1. Introduction
Radiomics is the practice of converting standard-of-care medical images into minable high-dimensional data (hereinafter referred to as
radiomic features) in order to support clinical decisions [
1]. It is based on the hypothesis that quantitative imaging of the tumor anatomy can yield information on its functional and progression mechanisms by investigating the spatial variations in the tumoral tissues and their surroundings [
2,
3] through texture analysis. As a matter of fact, when a tumor originates, many complex mechanisms occur, at the cellular level, possibly resulting in intra-tumoral heterogeneity, which might be captured by medical images. Tumors with higher heterogeneity are thought to be associated with a worse prognosis [
1].
Most radiomic features were initially developed to characterize two-dimensional rectangular aerial photographs, which shared the same pixel dimensions and therefore did not require normalization to differences in the size of the region of interest [
4]. In medical radiomics, these descriptors are extracted from volumes-of-interest (VOIs) encompassing the tissue under investigation, typically a tumor, which will vary in terms of area and volume. Several studies assessed the dependency of some radiomic features with volume and suggested corrections to remove such dependencies [
4,
5]. Identifying the dependencies of radiomic features with the size of the VOI is of major importance for their use as imaging biomarkers, as it allows the disentanglement of the texture component from the size component and, therefore, improves reliability [
5].
Nonetheless, even after the removal of the dependencies of radiomic features with size, it is possible that below a certain VOI size, not enough information is available for the proper calculation of the radiomic feature, making it inaccurate and hence unable to distinguish two different textures, potentially reflecting the different clinical outcomes radiomics aims to predict. Brooks and Grigsby [
6,
7] analyzed the behavior of texture quantifiers as a function of the tumor volume on
18F-fluorodeoxyglucose positron emission tomography (
18F-FDG PET) images. They concluded that small tumors (volume below 700 voxels) should not be included in studies of intratumoral heterogeneity of tracer uptake since they do not include a high enough number of voxels to adequately sample the underlying tracer distribution and properly calculate some radiomic features.
Similar research has not yet been performed in magnetic resonance imaging (MRI). We hypothesize that, also in MRI, some radiomic features may lose their texture-discriminative power when obtained using volumes below a certain threshold to be identified.
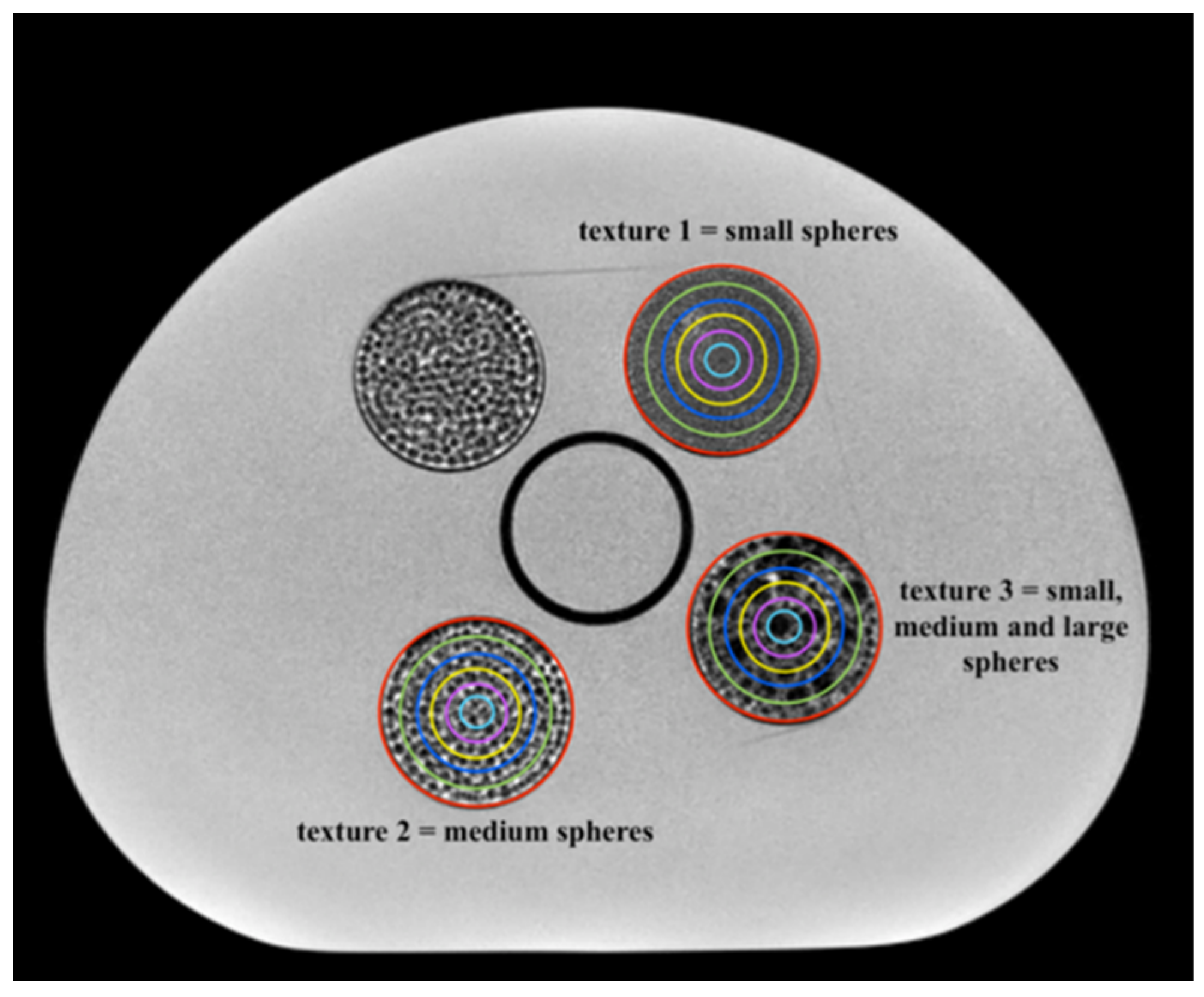
