Nephroprotective and Antioxidant Effects of Flavonoid-Rich Extract of Thymelaea microphylla Coss. et Dur Aerial Part
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoids Contents
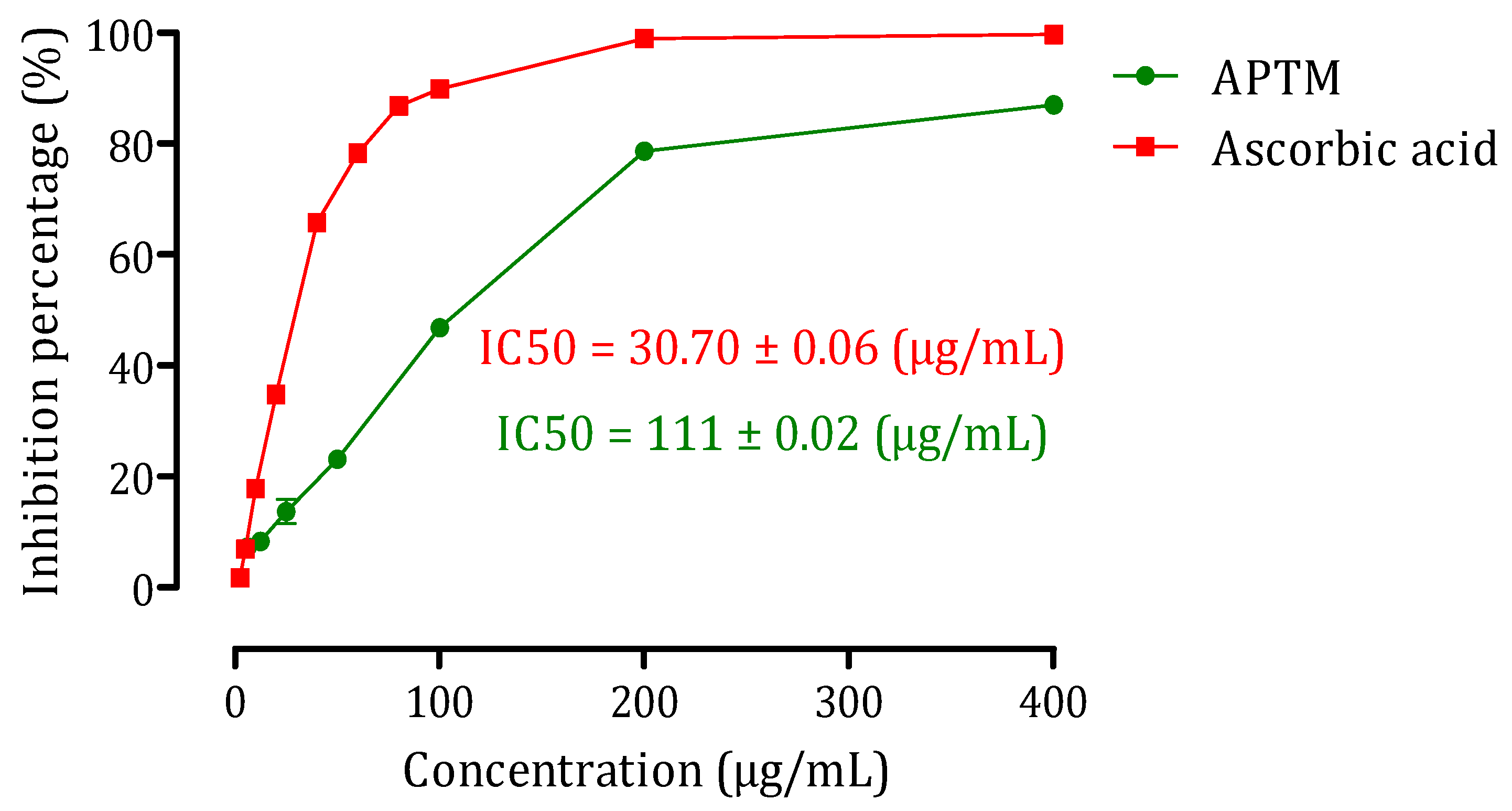
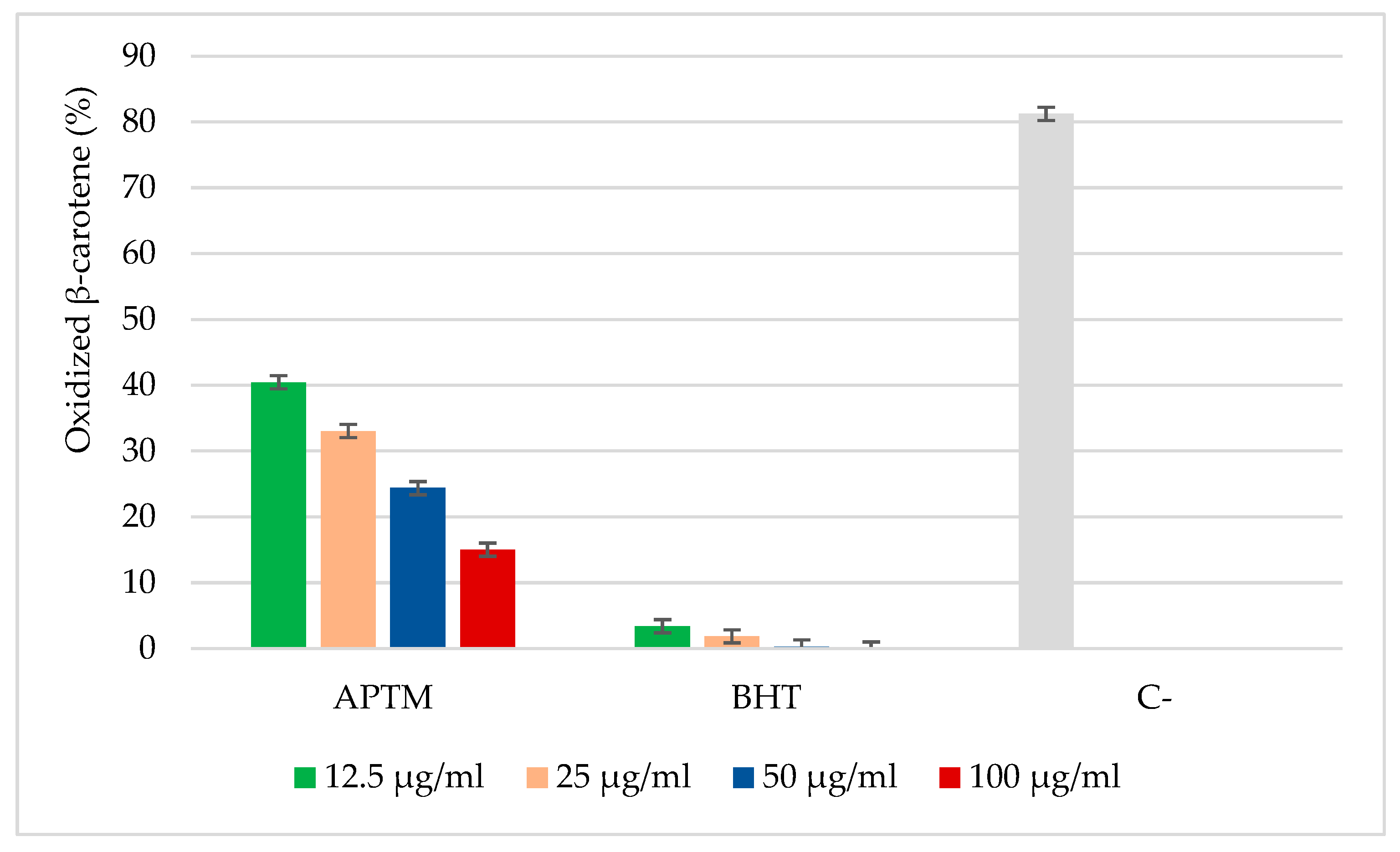
2.2. Antioxidant Activity of APTM Aqueous Extract
2.3. Acute Oral Toxicity Test of APTM Aqueous Extract
2.4. Nephroprotective Effect of APTM
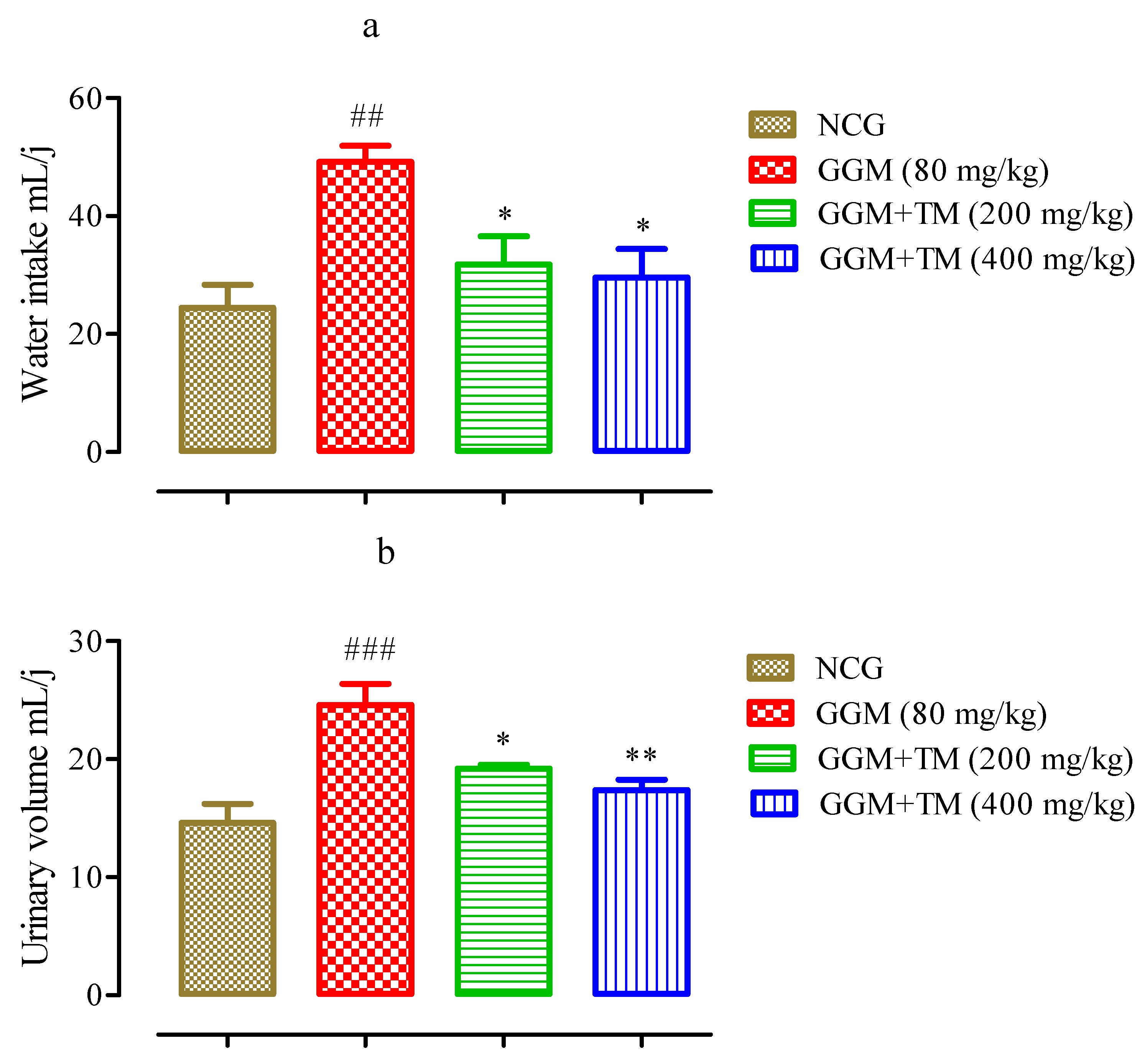
2.4.1. Effects of APTM on Urine Volume and Water Consumption
2.4.2. Weight Gain and Relative Kidney Weight to Body Weight
2.4.3. Effect of APTM on Serum and Urinary Creatinine, Uric Acid, and Urea Levels
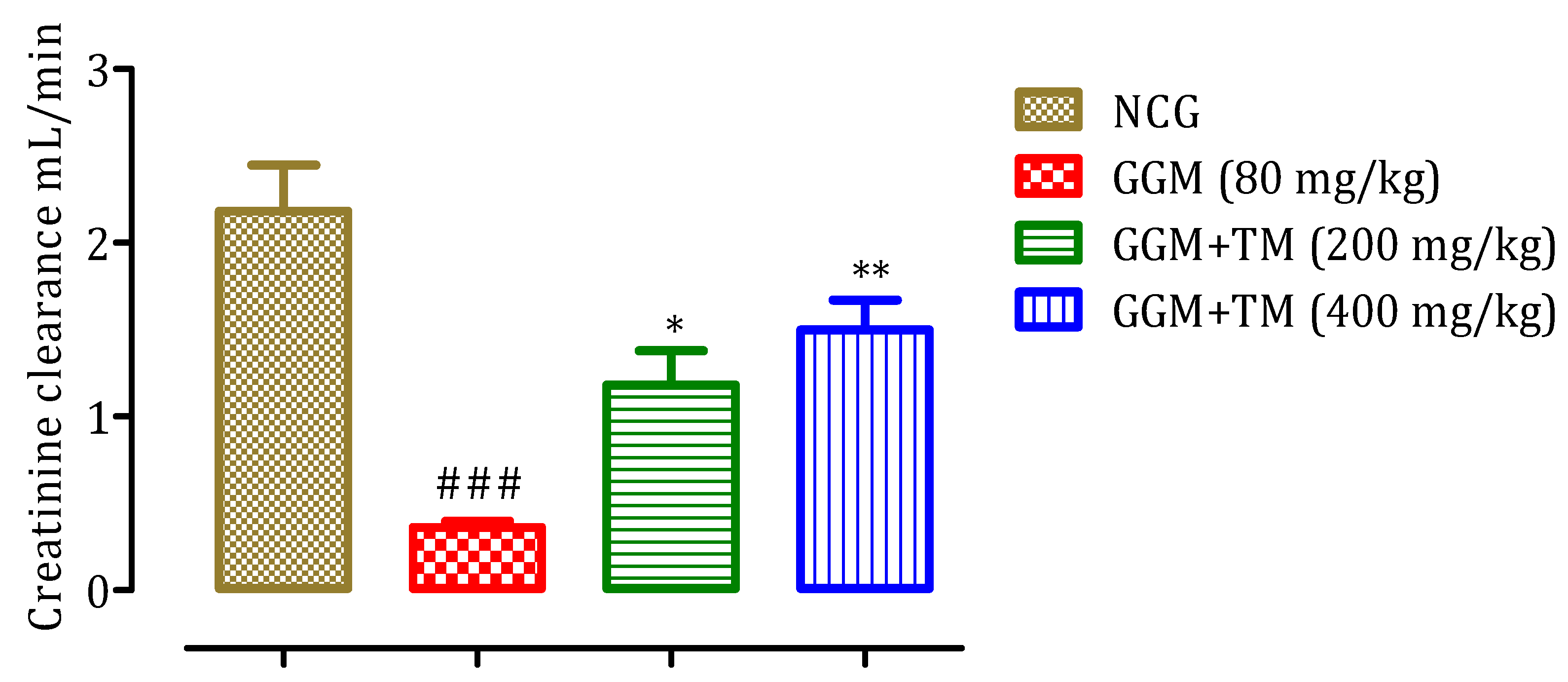
2.4.4. Effect of APTM on Creatinine Clearance
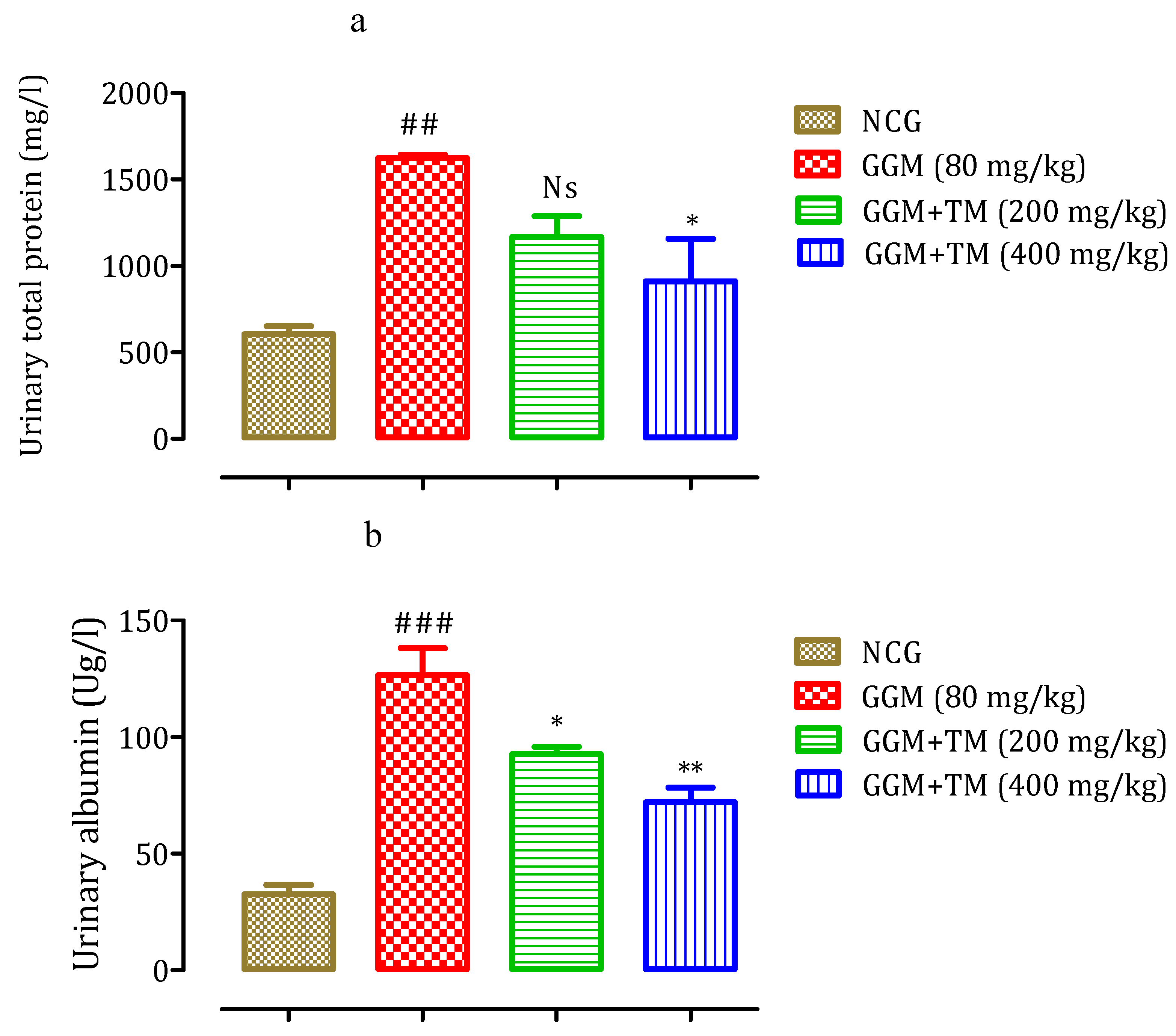
2.4.5. Effect of APTM on Urinary Total Protein and Albumin Levels
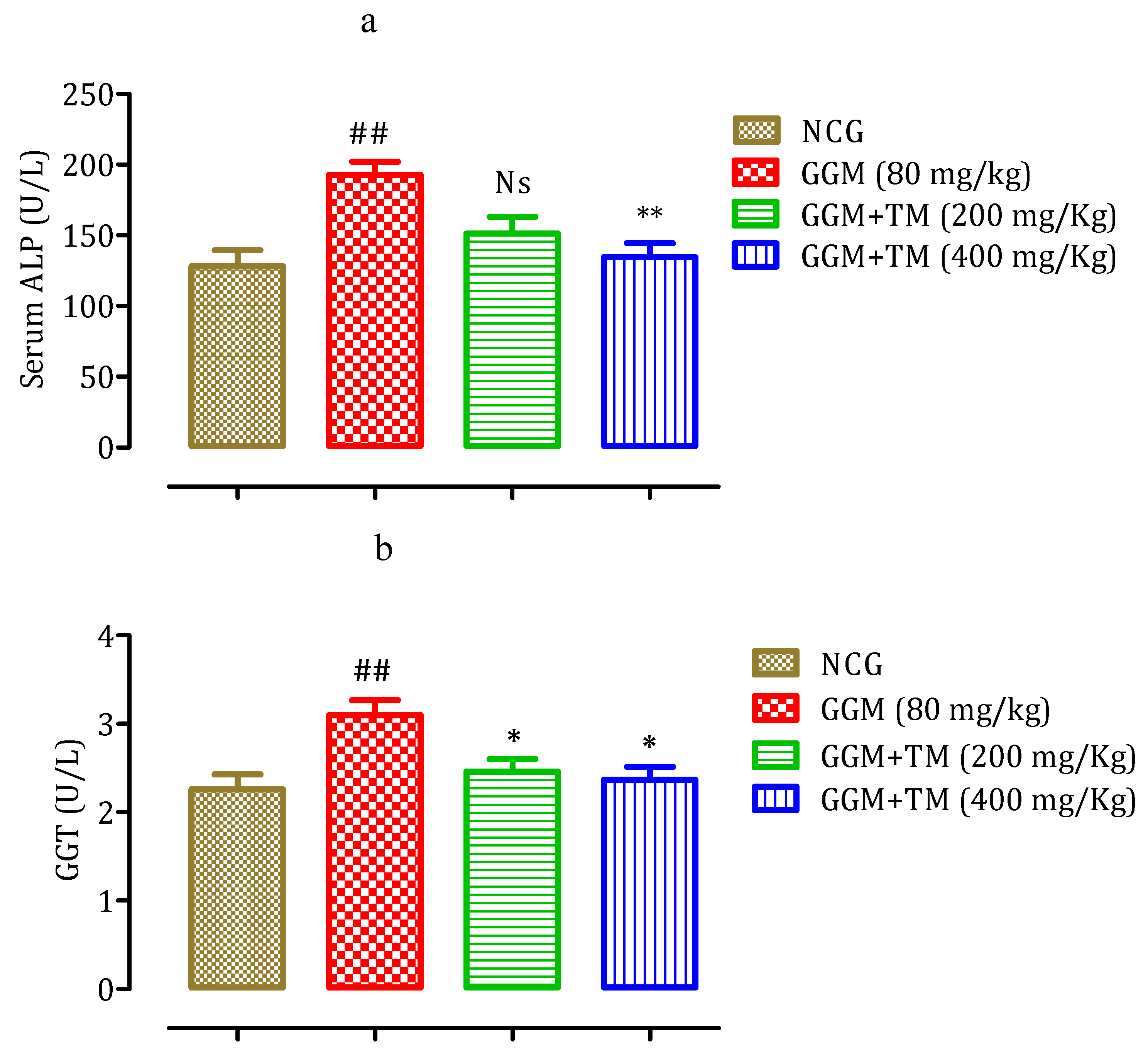
2.4.6. Effect of APTM on Serum Gamma-GT and Alkaline Phosphatase (ALP) Level
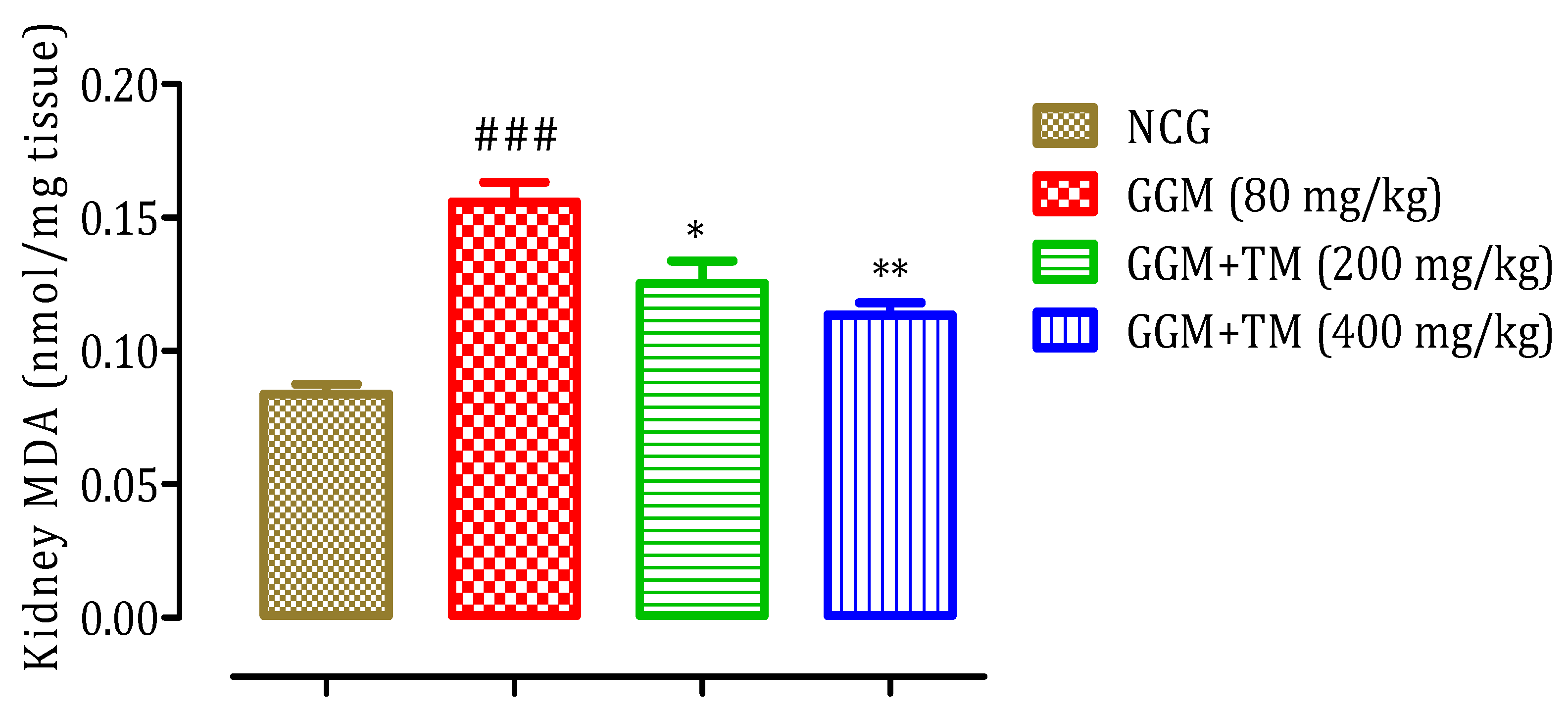
2.4.7. Effect of APTM on Renal Malondialdehyde (MDA) Level
2.4.8. Effect of APTM on Serum Electrolytes Level
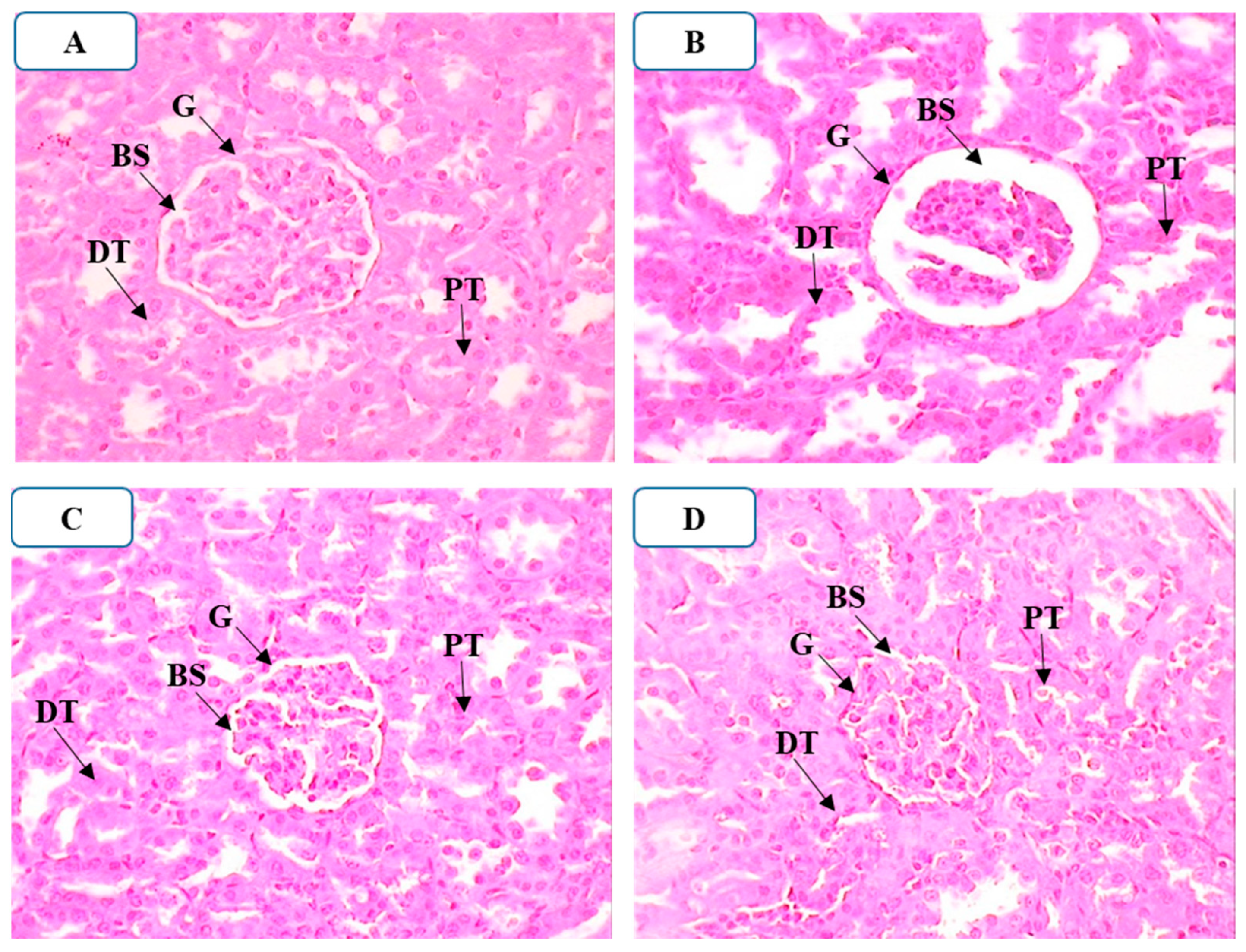
2.4.9. Effect of APTM on Histopathological Alterations in the Kidneys
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. APTM Extract Preparation
3.4. Estimation of Total Phenolic and Flavonoid Levels
3.5. Evaluation of the Antioxidant Properties
3.5.1. DPPH Free Radical-Scavenging Activity Test
3.5.2. β-Carotene Bleaching test
3.6. Acute Oral Toxicity Study
3.7. Nephroprotective Study
3.7.1. Experimental Design
3.7.2. Sample Collection
3.7.3. Biochemical Analysis
3.7.4. Creatinine Clearance (CCL)
3.7.5. Relative Kidney Weight (RKW)
3.7.6. Kidney Lipid Peroxidation
3.7.7. Histopathological Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jose, S.P.; Asha, S.; IM, K.; Ratheesh, M.; Santhosh, S.; Sandya, S.; Girish Kumar, B.; Pramod, C. Nephro-protective effect of a novel formulation of unopened coconut inflorescence sap powder on gentamicin induced renal damage by modulating oxidative stress and inflammatory markers. Biomed. Pharmacother. 2017, 85, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Martins, E. Nephrotoxicity and Nephroprotective Potential of African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 357–393. ISBN 9780128000182. [Google Scholar]
