Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Samples Preparation
2.3. Sugar Analysis
2.4. Glycosidic-Linkage Analysis
2.5. Colour
2.6. Statistical Analysis
3. Results and Discussion
3.1. Concentrate Elderberry Juice Carbohydrates Characterization
3.2. By-Products Characterization
3.3. Retentate Application as a Water-Soluble Colour Ingredient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food–A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Barsett, H.; Aslaksen, T.H.; Gildhyal, P.; Michaelsen, T.E.; Paulsen, B.S. Comparison of carbohydrate structures and immunomodulating properties of extracts from berries and flowers of Sambucus nigra L. Eur. J. Med. Plants 2012, 2, 216–229. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Ahmed, A.; Zou, Y.F.; Aslaksen, T.; Wangensteen, H.; Barsett, H. Structure-activity relationship of immunomodulating pectins from elderberries. Carbohydr. Polym. 2015, 125, 241–248. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT-Food Sci. Technol. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Stéger-Máté, M.; Abrankó, L. Effects of fruit juice concentrate production on individual anthocyanin species in elderberry. Int. J. Food Sci. Technol. 2016, 51, 641–648. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Buchweitz, M.; Brauch, J.; Carle, R.; Kammerer, D.R. Colour and stability assessment of blue ferric anthocyanin chelates in liquid pectin-stabilised model systems. Food Chem. 2013, 138, 2026–2035. [Google Scholar] [CrossRef]
- Da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin profile of elderberry juice: A natural-based bioactive colouring ingredient with potential food application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef] [PubMed]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Schillmöller, S.; Maier, O.; Schieber, A.; Carle, R. Colour stability of canned strawberries using black carrot and elderberry juice concentrates as natural colourants. Eur. Food Res. Technol. 2007, 224, 667–679. [Google Scholar] [CrossRef]
- Sampaio, S.L.; Lonchamp, J.; Dias, M.I.; Liddle, C.; Petropoulos, S.A.; Glamočlija, J.; Alexopoulos, A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Anthocyanin-rich extracts from purple and red potatoes as natural colourants: Bioactive properties, application in a soft drink formulation and sensory analysis. Food Chem. 2021, 342, 128526. [Google Scholar] [CrossRef]