This study aimed to investigate how the ability of MRI-based radiomic features to discriminate different textures varies as a function of the volume of the region considered for the feature extraction. For this purpose, an MRI phantom specifically designed for radiomic applications in the pelvic region, embedding inserts with different textures, was imaged on three scanners with a T2-weighted sequence included in the clinical protocol for the diagnosis of pelvic tumors.
4. Discussion
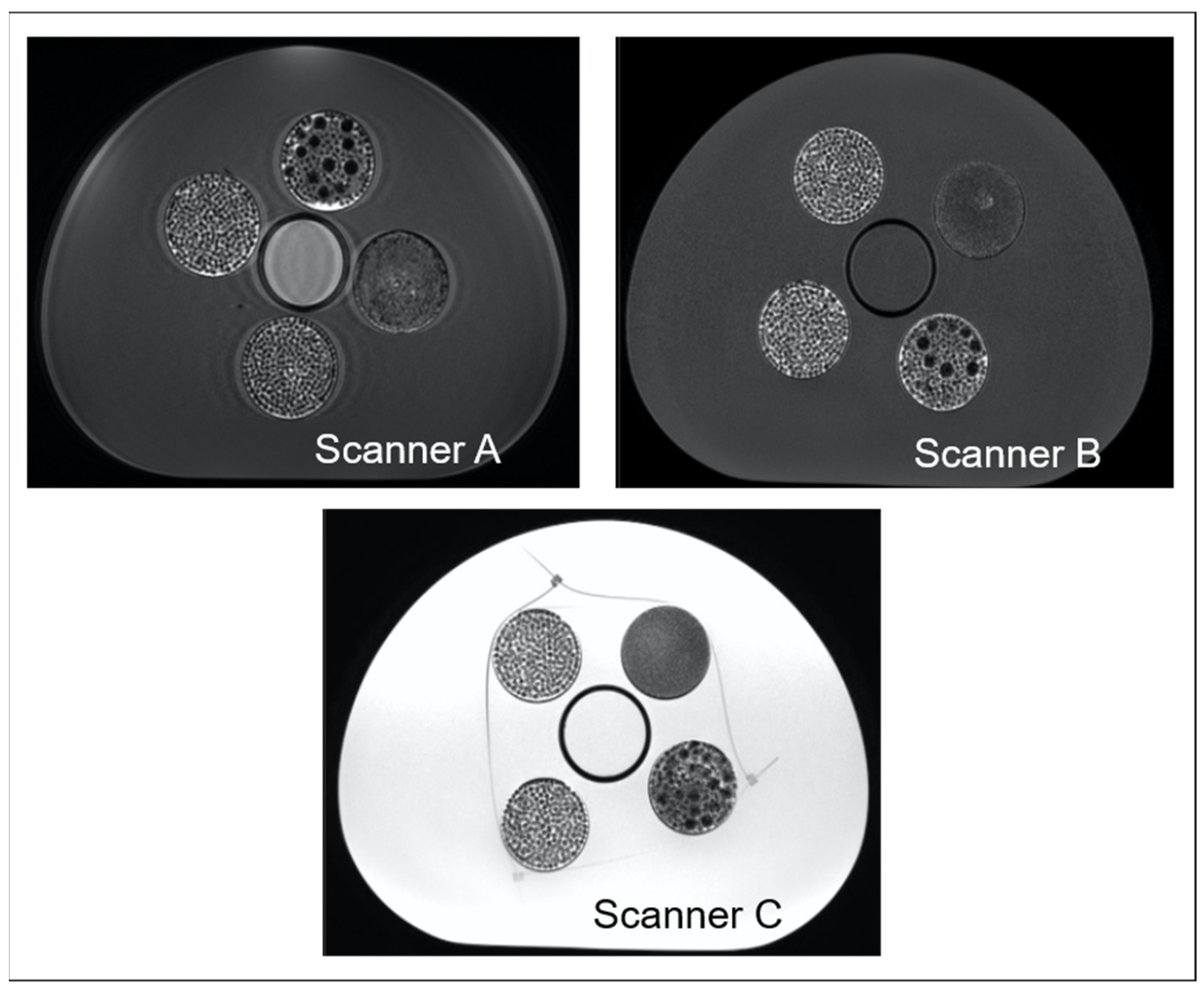
In this study, a phantom specifically designed for MRI radiomic studies in the pelvic region was used to investigate the dependency on VOI size of the ability of radiomic features to discriminate between different textures. Three scanners of different manufacturers and different magnetic field strengths were used to perform these assessments, aiming to understand to what extent such dependency may vary across these different settings.
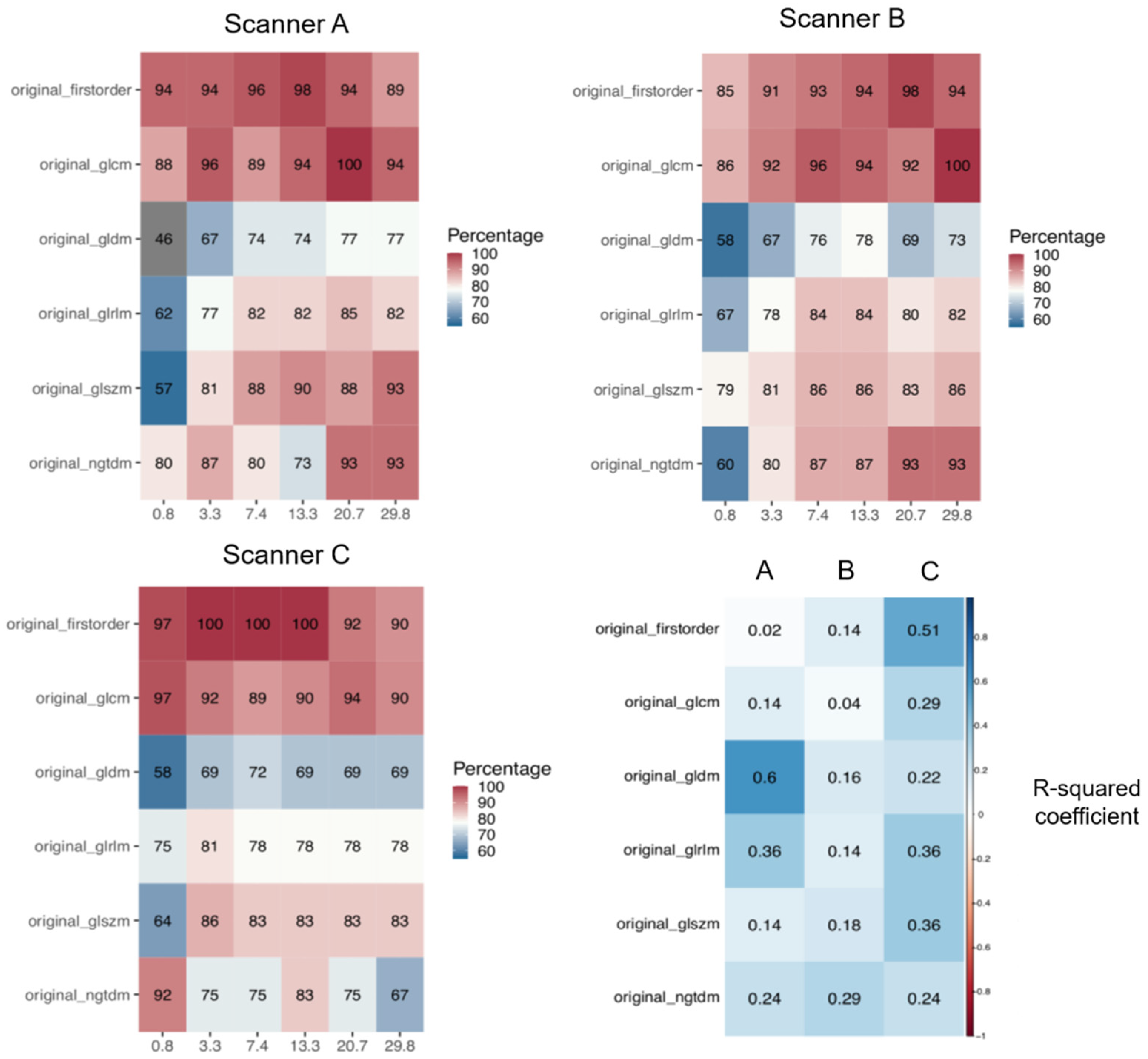
Only the radiomic features that had proved to be repeatable at test-retest imaging in a previous study performed on the same images [
9] were included in this analysis. As a result, the number of analyzed features was different for the three scanners: 829 for scanner A, 810 for scanner B, and 689 for scanner C (this lower number is likely due to the increased water-fat shift artifact).