- Schortgen, F. Néphrotoxicité et médicaments. Reanimation 2005, 14, 436–441. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.-P.; Tulkens, P.M. Aminoglycoside nephrotoxicity. Curr. Drug Targets Infect. Disord. 2004, 4, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nagai, J.; Takano, M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab. Pharmacokinet. 2004, 19, 159–170. [Google Scholar] [CrossRef]
- Edson, R.; Terrell, C.L. The Aminoglycosides. Mayo Clin. Proc. 1999, 74, 519–528. [Google Scholar] [CrossRef]
- Bencheikh, N.; Bouhrim, M.; Kharchoufa, L.; Al Kamaly, O.M.; Mechchate, H.; Es-safi, I.; Dahmani, A.; Ouahhoud, S.; El Assri, S.; Eto, B.; et al. The Nephroprotective Effect of Zizyphus lotus L. (Desf.) Fruits in a Gentamicin-Induced Acute Kidney Injury Model in Rats: A Biochemical and Histopathological Investigation. Molecules 2021, 26, 4806. [Google Scholar] [CrossRef]
- Bouhrim, M.; Bencheikh, N.; Imtara, H.; Daoudi, N.E.; Mechchate, H.; Ouassou, H.; Kharchoufa, L.; Elachouri, M.; Mekhfi, H.; Ziyyat, A.; et al. Protective Effect of Opuntia dillenii (Ker Gawl.) Haw. Seed Oil on Gentamicin-Induced Nephrotoxicity: A Biochemical and Histological Analysis. Sci. World J. 2021, 2021, 7. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef]
- Govindappa, P.K.; Gautam, V.; Tripathi, S.M.; Sahni, Y.P.; Raghavendra, H.L.S. Effect of Withania somnifera on gentamicin induced renal lesions in rats. Rev. Bras. Farmacogn. 2019, 29, 234–240. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Bekkara, F.A. Phenolic contents and antioxidant activities in vitro of some selected Algerian plants. J. Med. Plants Res. 2014, 8, 1198–1207. [Google Scholar]
- Dahamna, S.; Dehimi, K.; Merghem, M.; Djarmouni, M.; Bouamra, D.; Harzallah, D.; Khennouf, S. Antioxidant, Antibacterial and Hypoglycemic Activity of Extracts from Thymelaea microphylla Coss. et Dur. Int. J. Phytocosmetics Nat. Ingredients 2015, 2, 15. [Google Scholar] [CrossRef][Green Version]
