S. Molecular Profile and FDG-PET/CT Total Metabolic Tumor Volume Improve Risk Classification at Diagnosis for Patients with Diffuse Large B-Cell Lymphoma. Clin. Cancer Res. 2016, 22, 3801–3809. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Ben Bouallègue, F.; Al Tabaa, Y.; Kafrouni, M.; Cartron, G.; Vauchot, F.; Mariano-Goulart, D. Association between textural and morphological tumor indices on baseline PET-CT and early metabolic response on interim PET-CT in bulky malignant lymphomas. Med. Phys. 2017, 44, 4608–4619. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.; Hosono, M.; Tatsumi, Y.; Ishii, K.; Im, S.-W.; Tsuchiya, N.; Sakaguchi, K.; Matsumura, I. Heterogeneity of intratumoral 111In-ibritumomab tiuxetan and 18F-FDG distribution in association with therapeutic response in radioimmunotherapy for B-cell non-Hodgkin’s lymphoma. EJNMMI Res. 2015, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Parvez, A.; Tau, N.; Hussey, D.; Maganti, M.; Metser, U. 18F-FDG PET/CT metabolic tumor parameters and radiomics features in aggressive non-Hodgkin’s lymphoma as predictors of treatment outcome and survival. Ann. Nucl. Med. 2018, 32, 410–416. [Google Scholar] [CrossRef]
- Jiang, H.; Li, A.; Ji, Z.; Tian, M.; Zhang, H. Role of Radiomics-Based Baseline PET/CT Imaging in Lymphoma: Diagnosis, Prognosis, and Response Assessment. Mol. Imaging Biol. 2022, 24, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Primakov, S.; Beuque, M.; Woodruff, H.; Halilaj, I.; Wu, G.; Refaee, T.; Granzier, R.; Widaatalla, Y.; Hustinx, R.; et al. Radiomics for precision medicine: Current challenges, future prospects, and the proposal of a new framework. Methods 2020, 188, 20–29. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Barrington, S.F.; Meignan, M.A. Time to Prepare for Risk Adaptation in Lymphoma by Standardizing Measurement of Metabolic Tumor Burden. J. Nucl. Med. 2019, 60, 1096–1102. [Google Scholar] [CrossRef]
- Zwanenburg, A. Radiomics in nuclear medicine: Robustness, reproducibility, standardization, and how to avoid data analysis traps and replication crisis. Eur. J. Pediatr. 2019, 46, 2638–2655. [Google Scholar] [CrossRef]
- Orlhac, F.; Boughdad, S.; Philippe, C.; Stalla-Bourdillon, H.; Nioche, C.; Champion, L.; Soussan, M.; Frouin, F.; Frouin, V.; Buvat, I. A Postreconstruction Harmonization Method for Multicenter Radiomic Studies in PET. J. Nucl. Med. 2018, 59, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, L.; Milan, L.; Johnson, P.W.M.; Martelli, M.; Presilla, S.; Giovanella, L.; Zucca, E. Baseline PET features to predict prognosis in primary mediastinal B cell lymphoma: A comparative analysis of different methods for measuring baseline metabolic tumour volume. Eur. J. Pediatr. 2019, 46, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Zwezerijnen, B.G.; de Vet, H.C.; Heymans, M.W.; Mikhaeel, N.G.; Burggraaff, C.N.; Eertink, J.J.; Pike, L.C.; Hoekstra, O.S.; Zijlstra, J.M.; et al. Automated Segmentation of Baseline Metabolic Total Tumor Burden in Diffuse Large B-Cell Lymphoma: Which Method Is Most Successful? A Study on Behalf of the PETRA Consortium. J. Nucl. Med. 2020, 62, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Burggraaff, C.N.; On behalf of the PETRA Consortium; Rahman, F.; Kaßner, I.; Pieplenbosch, S.; Barrington, S.F.; Jauw, Y.W.; Zwezerijnen, G.J.; Müller, S.; Hoekstra, O.S.; et al. Optimizing Workflows for Fast and Reliable Metabolic Tumor Volume Measurements in Diffuse Large B Cell Lymphoma. Mol. Imaging Biol. 2020, 22, 1102–1110. [Google Scholar] [CrossRef]