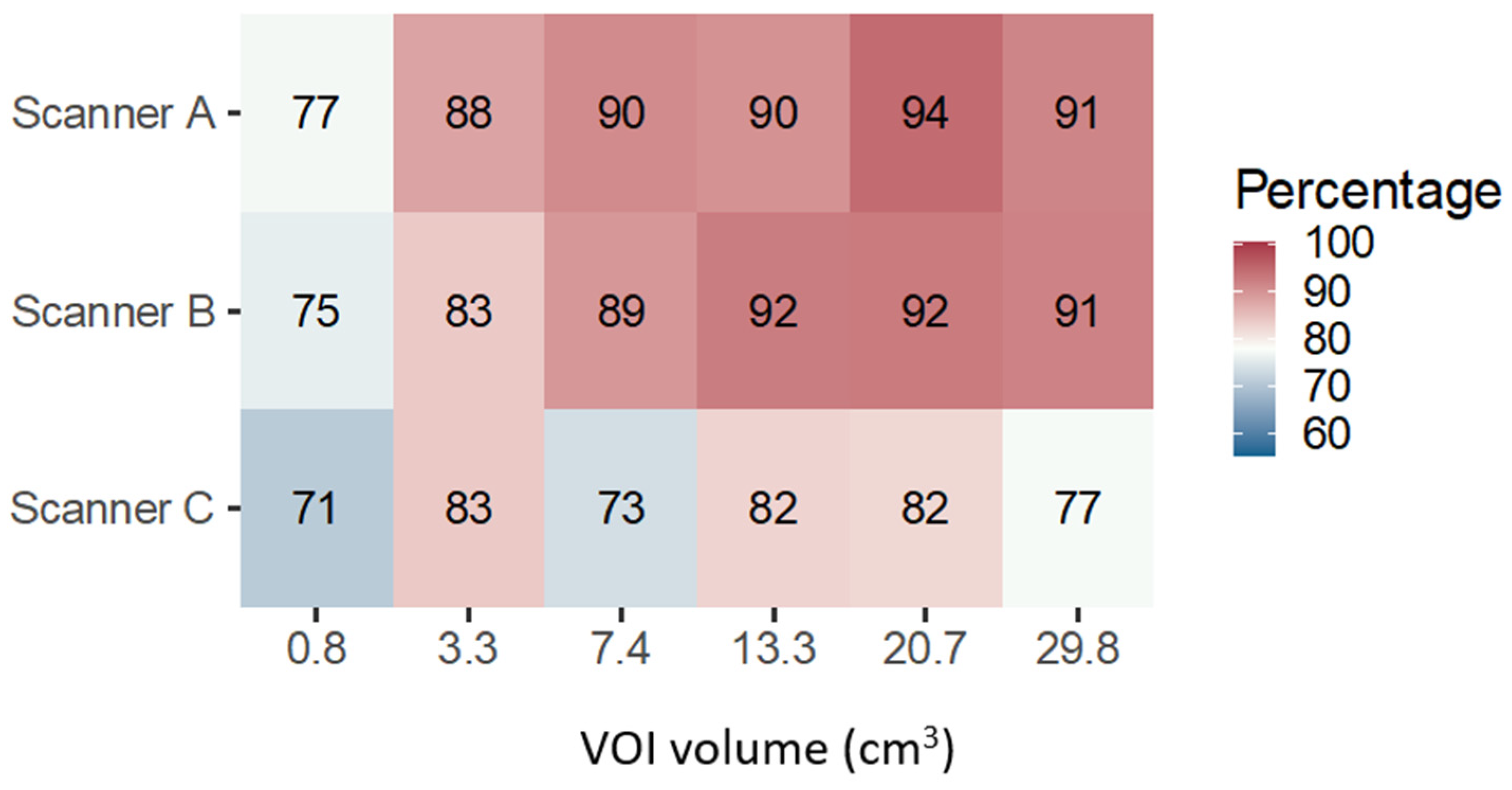
At the very first level of analysis, performed for each scanner separately, but aggregating the results for all feature classes, imaging filters, and texture couples, the main result emerged indicating that, for any scanner and VOI volume, a non-negligible fraction of features (>70%) was actually able to discriminate the different textures (
Figure 3). It was also observed that the smallest volume (0.8 cm
3) was less discriminative irrespectively of the scanner manufacturer and magnetic field strength; this is likely related to the reduced amount of information in the VOI (e.g., number of voxels), which might lead to inadequate sampling of the signal distribution therein, degrading the ability of the features to quantify the texture properties. Another common trend among scanners was the reduction in the proportion of discriminative features when increasing the VOI volume from 20.7 cm
3 to 29.8 cm
3, the latter being the largest VOI drawn in correspondence with the insert edge. In this regard, it might be argued that a slight amount of partial volume effect/background signal was included in the 29.8 cm
3 VOIs, possibly affecting the value of some features, which would have been discriminative otherwise. Finally, in the volume range from 3.3 cm
3 to 20.7 cm
3, an increasing trend in the proportion of discriminative features with volume was found for scanners A and B, supporting the hypothesis that a volume threshold (in terms of volume, or number of voxels) does exist for each feature to become discriminative, whereas noisy results were obtained for scanner C. While the number of features being considered was already different between scanners following the repeatability study performed on [
9], the percentage of discriminative features still varied across different scanners. Possible causes for such findings may be the different signal-to-noise ratios between scanners, artifacts affecting different scanners with different magnitudes, system types, e.g., digital versus analog, and coils used.