- Fakchich, J.; Elachouri, M. An overview on ethnobotanico-pharmacological studies carried out in Morocco, from 1991 to 2015: Systematic review (part 1). J. Ethnopharmacol. 2021, 267, 113–200. [Google Scholar] [CrossRef] [PubMed]
- Bencheikh, N.; Elbouzidi, A.; Kharchoufa, L.; Ouassou, H.; Merrouni, I.A.; Mechchate, H.; Es-Safi, I.; Hano, C.; Addi, M.; Bouhrim, M.; et al. Inventory of medicinal plants used traditionally to manage kidney diseases in north-eastern Morocco: Ethnobotanical fieldwork and pharmacological evidence. Plants 2021, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Noman, L.; Zellagui, A.; Hallis, Y.; Yaglioglu, A.S.; Demirtas, I.; Gherraf, N.; Rhouati, S. Antioxidant and antimicrobial activities of an endemic desert species Thymelea microphylla Coss. et Dur. Der Pharm. Lett. 2015, 7, 118–121. [Google Scholar]
- Allam, H.; Bennaceur, M.; Ksouri, R.; Sahki, R.; Marouf, A.; Benamar, H. Identification of phenolic compounds and assessment of the antioxidant and antibacterial properties of thymelaea microphylla coss. Et dur. from Western Algerian Sahara (Ain-Sefra Province). Jordan J. Pharm. Sci. 2020, 13, 363–377. [Google Scholar]
- Zrouri, H.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Kharchoufa, L.; Ouahhoud, S.; Ouassou, H.; El Assri, S.; Choukri, M. Phytochemical analysis, antioxidant activity, and nephroprotective effect of the Raphanus sativus aqueous extract. Mediterr. J. Chem. 2021, 11, 84. [Google Scholar] [CrossRef]
- Benhammou, N.; Bekkara, F.A.; Coustard, J. Antioxidant Activity of Methanolic and Water Extracts From Marrubium deserti (de Noë) and Thymelaea microphylla from Algerian sahara. Adv. Food Sci. 2009, 31, 194–201. [Google Scholar]
- Bencheikh, N.; Bouhrim, M.; Merrouni, I.A.; Boutahiri, S.; Legssyer, A.; Elachouri, M. Antihyperlipidemic and Antioxidant Activities of Flavonoid-Rich Extract of Ziziphus lotus (L.) Lam. Fruits. Appl. Sci. 2021, 11, 7788. [Google Scholar] [CrossRef]