- Eude, F.; Toledano, M.; Vera, P.; Tilly, H.; Mihailescu, S.-D.; Becker, S. Reproducibility of Baseline Tumour Metabolic Volume Measurements in Diffuse Large B-Cell LymphomA: Is There a Superior Method? Metabolites 2021, 11, 72. [Google Scholar] [CrossRef]
- Chihara, D.; Oki, Y.; Onoda, H.; Taji, H.; Yamamoto, K.; Tamaki, T.; Morishima, Y. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int. J. Hematol. 2011, 93, 502–508. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, D.; Kim, H.S.; Park, S.Y.; Kim, J.Y.; Cho, S.J.; Shin, J.H.; Kim, J.H. Quality of science and reporting of radiomics in oncologic studies: Room for improvement according to radiomics quality score and TRIPOD statement. Eur. Radiol. 2019, 30, 523–536. [Google Scholar] [CrossRef]
- Aide, N.; Fruchart, C.; Nganoa, C.; Gac, A.-C.; Lasnon, C. Baseline 18F-FDG PET radiomic features as predictors of 2-year event-free survival in diffuse large B cell lymphomas treated with immunochemotherapy. Eur. Radiol. 2020, 30, 4623–4632. [Google Scholar] [CrossRef]
- Aide, N.; Talbot, M.; Fruchart, C.; Damaj, G.; Lasnon, C. Diagnostic and prognostic value of baseline FDG PET/CT skeletal textural features in diffuse large B cell lymphoma. Eur. J. Pediatr. 2017, 45, 699–711. [Google Scholar] [CrossRef]
- Lue, K.-H.; Wu, Y.-F.; Lin, H.-H.; Hsieh, T.-C.; Liu, S.-H.; Chan, S.-C.; Chen, Y.-H. Prognostic Value of Baseline Radiomic Features of 18F-FDG PET in Patients with Diffuse Large B-Cell Lymphoma. Diagnostics 2020, 11, 36. [Google Scholar] [CrossRef]

| Shape | First-Order (HISTO) | Second-Order | |
|---|---|---|---|
| Sphericity | SUVmin | GLCM | Homogeneity (=Inverse Difference) |
| Compacity | SUVmean | Energy (=Angular Second Moment) | |
| SUVstd | Contrast (=Variance) | ||
| SUVmax (*) | Correlation | ||
| SUVQ1 | Entropy_log2 (=Joint Entropy) | ||
| SUVQ2 | Dissimilarity | ||
| SUVQ3 | GLRM | Short-Run Emphasis (SRE) | |
| SUVpeak (sphere 0.5 mL) | Long-Run Emphasis (LRE) | ||
| SUVpeak (sphere 1 mL) | Low Gray Level Run Emphasis (LGRE) | ||
| TLG | High Gray Level Run Emphasis (HGRE) | ||
| Skewness | Short-Run Low Gray-level Emphasis (SRLGE) | ||
| Kurtosis | Short-Run High Gray-level Emphasis (SRHGE) | ||
| ExcessKurtosis | Long-Run Low Gray-level Emphasis (LRLGE) | ||
| Entropy_log2 | Long-Run High Gray-level Emphasis (LRHGE) | ||
| Uniformity (=Energy) | Gray-Level Non-Uniformity (GLNU) | ||
| Run Length Non-Uniformity (RLNU) | |||
| Run Percentage (RP) | |||
| NGLDM | Coarseness | ||
| Contrast | |||
| Busyness | |||
| GLZLM | Short-Zone Emphasis (SZE) | ||
| Long-Zone Emphasis (LZE) | |||
| Low Gray-level Zone Emphasis (LGZE) | |||
| High Gray-level Zone Emphasis (HGZE) | |||
| Short-Zone Low Gray-level Emphasis (SZLGE) | |||
| Short-Zone High Gray-level Emphasis (SZHGE) | |||
| Long-Zone Low Gray-level Emphasis (LZLGE) | |||
| Long-Zone High Gray-level Emphasis (LZHGE) | |||
| Gray-Level Non-Uniformity (GLNU) | |||
| Zone Length Non-Uniformity (ZLNU) | |||
| Zone Percentage (ZP) | |||
| p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Method-1 | Method-2 | Method-3 | Method-4 | Method-5 | Method-6 | |||
| PFS | Univariate | TMTV | 0.01 | 0.01 | 0.06 | 0.04 | 0.05 | 0.08 |
| WTLG | 0.39 | 0.49 | 0.65 | 0.47 | 0.57 | 0.67 | ||
| Bivariate | TMTV IPI score | 0.11 0.13 | 0.13 0.08 | 0.27 0.05 | 0.25 0.08 | 0.24 0.08 | 0.30 0.05 | |
| WTLG IPI score | 0.92 0.03 | 0.98 0.02 | 0.90 0.02 | 0.98 0.02 | 0.94 0.02 | 0.88 0.02 | ||
| OS | Univariate | TMTV | 0.91 | 0.51 | 0.57 | 0.56 | 0.87 | 0.75 |