While the aggregated results captured interesting, broad, and succinct information, a more detailed analysis allows a deeper understanding and potentially provides more practical indications.
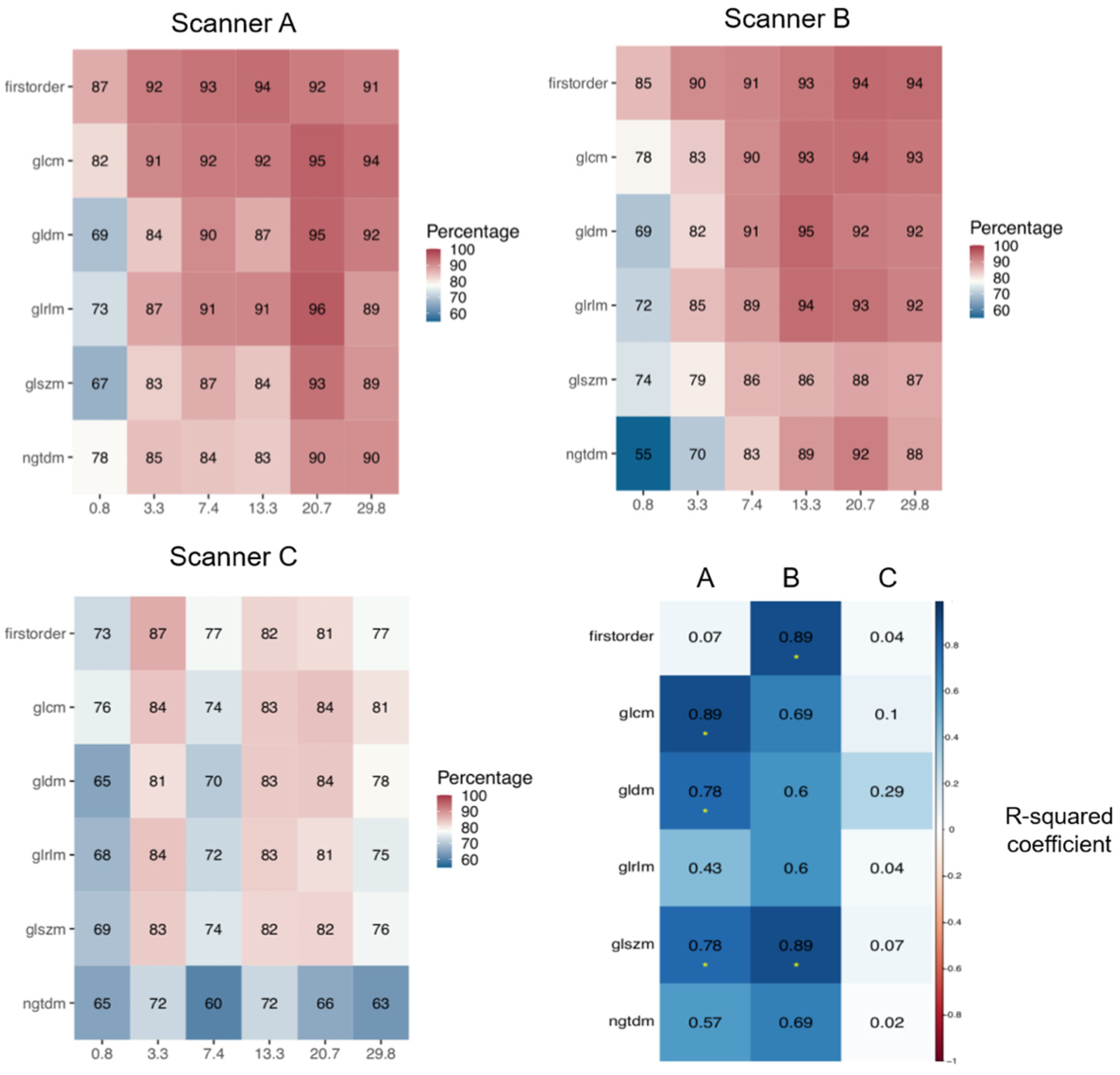
For this reason, the analysis of discriminative features was first stratified, for each scanner, according to feature class, still aggregating data by imaging filters (
Figure 4). For scanners A and B, while confirming the lower discriminability of the 0.8 cm
3 volume with respect to the larger volumes across all feature classes, the first-order class distinguished itself for better discrimination, even with the 0.8 cm
3 volume, with >85% of discriminative features, versus 55–81% for the other classes. In addition, a monotonic trend for the percentage of discriminative features as a function of volume (R
2 > 0.85) was obtained for GLCM, GLDM, and GLSZM classes for scanner A and for first-order and GLSZM classes for scanner B (
Figure 4, bottom right). In the case of scanner C, a general worse performance across volumes and feature classes was obtained, with no apparent trends.
Supplementary Figures S2–S10 report detailed results for each feature and separately for each couple of textures.
Aggregating data by image filters might mask some additional and informative trends. Indeed, as shown in
Supplementary Figure S13, the differences among the three textures might be suppressed or highlighted by specific filters. Hence, the inability to discriminate textures of some features might be wrongly attributed to VOI size when it might be an effect of the filter instead. For this reason, in
Figure 5, we report the analysis of discriminative features stratified by scanner and feature class for unfiltered (original) images only. For all scanners, a very interesting result emerged about the discriminative reliability of almost all features belonging to first-order and GLCM classes, for all volumes, without an apparent association between the percentage of discriminative features and volume (