- Obame, L.C.; Edou, P.; Bassolé, I.H.N.; Koudou, J.; Agnaniet, H.; Eba, F.; Traore, A.S. Chemical composition, antioxidant and antimicrobial properties of the essential oil of Dacryodes edulis (G. Don) H.J. Lam from Gabon. Adv. J. Microbiol. Res. 2012, 2012, 148. [Google Scholar]
- Khoulati, A.; Ouahhoud, S.; Mamri, S.; Alaoui, K.; Lahmass, I. Saffron extract stimulates growth, improves the antioxidant components of Solanum lycopersicum L., and has an antifungal effect Annals of Agricultural Sciences Saffron extract stimulates growth, improves the antioxidant components of Solanum lycopersic. Ann. Agric. Sci. 2019, 64, 138–150. [Google Scholar] [CrossRef]
- El-Zawahry, B.H.; Abu El Kheir, E.M. The Protective Effect of Curcumin against Gentamicin-Induced Renal Dysfunction and Oxidative Stress in Male Albino Rats. Egypt. J. Hosp. Med. 2007, 29, 546–556. [Google Scholar] [CrossRef]
- Erdem, A.; Gündoǧan, N.Ü.; Usubütün, A.; Kilinç, K.; Erdem, Ş.R.; Kara, A.; Bozkurt, A. The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol. Dial. Transplant. 2000, 15, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- El-Kashef, D.H.; El-Kenawi, A.E.; Rahim, M.A.; Suddek, G.M.; Salem, H.A. Agmatine improves renal function in gentamicin-induced nephrotoxicity in rats. Can. J. Physiol. Pharmacol. 2015, 94, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Rashid, U.; Khan, M.R. Fagonia olivieri prevented hepatorenal injuries induced with gentamicin in rat. Biomed. Pharmacother. 2017, 88, 469–479. [Google Scholar] [CrossRef]
- Salih, N.A. Effect of nettle (Urtica dioica) extract on gentamicin induced nephrotoxicity in male rabbits. Asian Pac. J. Trop. Biomed. 2015, 5, 756–760. [Google Scholar] [CrossRef]
- El Gamal, A.A.; Alsaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; Al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediators Inflamm. 2014, 2014, 12. [Google Scholar] [CrossRef]
- Salem, N.A.; Salem, E.A. Renoprotective effect of grape seed extract against oxidative stress induced by gentamicin and hypercholesterolemia in rats. Ren. Fail. 2011, 33, 824–832. [Google Scholar] [CrossRef]