| WTLG | 0.59 | 0.78 | 0.75 | 0.48 | 0.67 | 0.71 | ||
| Bivariate | TMTV IPI score | 0.70 0.30 | 0.59 0.41 | 0.69 0.39 | 0.35 0.24 | 0.95 0.34 | 0.86 0.35 | |
| WTLG IPI score | 0.41 0.25 | 0.64 0.30 | 0.60 0.30 | 0.30 0.23 | 0.52 0.28 | 0.57 0.29 | ||
| Variable | PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Radiomic | Clinical–Radiomic | Radiomic | Clinical–Radiomic | ||||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| SUVmax | 0.98 (0.94–1.01) | 0.15 | - | - | 1.00 (0.96–1.04) | 0.92 | - | - | |
| TMTV * | 1.07 (1.01–1.13) | 0.02 | 0.99 (0.93–1.05) | 0.66 | 1.09 (1.0–1.16) | 0.02 | 0.98 (0.92–1.05) | 0.54 | |
| Vol(SUVmax lesion) * | 1.04 (0.98–1.10) | 0.17 | - | - | 1.06 (0.99–1.12) | 0.10 | - | - | |
| WTLG ** | 1.02 (0.96–1.07) | 0.55 | - | - | 1.04 (0.99–1.10) | 0.11 | - | - | |
| IPI score | Low | 1.00 (Ref) | - | 1.00 (Ref) | - | 1.00 (Ref) | - | 1.00 (Ref) | - |
| High | 2.60 (1.25–5.48) | 0.01 | 2.68 (1.22–5.86) | 0.01 | 10.20 (2.97–35.01) | 0.0002 | 8.12 (2.25–29.4) | 0.001 | |
| Radiomic Score | 3.56 (2.29–5.54) | <0.0001 | 3.65 (2.26–5.89) | <0.0001 | 3.83 (2.37–6.20) | <0.0001 | 3.08 (1.94–4.88) | <0.0001 | |
| Analysis | Segmentation Method | Representative Lesion for Radiomic Analysis | Model | C-Index (95% CI) | |
|---|---|---|---|---|---|
| PFS | OS | ||||
| Main | Method-2 | SUVmax lesion | Radiomic | 0.81 (0.75–0.88) | 0.84 (0.76–0.91) |
| Clinical–Radiomic | 0.83 (0.76–0.90) | 0.90 (0.82–0.98) | |||
| Sensitivity Case 1 | Method-1 | SUVmax lesion | Radiomic | 0.79 (0.72–0.86) | 0.82 (0.72–0.92) |
| Clinical–Radiomic | 0.80 (0.72–0.87) | 0.87 (0.78–0.96) | |||
| Sensitivity Case 2 | Method-2 | VOLmax lesion | Radiomic | 0.80 (0.72–0.87) | 0.87 (0.78–0.96) |
| Clinical–Radiomic | 0.82 (0.74–0.90) | 0.85 (0.76–0.94) | |||
| Sensitivity Case 3 | Method-1 | VOLmax lesion | Radiomic | 0.79 (0.72–0.89) | 0.84 (0.73–0.94) |
| Clinical–Radiomic | 0.81 (0.73–0.89) | 0.83 (0.73–0.94) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botta, F.; Ferrari, M.; Raimondi, S.; Corso, F.; Lo Presti, G.; Mazzara, S.; Airò Farulla, L.S.; Radice, T.; Vanazzi, A.; Derenzini, E.; et al. The Impact of Segmentation Method and Target Lesion Selection on Radiomic Analysis of 18F-FDG PET Images in Diffuse Large B-Cell Lymphoma. Appl. Sci. 2022, 12, 9678. https://doi.org/10.3390/app12199678
Botta F, Ferrari M, Raimondi S, Corso F, Lo Presti G, Mazzara S, Airò Farulla LS, Radice T, Vanazzi A, Derenzini E, et al. The Impact of Segmentation Method and Target Lesion Selection on Radiomic Analysis of 18F-FDG PET Images in Diffuse Large B-Cell Lymphoma. Applied Sciences. 2022; 12(19):9678. https://doi.org/10.3390/app12199678
Chicago/Turabian StyleBotta, Francesca, Mahila Ferrari, Sara Raimondi, Federica Corso, Giuliana Lo Presti, Saveria Mazzara, Lighea Simona Airò Farulla, Tommaso Radice, Anna Vanazzi, Enrico Derenzini, and et al. 2022. "The Impact of Segmentation Method and Target Lesion Selection on Radiomic Analysis of 18F-FDG PET Images in Diffuse Large B-Cell Lymphoma" Applied Sciences 12, no. 19: 9678. https://doi.org/10.3390/app12199678
APA StyleBotta, F., Ferrari, M., Raimondi, S., Corso, F., Lo Presti, G., Mazzara, S., Airò Farulla, L. S., Radice, T., Vanazzi, A., Derenzini, E., Travaini, L. L., & Ceci, F. (2022). The Impact of Segmentation Method and Target Lesion Selection on Radiomic Analysis of 18F-FDG PET Images in Diffuse Large B-Cell Lymphoma. Applied Sciences, 12(19), 9678. https://doi.org/10.3390/app12199678