Figure 5, bottom right). According to these results, these categories should be favored when analyzing original images, especially if small volumes are present in the data set under investigation. As for the remaining feature classes, with 1.5T scanners, GLRLM, GLSZM, and NGTDM categories appear to be discriminative with a quite high percentage of features (80–93%) but only above the 3.3 cm
3 volume threshold. Similar considerations can be derived for each filtered image by inspecting
Supplementary Materials Figures S14–S22. As a general comment, focusing on 1.5T scanners only, for most filtered images, it can be observed that first-order and GLCM features confirm their discriminative superiority across all volumes, even the small ones. The GLDM and GLRLM features are generally less discriminative, and finally, GLSZM and NGTDM features can equal first-order and GLCM performance but in most cases for larger volumes only. The worst discriminative performance was found with the wavelet-HH filter and with the Laplacian of Gaussian (LoG) filter, in the latter case possibly due to the high sigma value considered (6 mm), resulting in a smoothing level that reduces the texture differences among inserts; different values of sigma might be investigated to improve the discriminative power of the features. Conversely, wavelet-LL filtered images, meant as an approximation of original images, which likely reduces noise and other artifacts affecting radiomic features, provided very high discriminative performance, not only confirming the results observed with first-order and GLCM features on original images but also increasing the performance of NGTDM features in the case of scanners A and B, and even of GLRLM and GLSZM features in the case of scanner B.