- Mestry, S.N.; Gawali, N.B.; Pai, S.A.; Gursahani, M.S.; Dhodi, J.B.; Munshi, R.; Juvekar, A.R. Punica granatum improves renal function in gentamicin-induced nephropathy in rats via attenuation of oxidative stress. J. Ayurveda Integr. Med. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Nafiu, A.O.; Akomolafe, R.O.; Alabi, Q.K.; Idowu, C.O.; Odujoko, O.O. Effect of fatty acids from ethanol extract of Moringa oleifera seeds on kidney function impairment and oxidative stress induced by gentamicin in rats. Biomed. Pharmacother. 2019, 117, 109154. [Google Scholar] [CrossRef]
- Ogundipe, D.J.; Akomolafe, R.O.; Sanusi, A.A.; Imafidon, C.E.; Olukiran, O.S.; Oladele, A.A. Effects of two weeks administration of Ocimum gratissimum leaf on feeding pattern and markers of renal function in rats treated with gentamicin. Egypt. J. Basic Appl. Sci. 2016, 3, 219–231. [Google Scholar]
- Kuatsienu, L.E.; Ansah, C.; Adinortey, M.B. Toxicological evaluation and protective effect of ethanolic leaf extract of Launaea taraxacifolia on gentamicin induced rat kidney injury. Asian Pac. J. Trop. Biomed. 2017, 7, 640–646. [Google Scholar] [CrossRef]
- Raju, S.; Kavimani, S.; Uma Maheshwara Rao, V.; Sreeramulu Reddy, K.; Vasanth Kumar, G. Floral extract of Tecoma stans: A potent inhibitor of gentamicin-induced nephrotoxicity in vivo. Asian Pac. J. Trop. Med. 2011, 4, 680–685. [Google Scholar] [CrossRef]
- Perazella, M.A. Pharmacology behind common drug nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Randjelović, P.; Veljković, S.; Stojiljković, N.; Sokolović, D.; Ilić, I. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 2017, 16, 388–399. [Google Scholar] [PubMed]
- Abdel-Rahmeen, T.I.; Ahmed Ali, A.-G.; Gamal Abdallah, M. Protective Effect of Quercetin against Gentamicin-Induced Nephrotoxicity in Rats. Biol. Pharm. Bull. 2009, 32, 61–67. [Google Scholar] [CrossRef]
- Khan, M.R.; Badar, I.; Siddiquah, A. Prevention of hepatorenal toxicity with Sonchus asper in gentamicin treated rats. BMC Complement. Altern. Med. 2011, 11, 3–11. [Google Scholar] [CrossRef]