- Bridle, P.; García-Viguera, C. A simple technique for the detection of red wine adulteration with elderberry pigments. Food Chem. 1996, 55, 111–113. [Google Scholar] [CrossRef]
- Landbo, A.K.; Kaack, K.; Meyer, A.S. Statistically designed two step response surface optimization of enzymatic prepress treatment to increase juice yield and lower turbidity of elderberry juice. Innov. Food Sci. Emerg. Technol. 2007, 8, 135–142. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; De Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Coelho, E.; Pinto, M.; Bastos, R.; Cruz, M.; Nunes, C.; Rocha, S.M.; Coimbra, M.A. Concentrate apple juice industry: Aroma and pomace valuation as food ingredients. Appl. Sci. 2021, 11, 2443. [Google Scholar] [CrossRef]
- Cruz, M.G.; Bastos, R.; Pinto, M.; Ferreira, J.M.; Santos, J.F.; Wessel, D.F.; Coelho, E.; Coimbra, M.A. Waste mitigation: From an effluent of apple juice concentrate industry to a valuable ingredient for food and feed applications. J. Clean. Prod. 2018, 193, 652–660. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Salvador, T.C.; Rocha, S.M.; Silvestre, A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): Influence of ripening, cultivar and season. Ind. Crops Prod. 2015, 71, 15–23. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.P.; de Sousa, H.C. Fractioned high pressure extraction of anthocyanins from elderberry (Sambucus nigra L.) pomace. Food Bioprocess Technol. 2010, 3, 674–683. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef]
- Santos, P.S.M.; Santos, G.T.A.D.; Cachada, A.; Patinha, C.; Coimbra, M.A.; Coelho, E.; Duarte, A.C. Sources of carbohydrates on bulk deposition in South-Western of Europe. Chemosphere 2021, 263, 127982. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple pomace extract as a sustainable food ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Wu, J.; Xu, W.; Wang, X.; Lü, X. Improvement of simultaneous determination of neutral monosaccharides and uronic acids by gas chromatography. Food Chem. 2017, 220, 198–207. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, S.M.; Coimbra, M.A. Foamability and foam stability of molecular reconstituted model sparkling wines. J. Agric. Food Chem. 2011, 59, 8770–8778. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Waldron, K.W.; Selvendran, R.R. Isolation and characterisation of cell wall polymers from olive pulp (Olea europaea L.). Carbohydr. Res. 1994, 252, 245–262. [Google Scholar] [CrossRef]