Scanner C, the 3T scanner, provided, in general, worse discriminative performance and noisier results without evidence of specific trends. More in detail, it demonstrated an overall lower discriminability of radiomic features across sizes when compared with the 1.5T scanners (scanners A and B). This result is likely due to the higher magnetic field that leads to higher water-fat shift artifacts and possibly other artifacts (related to hardware, differences of SNR, parallel imaging not used in the current study, susceptibility, and bandwidth) that may reduce the discriminative ability of radiomic features between textures, as also seen in terms of repeatability.
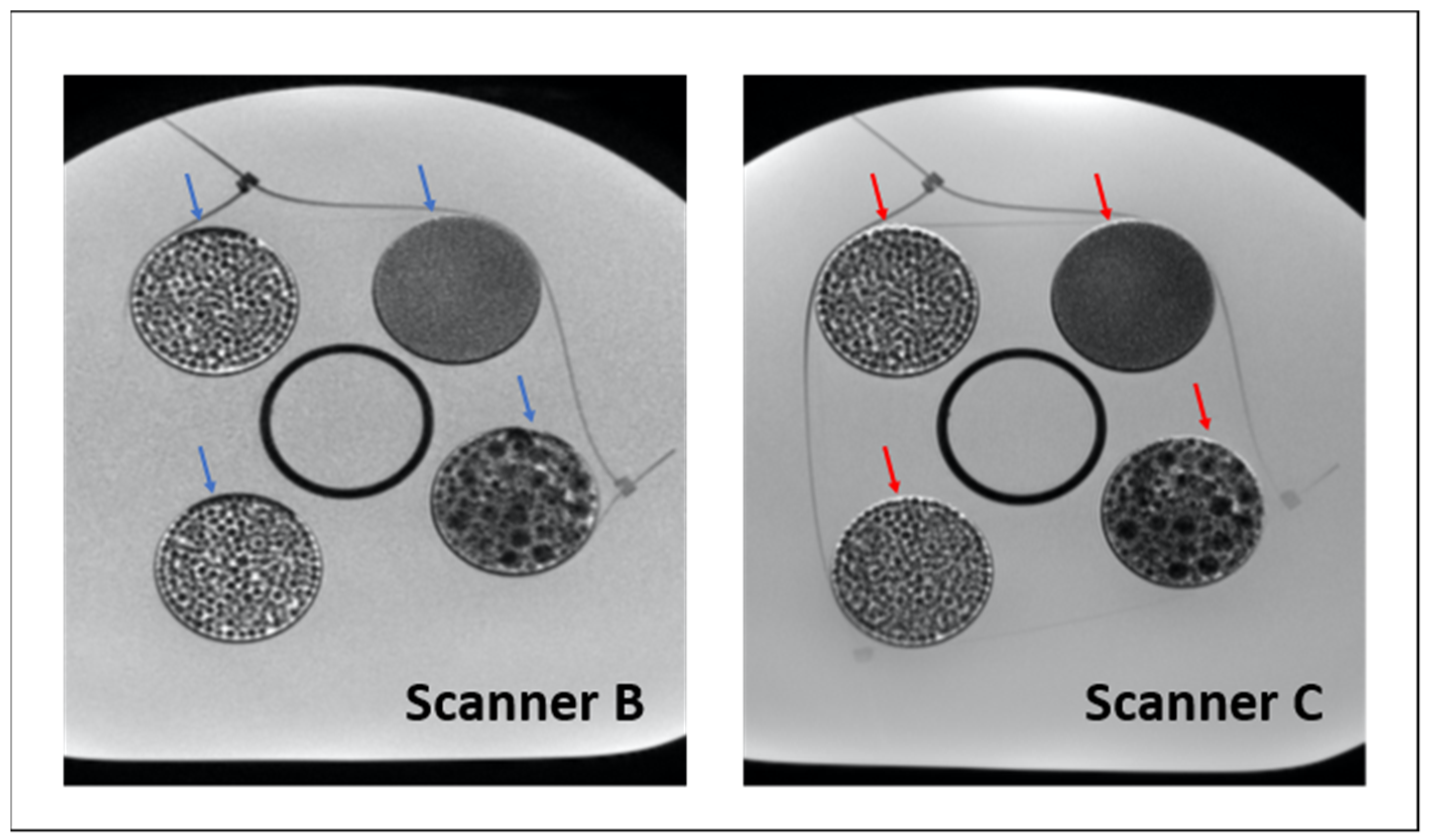
Figure 6 provides an example of the increased water-shift artifact in scanner C, particularly visible at the inserts’ edges but potentially present also within the inserts, where many interfaces occur. In addition, it appears evident, especially in the case of texture 2, that scanner C images are more blurry, which might have reduced the difference among textures, resulting in a lower ability of radiomic features to distinguish them. Hence, in the case of 3T scanners, additional investigation is required, especially with further adjustment of the bandwidth, which could reduce the chemical shift observed and improve the discriminability of radiomic features.