- Aboubakr, M.; Abdelazem, A.M. Hepatoprotective effect of aqueous extract of cardamom against gentamicin induced hepatic damage in rats. Int. J. Basic Appl. Sci. 2015, 5, 1. [Google Scholar] [CrossRef]
- Nale, L.P.; More, P.R.; More, B.K.; Ghumare, B.C.; Shendre, S.B.; Mote, C.S. Protective Effect of Carica papaya L. Seed Extract in Gentamicin Induced Hepatotoxicity and Nephrotoxicity in Rats. Int. J. Pharma Bio Sci. 2012, 3, 508–515. [Google Scholar]
- Parlakpinar, H.; Tasdemir, S.; Polat, A.; Bay-Karabulut, A.; Vardi, N.; Ucar, M.; Acet, A. Protective role of caffeic acid phenethyl ester (cape) on gentamicin-induced acute renal toxicity in rats. Toxicology 2005, 207, 169–177. [Google Scholar] [CrossRef]
- Boroushaki, M.T.; Asadpour, E.; Sadeghnia, H.R.; Dolati, K. Effect of pomegranate seed oil against gentamicin-induced nephrotoxicity in rat. J. Food Sci. Technol. 2014, 51, 3510–3514. [Google Scholar] [CrossRef]
- Baliga, R.; Ueda, N.; Walker, P.D.; Shah, S.V. Oxidant mechanisms in toxic acute renal failure. Drug Metab. Rev. 1999, 31, 971–997. [Google Scholar] [CrossRef] [PubMed]
- Dungca, N.T.P. Protective effect of the methanolic leaf extract of Eclipta alba (L.) Hassk. (Asteraceae) against gentamicin-induced nephrotoxicity in Sprague Dawley rats. J. Ethnopharmacol. 2016, 184, 18–21. [Google Scholar] [CrossRef]
- Chatterjee, P.; Mukherjee, A.; Nandy, S. Protective effects of the aqueous leaf extract of Aloe barbadensis on gentamicin and cisplatin-induced nephrotoxic rats. Asian Pac. J. Trop. Biomed. 2012, 2, S1754–S1763. [Google Scholar] [CrossRef]
- Raghavan, V.; Weisz, O.A. Discerning the role of mechanosensors in regulating proximal tubule function. Am. J. Physiol. Ren. Physiol. 2021, 15261, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rai, D.; Bhatia, G.; Sen, T.; Palit, G. Anti-stress Effects of Ginkgo biloba and Panax ginseng: A Comparative Study. J. Pharmacol. Sci. 2003, 464, 458–464. [Google Scholar] [CrossRef]