- Hilz, H.; Williams, P.; Doco, T.; Schols, H.A.; Voragen, A.G.J. The pectic polysaccharide rhamnogalacturonan II is present as a dimer in pectic populations of bilberries and black currants in muro and in juice. Carbohydr. Polym. 2006, 65, 521–528. [Google Scholar] [CrossRef]
- Aura, A.M.; Holopainen-Mantila, U.; Sibakov, J.; Kössö, T.; Mokkila, M.; Kaisa, P. Bilberry and bilberry press cake as sources of dietary fibre. Food Nutr. Res. 2015, 59, 28367. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K.K.; Söderling, E. A quantitative study of mannitol, sorbitol, xylitol, and xylose in wild berries and commercial fruits. J. Food Sci. 1980, 45, 367–371. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Silva, A.M.S.; Evtuguin, D.V.; Nunes, F.M.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. The hydrophobic polysaccharides of apple pomace. Carbohydr. Polym. 2019, 223, 115132. [Google Scholar] [CrossRef] [PubMed]
- Hilz, H.; Bakx, E.J.; Schols, H.A.; Voragen, A.G.J. Cell wall polysaccharides in black currants and bilberries-Characterisation in berries, juice, and press cake. Carbohydr. Polym. 2005, 59, 477–488. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Lecas, M.; Brillouet, J.M. Cell wall composition of grape berry skins. Phytochemistry 1994, 35, 1241–1243. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.M.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A. Revisiting the structural features of arabinoxylans from brewers’ spent grain. Carbohydr. Polym. 2016, 139, 167–176. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef]
- Fernandes, A.; Raposo, F.; Evtuguin, D.V.; Fonseca, F.; Ferreira-da-Silva, F.; Mateus, N.; Coimbra, M.A.; de Freitas, V. Grape pectic polysaccharides stabilization of anthocyanins red colour: Mechanistic insights. Carbohydr. Polym. 2021, 255, 117432. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Xu, Z.; Wicker, L. Blueberry pectin and increased anthocyanins stability under in vitro digestion. Food Chem. 2020, 302, 125343. [Google Scholar] [CrossRef] [PubMed]
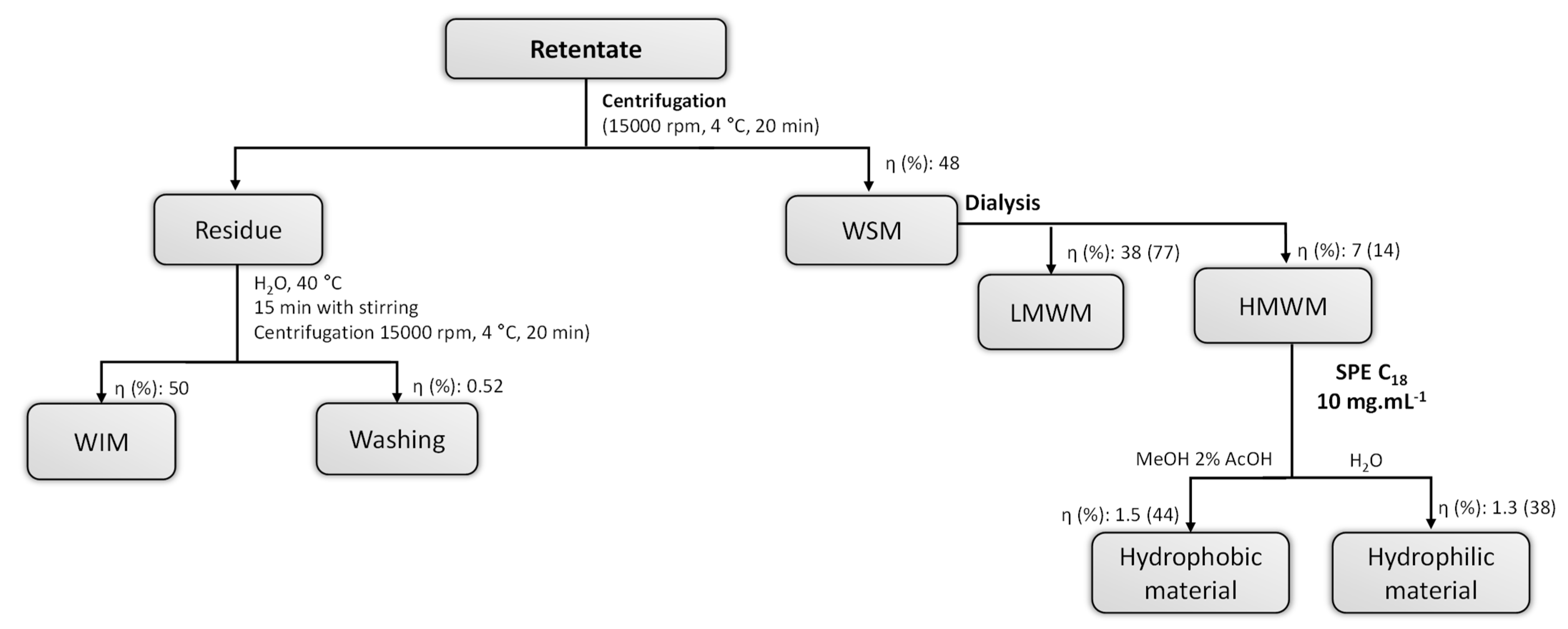
| ɳ% 1 | (%mol) | Total Sugars | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rha | Fuc | Ara | Xyl | Man | Gal | Glc | Fru | UA | (μg·mg−1) | ||
| ECJ Free sugars | 0.3 | 0.5 | 0.4 | 2 | 57 | 40 | 552 ± 0.3 | ||||
| ECJ Total sugars | 0.2 | 0.5 | 0.3 | 3 | 6 | 59 | 30 | 1 | 738 ± 0.1 | ||