The results discussed so far refer to the specific settings used in this study for the calculation of features; future studies might investigate a filter-dependent optimization of such parameters, aiming to improve the ability of features to discriminate different textures at different volumes.
A similar study with both 1.5T and 3T scanners was performed by Ammari et al. [
12], who acquired images of phantom embedding inserts with different textures. Radiomic features were extracted for each insert considering a fixed VOI volume (6 cm
3). The authors report that texture features that are able to differentiate phantom textures were significantly influenced by field strength, in line with our findings. However, they did not report whether the number of discriminative features was higher in the case of 1.5T or 3T images, not allowing a straightforward comparison with our data. It must be highlighted, however, that to explain the different behavior of 1.5T and 3T scanners, not only the field strength has to be taken into account, but also the coil properties, which might be different between the two scanners, and the sequence parameters, which can be varied and optimized in different ways. For example, Waugh et al. [
13] found comparable texture misclassification with 1.5T images and 3T images acquired with a high temporal resolution and low spatial resolution sequence, whereas misclassification at 3T was notably reduced when using a high spatial resolution sequence. In our study, 3T images were acquired with the same acquisition parameters used on the 1.5T scanner from the same vendor.
In general terms, these data are a first attempt to quantify to what extent the ability of radiomic features to discriminate different textures depends on the VOI size, the textures to be discriminated, the magnetic field strength, the radiomic feature, and the image filter type, in case of T
2-weighted imaging of pelvic region. Despite not being directly generalizable to every clinical scenario, they are important for offering the first guide in the absence of more adequate data and, most importantly, for setting the basis and methodology for further investigation. To allow other researchers to adapt this analysis to their clinical scenario, the source code and data files used in this study are publicly available in the Github repository:
https://github.com/ReliabilityRadiomicsIEOFC/Radiomics_Size_Dependency, created on 7 March 2022.
Aiming at a clinical application of radiomics, especially in the case of the classical radiomic approach with the calculation of handcrafted radiomic features, it should be of paramount importance to assess these dependencies before designing a new radiomic study, along with other methodological investigations such as radiomic feature repeatability and reproducibility upon image acquisition and reconstruction parameters. If available, such information would allow customizing the patient inclusion criteria, setting a threshold/range to the size of the regions that can be properly investigated with radiomics, and would guide the choice of the most suitable image filter and feature categories for each study, avoiding an unnecessary proliferation of the parameters calculated from the images. Conversely, in the absence of this information, noise could be introduced, which might ultimately mask the information radiomics is expected to quantify and prevent the possibility of identifying useful associations between radiomic features and clinically relevant information.
Despite its relevance, this issue has not been extensively investigated in the case of MRI, probably due to the lack of suitable phantoms to perform systematic acquisitions under controlled settings. Jensen et al. [
14] investigated the stability of radiomic features across different VOI sizes using a homogeneous phantom and evidenced the need to repeat the analysis with heterogeneous objects to allow clinical translation of the phantom results. Ammari et al. [
12] developed a phantom with heterogeneous objects, but they considered one VOI volume only. To assess the volume dependency, instead, it is necessary not only to have objects with different textures, possibly simulating the texture of the tissues under investigation in the clinical application, but also that each object is homogeneous and large enough to incorporate different VOIs with different volumes but fixed texture. In this sense, the phantom developed by our group appears suitable for the purpose, even if the pattern within each insert is not obtained via replication of a texture kernel to increasingly larger volumes, as would be more appropriate, and even if until now only texture 1—small spheres—was demonstrated to be comparable to a real clinical scenario (gynecological diseases [
8]). Nonetheless, despite these two limitations, this phantom incorporates three textures that are different by construction, a difference that radiomic features should theoretically be able to reflect. In addition, despite the random distribution of small, medium, and large spheres in each insert, it is still true that the textures within different VOIs of the same insert are far more similar among them than the textures within VOIs drawn on different inserts. For these reasons, important information can be obtained from this phantom prototype, laying the basis for further studies, possibly with improved phantom versions to match more closely specific clinical scenarios.