- Helal, M.G.; Zaki, M.M.A.F.; Said, E. Nephroprotective effect of saxagliptin against gentamicin-induced nephrotoxicity, emphasis on anti-oxidant, anti-inflammatory and anti-apoptic effects. Life Sci. 2018, 208, 64–71. [Google Scholar] [CrossRef]
- Kojom, J.J.W.; Nguemfo, E.L.; Djouatsa, Y.N.N.; Bogning, C.Z.; Azebaze, A.G.B.; Llorent-Martínez, E.J.; Córdova, M.L.F.D.; Dongmo, A.B. Phytochemical, antihypertensive and nephroprotective study of aqueous extract of the stems and roots of Selaginella vogelii Mett (Selaginellaceae) in rats. S. Afr. J. Bot. 2019, 127, 256–264. [Google Scholar] [CrossRef]
- Abdelrahman, R.S.; Abdelmageed, M.E. Renoprotective effect of celecoxib against gentamicin-induced nephrotoxicity through suppressing NFκB and caspase-3 signaling pathways in rats. Chem. Biol. Interact. 2020, 315, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteau, V. Tyrosine and Tryptophane in Proteins. J. Biol. Chem. 1927, 73, 627–648. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Ho, C.T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- OECD. Guideline 425: Acute Oral Toxicity–Up-and-Down-Procedure (UDP); OECD: Paris, France, 2008. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
| Route of Administration | Doses (mg/kg Body Weight) | Toxicity Symptoms |
|---|---|---|
| Oral | Control Group (Distilled Water) | None |
| APTM (50 mg/kg) | None | |
| APTM (300 mg/kg) | None | |
| APTM (500 mg/kg) | None | |
| APTM (700 mg/kg) | None | |
| APTM (2000 mg/kg) | None |
| Groups | Weight Gain (g) | Relative Kidney to Body Weight (g) |
|---|---|---|
| NCG | 20.3 ± 2.73 | 0.25 ± 0.02 |
| GGM (80 mg/kg) | 7.15 ± 0.69 ### | 0.45 ± 0.05 ### |
| GGM+TM (200 mg/kg) | 11.07 ± 2.33 Ns | 0.30 ± 0.02 *** |
| GGM+TM (400 mg/kg) | 14.15 ± 2.27 ** | 0.25 ± 0.03 *** |
| In Serum | In Urine | |||||
|---|---|---|---|---|---|---|
| Groups | Creatinine (mg/L) | Uric Acid (mg/L) | Urea (g/L) | Creatinine (mg/L) | Uric Acid (mg/L) | Urea (g/L) |
| NCG | 3.75 ± 0.96 | 22.2 ± 1.92 | 0.268 ± 0.061 | 551.76 ± 48.40 | 76.7 ± 12.18 | 22.79 ± 2.46 |
| GGM (80 mg/kg) | 10.05 ± 1.73 ### | 29.50 ± 3.70 # | 0.454 ± 0.068 ## | 206.71 ± 15.13 ### | 49.76 ± 4.11 ## | 10.81 ± 3.66 ## |