| Polymeric material | 1 | 1 | 2 | 5 | 22 | 13 | 2 | 44 | 11 | 279 ± 21 | |
| Hydrophilic material | 0.2 (17) | 2 | 1 | 4 | 5 | 1 | 5 | 6 | 77 | 687 ± 34 | |
| Hydrophobic material | 0.5 (40) | 1 | 5 | 28 | 65 | 1 | 204 ± 1 | ||||
| ɳ% 1 | (mol%) | Total Sugars | Mannitol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rha | Fuc | Ara | Xyl | Man | Gal | Glc | Fru | GalA | (μg·mg−1) | (μg·mg−1) | ||
| Pomace | ||||||||||||
| Total sugars | 0.5 | 0.2 | 4 | 24 | 4 | 3 | 50 | 1 | 14 | 496 ± 6 | ||
| Free sugars | 9 | 61 | 5 | 19 | 5 | 152 ± 0.01 | ||||||
| Retentate | ||||||||||||
| WSM | 48 | |||||||||||
| Total sugars | 2 | 0.1 | 3 | 6 | 11 | 4 | 47 | 27 | 685 ± 34 | 9 ± 0.03 | ||
| Free sugars | 4 | 0.4 | 4 | 5 | 1 | 80 | 8 | 466 ± 0.05 | ||||
| LMWM | 38 (77) | 1 | 2 | 3 | 1 | 79 | 14 | 317 ± 0.2 | 10 ± 0.01 | |||
| HMWM | 7 (14) | 6 | 12 | 10 | 4 | 13 | 1 | 4 | 47 | 526 ± 36 | 4 ± 0.01 | |
| Hydrophilic material | 1.3 (38) | 8 | 1 | 8 | 4 | 1 | 16 | 3 | 61 | 719 ± 12 | ||
| Hydrophobic material | 1.5 (44) | 3 | 8 | 23 | 7 | 44 | 15 | 201 ± 0.05 | ||||
| Washing | 0.52 | |||||||||||
| Total sugars | 2 | 0.1 | 2 | 7 | 16 | 2 | 39 | 31 | 639 ± 11 | 8 ± 0.03 | ||
| Free sugars | 5 | 0.2 | 6 | 19 | 7 | 63 | 182 ± 0.02 | |||||
| WIM | 50 | |||||||||||
| Total sugars | 1 | 3 | 12 | 9 | 10 | 46 | 8 | 256 ± 6 | ||||
| Glycosidic-Linkage | %mol |
|---|---|
| t-Rhap | 3.3 |
| 2-Rhap | 5.8 |
| 2,4-Rhap | 3.5 |
| 2,3-Rhap | 1.5 |
| 2,3,4-Rhap | 1.6 |
| Total Rhap | 15.8 (19.5) |
| t-Fucp | 1.2 |
| Total Fucp | 1.2 (2.4) |
| t-Araf | 6.2 |
| 2-Araf | 0.7 |
| 3-Araf | 2.7 |
| 5-Araf | 1.1 |
| 4-Arap | 1.6 |
| 3,5-Araf | 0.5 |
| 2,3-Arap | 6.4 |
| 2,3,5-Araf | 2.0 |
| Total Ara | 21.3 (19.5) |
| t-Xylp | 3.7 |
| 2-Xylp | 0.5 |
| 4-Xylp | 6.2 |
| 2,4-Xylp | 2.9 |
| Total Xylp | 13.2 (9.8) |
| t-Manp | 0.3 |
| 3,4-Manp | 1.7 |
| 2,6-Manp | 0.6 |
| Total Manp | 2.7 (2.4) |
| t-Galp | 6.8 |
| 2-Galp | 0.5 |
| 4-Galp | 1.0 |
| 3-Galp | 0.5 |
| 6-Galp | 4.2 |
| 2,4-Galp | 2.6 |
| 3,6-Galp | 7.0 |
| 2,3,4-Galp | 1.1 |
| 2,3,4,6-Galp | 1.3 |
| Total Galp | 25.0 (39.0) |
| t-GalpA | 2.8 |
| 4-GalpA | 2.5 |
| 2,4-GalpA | 1.0 |
| Total GalpA | 6.3 |
| t-GlcpA | 7.3 |
| 3,4-GlcpA | 2.1 |
| Total GlcpA | 9.3 |
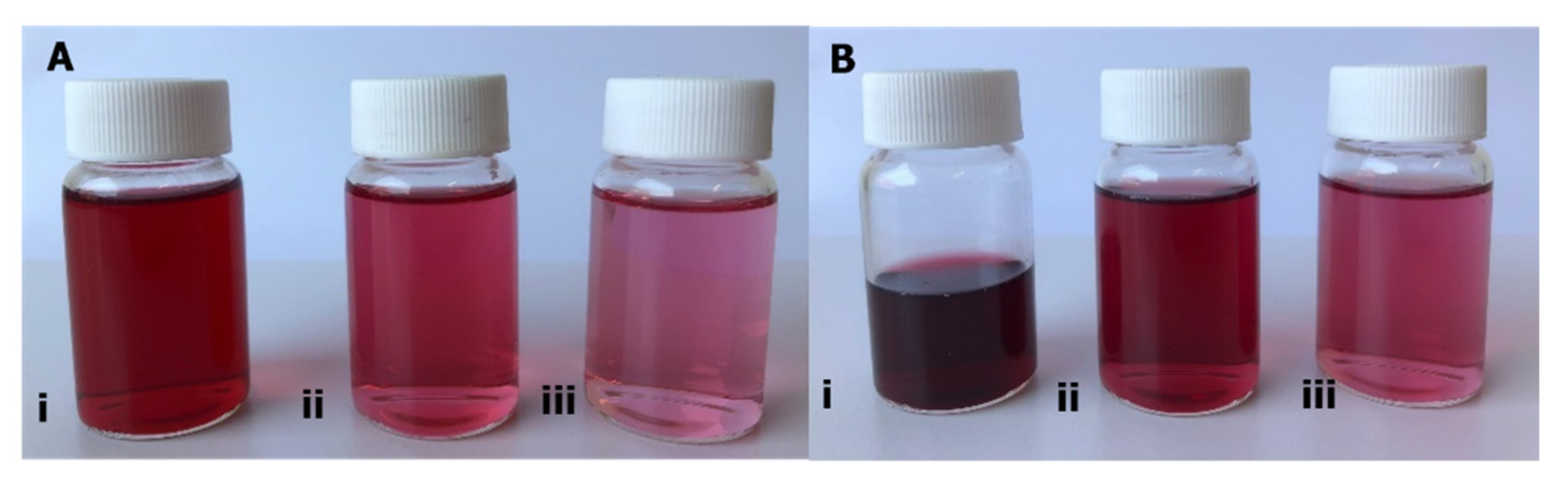
| Tonic Water Formulations | Day 0 | Day 22 | Day 72 | |||
|---|---|---|---|---|---|---|
| WSM | hydrophobic | WSM | hydrophobic | WSM | hydrophobic | |