| GGM+TM (200 mg/kg) | 7.00 ± 0.81 ** | 27.20 ± 5.17 Ns | 0.336 ± 0.058 * | 383.96 ± 41.19 ** | 66.33 ± 5.50 * | 20.07 ± 3.90 * |
| GGM+TM (400 mg/kg) | 4.66 ± 1.13 *** | 22.60 ± 1.95 * | 0.314 ± 0.065 * | 469.35 ± 56.27 *** | 70.17 ± 2.47 ** | 22.21 ± 2.59 * |
| Groups | Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) | Calcium (mg/L) |
|---|---|---|---|---|
| NCG | 117.00 ± 20.70 | 3.45 ± 1.20 | 77.50 ± 18.94 | 70.55 ± 19.67 |
| GGM (80 mg/kg) | 154.20 ± 12.00 ## | 2.60 ± 0.78 Ns | 102.25 ± 3.59 # | 104.27 ± 4.18 ## |
| GGM+TM (200 mg/kg) | 145.50 ± 4.65 Ns | 2.84 ± 0.53 Ns | 99.25 ± 5.85 Ns | 97.06 ± 6.94 Ns |
| GGM+TM (400 mg/kg) | 143.50 ± 11.93 Ns | 3.11 ± 0.29 Ns | 94.60 ± 7.60 Ns | 94.17 ± 10.96 Ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencheikh, N.; Ouahhoud, S.; Cordero, M.A.W.; Alotaibi, A.; Fakchich, J.; Ouassou, H.; Assri, S.E.; Choukri, M.; Elachouri, M. Nephroprotective and Antioxidant Effects of Flavonoid-Rich Extract of Thymelaea microphylla Coss. et Dur Aerial Part. Appl. Sci. 2022, 12, 9272. https://doi.org/10.3390/app12189272
Bencheikh N, Ouahhoud S, Cordero MAW, Alotaibi A, Fakchich J, Ouassou H, Assri SE, Choukri M, Elachouri M. Nephroprotective and Antioxidant Effects of Flavonoid-Rich Extract of Thymelaea microphylla Coss. et Dur Aerial Part. Applied Sciences. 2022; 12(18):9272. https://doi.org/10.3390/app12189272
Chicago/Turabian StyleBencheikh, Noureddine, Sabir Ouahhoud, Mary Anne W. Cordero, Amal Alotaibi, Jamila Fakchich, Hayat Ouassou, Soufiane El Assri, Mohammed Choukri, and Mostafa Elachouri. 2022. "Nephroprotective and Antioxidant Effects of Flavonoid-Rich Extract of Thymelaea microphylla Coss. et Dur Aerial Part" Applied Sciences 12, no. 18: 9272. https://doi.org/10.3390/app12189272
APA StyleBencheikh, N., Ouahhoud, S., Cordero, M. A. W., Alotaibi, A., Fakchich, J., Ouassou, H., Assri, S. E., Choukri, M., & Elachouri, M. (2022). Nephroprotective and Antioxidant Effects of Flavonoid-Rich Extract of Thymelaea microphylla Coss. et Dur Aerial Part. Applied Sciences, 12(18), 9272. https://doi.org/10.3390/app12189272