| 1.0 mg·mL−1 | 1.40 ± 0.02aaa | 3.81 ± 0.02baa | 1.39 ± 0.01aaa | 2.01 ± 0.02bab | 0.95 ± 0.00aab | 2.50 ± 0.08bac |
| 0.25 mg·mL−1 | 0.46 ± 0.01aba | 1.17 ± 0.00bba | 0.36 ± 0.01abb | 0.61 ± 0.01bbb | 0.22 ± 0.00abc | 0.87 ± 0.15bbb |
| 0.10 mg·mL−1 | 0.19 ± 0.00aca | 0.51 ± 0.03bca | 0.17 ± 0.00acb | 0.24 ± 0.00bcb | 0.08 ± 0.00acc | 0.25 ± 0.00bcb |
| Tonic Water Formulations | L* | a* | b* | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 22 | Day 72 | Day 0 | Day 22 | Day 72 | Day 0 | Day 22 | Day 72 | ||||||||||
| WSM | hydrophobic | WSM | hydrophobic | WSM | hydrophobic | WSM | hydrophobic | WSM | hydrophobic | WSM | hydrophobic | WSM | hydrophobic | WSM | hydrophobic | WSM | hydrophobic | |
| 1.0 mg·mL−1 | 43.9 ± 0.3aaa | 35.5 ± 0.4baa | 43.8 ± 0.7aaa | 35.9 ± 0.2baa | 32.7± 0.9aab | 38.0 ± 0.7bab | 20.6 ± 0.4aaa | 12.4 ± 1.6baa | 20.7 ± 0.7aaa | 13.7 ± 0.6baa | 26.4 ± 1.2aab | 13.8 ± 0.3baa | 3.3 ± 0.5aaa | 1.7 ± 0.2baa | 3.7 ± 0.3aaa | 2.4 ± 0.2bab | 6.5 ± 0.7aab | 3.6 ± 0.2bac |
| 0.25 mg·mL−1 | 48.7 ± 0.5aba | 38.3 ± 0.7bba | 53.8 ± 0.4aba | 40.1 ± 0.4bbb | 42.4 ± 0.3abb | 40.9 ± 0.1bbb | 10.8 ± 0.5aba | 17.5 ± 0.8bba | 9.9 ± 0.2aba | 16.1 ± 0.4bba | 14.0 ± 0.2aba | 11.7 ± 0.8bbc | −0.7 ± 0.4aba | 2.0 ± 0.2baa | −0.6 ± 0.1aba | 2.9 ± 0.2bbb | 1.8 ± 0.1abb | 3.2 ± 0.4bab |
| 0.10 mg·mL−1 | 56.3 ± 0.4aba | 43.6 ± 0.3bca | 53.0 ± 1.5abb | 46.5 ± 0.3bca | 49.7 ± 0.2acc | 45.9 ± 0.8bca | 5.0 ± 0.1aca | 17.5 ± 0.4bba | 4.4 ± 0.2acb | 13.1 ± 0.3baa | 7.1 ± 0.3acc | 8.0 ± 0.2bca | −1.5 ± 0.0aca | 0.9 ± 0.1bba | −1.1 ± 0.2aca | 1.9 ± 0.0bcb | 0.3 ± 0.1acc | 2.5 ± 0.1bbb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso, M.I.; Coelho, E.; Trabulo, O.; Coimbra, M.A. Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation. Appl. Sci. 2022, 12, 9463. https://doi.org/10.3390/app12199463
Veloso MI, Coelho E, Trabulo O, Coimbra MA. Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation. Applied Sciences. 2022; 12(19):9463. https://doi.org/10.3390/app12199463
Chicago/Turabian StyleVeloso, Maria Inês, Elisabete Coelho, Oswaldo Trabulo, and Manuel A. Coimbra. 2022. "Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation" Applied Sciences 12, no. 19: 9463. https://doi.org/10.3390/app12199463
APA StyleVeloso, M. I., Coelho, E., Trabulo, O., & Coimbra, M. A. (2022). Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation. Applied Sciences, 12(19), 9463. https://doi.org/10.3390/app12199463








